Abstract
INTRODUCTION
mTOR activity is increased in advanced prostate cancer (CaP) as a result of a high rate of PTEN mutations. RAD001 (Everolimus) is a new orally available mTOR inhibitor. The objective of our study was to evaluate the effects of RAD001 on the growth of CaP in the bone, both alone and in combination with docetaxel and zoledronic acid.
METHODS
C4-2 CaP cells were injected into tibiae of mice and the animals were treated with RAD001, docetaxel, and zoledronic acid alone or in combination. Histomorphometrical analysis, serum PSA measurements, bone mineral density (BMD), and µCT were used to determine the effects of treatment on tumor and bone.
RESULTS
All three agents alone decreased tumor volume, and RAD001 and docetaxel also decreased levels of serum PSA by 68% and 65%, respectively (both P < 0.01). Combinations of the agents were more effective in decreasing tumor volume than single agents. Three-drug treatment showed the greatest effect: 64% inhibition versus control (P < 0.01). Treatment with RAD001 interfered with the weight loss associated with growth of this tumor in the bone (non-RAD001 groups: 4.0% decrease in body weight, P = 0.0014; RAD001 groups: increase of 3.6% in body weight, P = 0.0037).
CONCLUSIONS
RAD001 inhibited growth of C4-2 cells in bone, an effect augmented by addition of docetaxel and zoledronic acid. Moreover RAD001 had a significant impact on maintenance of body weight. RAD001 may hold promise for its effects on both metastatic CaP and the important syndrome of tumor cachexia.
Keywords: mTOR, RAD001, prostate cancer, bone metastasis, combination chemotherapy
INTRODUCTION
Patients with advanced prostate cancer (CaP) suffer from the severe consequences of advanced disease, which commonly include bone metastases and cachexia. At present, there is no curative or effective long-term treatment for this stage of the disease. Therapeutic modalities that would eliminate or slow the growth of advanced metastatic CaP and improve quality of life are therefore of great interest.
Mutations or chromosomal abnormalities in cancer cells often result in altered cell-cycle regulation and growth-signal transduction. Pathways playing roles in these processes may be strategic targets for cancer therapy. One such pathway of possible clinical relevance to CaP is the PTEN tumor-suppressor pathway. Although deletion and mutation of the PTEN gene have been reported only infrequently in primary CaP [1], a high rate of mutations and deletions is seen in metastatic lesions of CaP as well as CaP cell lines and xenografts [2]. Absence of PTEN expression is correlated with a Gleason score 7 or higher in patients with advanced CaP [3]. Additionally, a number of other recent reports support a tumor-suppressor role of PTEN in CaP [4–8].
Deregulated signaling through the mTOR pathway is a prominent consequence of PTEN inactivation. Consistent with this, PTEN-negative tumors are particularly good targets for mTOR inhibitors [9,10]. mTOR, originally identified as the mammalian target of rapamycin, is a serine/threonine kinase that plays a central role in control of translation and also regulates anti-apoptotic signals (reviewed in Castedo et al. [11]). The potential clinical utility of inhibition of the mTOR pathway in CaP is supported by published reports of inhibition of CaP growth by rapamycin and its derivatives [12–17]. RAD001 (Everolimus), an orally bioavailable mTOR inhibitor, induces apoptosis of epithelial cells and completely reverses the neoplastic phenotype of mice expressing human AKT1 in the prostate [18]. It has also been shown to decrease tumor growth in a pancreatic cancer model [19].
Because of significant heterogeneity of CaP tumors and their capacity to mutate and adapt, treatment with a single agent is unlikely to result in prolonged clinical responses. Therefore, it is important to identify agents that may be useful in combination [20–22]. There are two prevalent ways of selecting agents for combination therapy: identification of compounds that act by clearly distinct mechanisms and selection of compounds affecting different points in a specific signaling pathway critical to cancer progression. In this study we have chosen the former strategy, evaluating the simultaneous inhibition of mTOR (RAD001) and tubulin polymerization (docetaxel, TAX), while also targeting the bone microenvironment (zoledronic acid, ZOL). Our choice of these compounds for combined testing with RAD001 was based on the following reasoning. TAX, an inhibitor of tubulin depolymerization, is the only agent that has been shown to prolong survival in patients with advanced CaP and is considered a standard therapy for these patients. However, this survival advantage was modest (1.9–2.4 months), and the drug is also associated with significant adverse side effects [23,24]. ZOL is a bisphosphonate which exerts beneficial effects in CaP patients with bone metastases, reducing both pain and skeletal related events [25–30]. Our previously published results suggested that the most likely mode of action of ZOL in retarding the growth of bone metastases is indirect, involving diminished availability of growth factors otherwise released through osteolysis, although we cannot rule out the possibility that ZOL directly inhibits the growth of CaP cells as well [26]. Based on these facts we hypothesize that the combination of RAD001, TAX, and ZOL—all acting on different pathways—will result in more pronounced inhibition of CaP tumor growth in the bone environment than any single treatment alone.
METHODS
Cell Line
The C4-2 cell line, a subline of LNCaP cells [31,32], is an androgen-independent line that expresses the androgen receptor and mutated PTEN. Cells were maintained under standard tissue culture conditions as previously described.
Intra-Tibial Tumors
All animal procedures were performed in compliance with the University of Washington Institutional Animal Care and Use Committee, and NIH guidelines. Four- to 6-week-old male SCID mice (Fox Chase SCID mice, Charles River, Wilmington, MA) were injected with approximately 2 × 105 cells into the proximal end of the right tibia as we have described previously [33–35]. Tumor growth was monitored by serum levels of prostate specific antigen (PSA). Mice were randomized into groups when the tumors were well established in the bone (mean of serum PSA >5 ng/ml in each group; 4–5 weeks after tumor-cell injections).
RAD001 and Docetaxel Dose Determinations
To gauge the efficacy of RAD001 and TAX in treating intra-tibial C4-2 tumors, we conducted initial experiments with two different doses of each drug. RAD001 for these studies was provided by Novartis Institute for BioMedical Research, Oncology, Basel, Switzerland. In the RAD001 portion of the study, 14 animals with tumors were randomized to three groups: (1) a control group receiving placebo gavage; (2) a group receiving 5 mg/kg RAD001 gavage five times per week; and (3) a group receiving 10 mg/kg RAD001 gavage five times per week. In the TAX portion of the study, 14 animals were randomized to three groups: (1) a control group receiving weekly placebo intraperitoneal (IP) injections; (2) a TAX group which received 10 mg/kg TAX via weekly IP injections; and (3) a TAX group which received 20 mg/kg TAX via weekly IP injections. Two mice were excluded from analysis due to tumor invasion of muscle, indicated by PSA > 100 ng/ml (one each in control group and TAX 20 mg/kg group). In both studies, blood samples were collected weekly for determination of serum PSA levels (IMx Total PSA assay, Abbott Laboratories, Abbott Park, IL). Body weight was measured weekly and BMD was measured (PIXImus Lunar densitometer, GE Healthcare, Waukesha, WI) prior to sacrifice. Animals were sacrificed 5 weeks after enrollment in the RAD001 study and 4 weeks after enrollment in the TAX study, since significant differences in PSA were observed after a shorter duration of TAX treatment.
Effects of RAD001, Docetaxel, and Zoledronic Acid on Growth of Tumor in the Bone
Animals with established tumors were randomized into eight groups treated with single agents or combinations of agents. After exclusion of animals with tumor in the muscle at the end of the study, each group had 7–9 animals (total 66 animals). Dosing regimens were determined based on the results of the preliminary individual studies with RAD001 and TAX and our previously published results with ZOL [25,26]. We sought to use low doses to elicit better-defined differences between groups and also to minimize adverse side effects of the treatments in a manner consistent with clinical practice. The selected dosing regimens were RAD001: 2.5 mg/kg gavage five times per week, TAX: 5 mg/kg IP every other week, and ZOL: 0.1 mg/kg injected subcutaneously (SC) twice per week. Placebo, given in place of each drug not administered, was 13% ethanol in saline by IP injection every other week in place of TAX, 5%glucose in place of RAD001, and saline SC injection twice per week in place of ZOL. The groups were as follows: (1) control (placebos only), (2) TAX, (3) RAD001, (4) ZOL, (5) TAX + ZOL, (6) RAD001 + ZOL, (7) TAX + RAD001, and (8) TAX + RAD001 + ZOL. Tumor growth was monitored by weekly serum PSA levels. Body weight was measured weekly. Animals were sacrificed 46 days after enrollment. Prior to sacrifice, bone mineral density (BMD) and lean body mass were determined by densitometry (PIXImus Lunar densitometer, GE Healthcare), and tibiae were harvested for µCT and histomorphometrical analyses.
Analysis of Intra-Osseus Tumors
Histomorphometrical analysis
Five micrometer sections of methacrylate-embedded tibiae stained with Goldner’s stain as described [25,26,36] were used for this analysis (n = 4–5 per group). Tumor volume (TuV, mm2), tissue volume (TV, mm2), and bone volume (BV, mm2) were measured in a blinded fashion on longitudinal sections of the tibiae using Bioquant Analysis Software (Bioquant Image Analysis Corporation, Nashville, TN).
Micro-computed tomography (µCT)
A Scanco vivaCT 40 high resolution µCT scanner was used to obtain 10.5-µm voxel resolution images spanning the proximal region and mid-shaft of tumored and non-tumored contralateral tibiae (n = 5 per group). Specific regions of analysis included a 0.85-mm section spanning the proximal tibia metaphysis and a 0.55-mm section of mid-diaphyseal cortical bone centered at a distance of 4.35 mm proximal to the tibia–fibula junction. Given the prolific woven bone induction observed in many of the samples, analyses of µCT data were confined to measurements of bone volume (BV; mm3), tissue volume (TV; mm3), and the ratio of bone volume to tissue volume in the metaphysis (BV/TV, %), and determinations of cortical volume (mm3) and periosteal volume (mm3) at the mid-diaphysis. All imaging and analyses were conducted with the identity of the specimens blinded.
Statistical Analyses
GraphPad Prism 3.0 (GraphPad Software, Inc., San Diego, CA) was used for the statistical analyses. All quantitative data are expressed as mean ± standard error of the mean (SEM). In initial evaluations of the effects of single agents on tumor growth, the unpaired Student’s t-test was used to determine the statistical significance of differences in serum PSA levels. To determine the statistical significance of differences among multiple groups, a one-way analysis of variance (ANOVA) with the Dunnett multiple comparison test was used to compare treatment groups with the placebo group while controlling for multiple testing errors. Results were determined to be significantly different when P < 0.05.
RESULTS
Effects of RAD001 on Growth of C4-2 in the Bone Environment
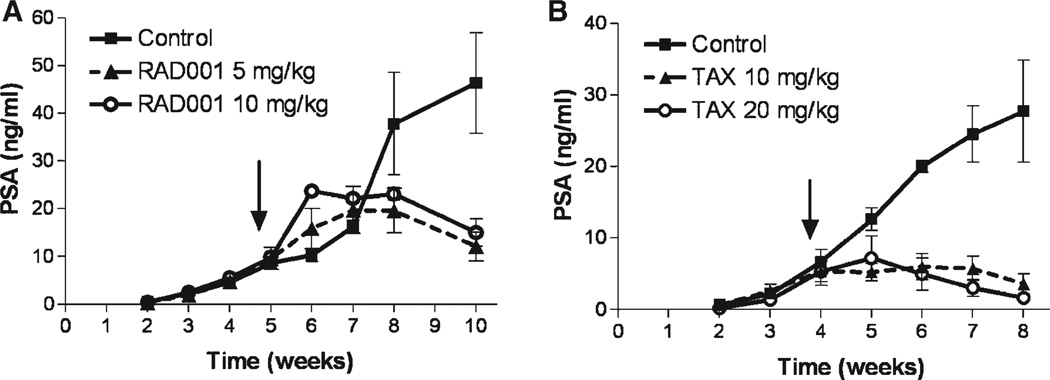
In this pilot study we investigated whether RAD001 inhibits growth of CaP in the bone and sought an optimal dose to use in combination with other agents. Our results show that RAD001 inhibits the growth of C4-2 xenografts in the tibiae of SCID male mice (Fig. 1A). At sacrifice, 5 weeks after the start of treatment, decreases in serum PSA levels of 69.42 ± 6.18% (P = 0.026) and 67.64 ± 6.18% (P = 0.029) compared to control-group levels were observed in the 5 mg/kg and the 10 mg/kg groups, respectively. Differences between the RAD001 groups were not significant. To further examine the effects of treatment on tumor growth we determined the weight of tumored tibiae. Relative to tibial weight in the control group (105.80 ± 7.92 mg), the weight of tumored-tibiae in RAD001-treated groups was significantly decreased in both the 5 mg/kg group (66.80 ± 2.85 mg, P = 0.0014) and the 10 mg/kg group (71.75 ± 1.65 mg; P = 0.0057). Again there was no significant difference between the RAD001 groups. Additionally, growth of C4-2 in tibiae showed a trend towards decreased overall body weight in control animals. Mean weight at the end of the study in the animals with C4-2 cells in the tibiae was 85.8 ± 4.6% of that at the initiation of treatment (P = 0.0047). The lower dose of RAD001 (5 mg/kg) inhibited this effect of the tumor, with a final weight 101.7 ± 3.6% of the weight at the start of treatment (P = 0.71). Animals treated with 5 mg/kg RAD001 had a final weight 20.9 ± 4.3% greater than the controls (P = 0.018). RAD001 (10 mg/kg) did not have this effect. Evaluation by bone densitometry revealed that C4-2 growth in the tibiae decreased BMD by ~35% versus contralateral non-tumored tibiae; this effect was not altered by RAD001. Because the inhibition of tumor growth with these two doses of RAD001 was similar, we selected an even lower dose of RAD001, 2.5 mg/kg, for the larger study in order to best elicit differential effects with combination therapy.
Fig. 1.
Effects of RAD001 and TAX on serum PSA. RAD001 and TAX inhibited tumor growth in bone as demonstrated by decreases in serum PSA levels. Arrows mark start of treatment. A: RAD001: 5 mg/kg, P = 0.026; and 10 mg/kg, P = 0.029. B: TAX: 10 mg/kg, P = 0.0070; and 20 mg/kg, P = 0.0011. Results are plotted as mean ± SEM.
Effects of TAX on Growth of C4-2 in the Bone Environment
TAX has been used in preclinical studies with tumors of various histological types. Since the efficacy of this agent depends on the tumor type as well as the growth environment [38], we tested two doses of TAX for inhibition of growth of C4-2 tumors in the bone. Both of the TAX doses inhibited tumor growth, as determined by lower levels of serum PSA in treated versus untreated animals (Fig. 1B). At sacrifice, serum levels of PSA were decreased by 86.9 ± 5.26% (P = 0.0007) and 93.9 ± 2.6% (P = 0.0011) in the 10 and 20 mg/kg TAX groups, respectively, versus the untreated group. No significant differences were observed between the TAX groups (P = 0.30). Compared with the control group (97.7 ± 15.3 mg), the weight of tibiae with tumors was significantly decreased in the TAX 20 mg/kg group (59.0 ± 4.0 mg; P = 0.049), and decreases that did not reach significance were detected in the TAX 10 mg/kg group (64.0 ± 4.8 mg; P = 0.053). The decrease in BMD caused by these cells was not significantly altered by TAX treatment (P = 0.20). As with RAD001, based on these results we selected a lower dose of 5 mg/kg every 2 weeks for the combination studies, anticipating that this dose of TAX would not eradicate the whole tumor.
Effects of RAD001, TAX, and ZOL on Growth of C4-2 in the Bone Environment
In comparison with the control group, serum PSA levels at the end of treatment were significantly lower in groups treated with TAX (67.5 ± 7.4% decrease, P < 0.01), RAD001 (64.6 ± 6.5% decrease, P < 0.01), RAD001 + ZOL (75.4 ± 2.9% decrease, P < 0.01), TAX + RAD001 (67.2 ± 4.8% decrease, P < 0.01), and TAX + RAD001 + ZOL (52.7 ± 10.7% decrease, P < 0.05), consistent with inhibition of tumor growth (Fig. 2A,B). As we have found previously [38], administration of ZOL alone did not result in decreased levels of serum PSA versus control animals.
Fig. 2.
Effects of RAD001, TAX, and ZOL alone and in combination on serum PSA. Treatment of C4-2 tumors in bone resulted in decreases in serum PSA levels. Treatment began at week 4 as indicated by arrow. A: Single-agent therapy. B: Combination therapy. C: Comparison of sacrifice serum PSA levels among groups. Results are plotted as mean ± SEM. Significant differences versus controls are denoted by: *P < 0.05, **P < 0.01.
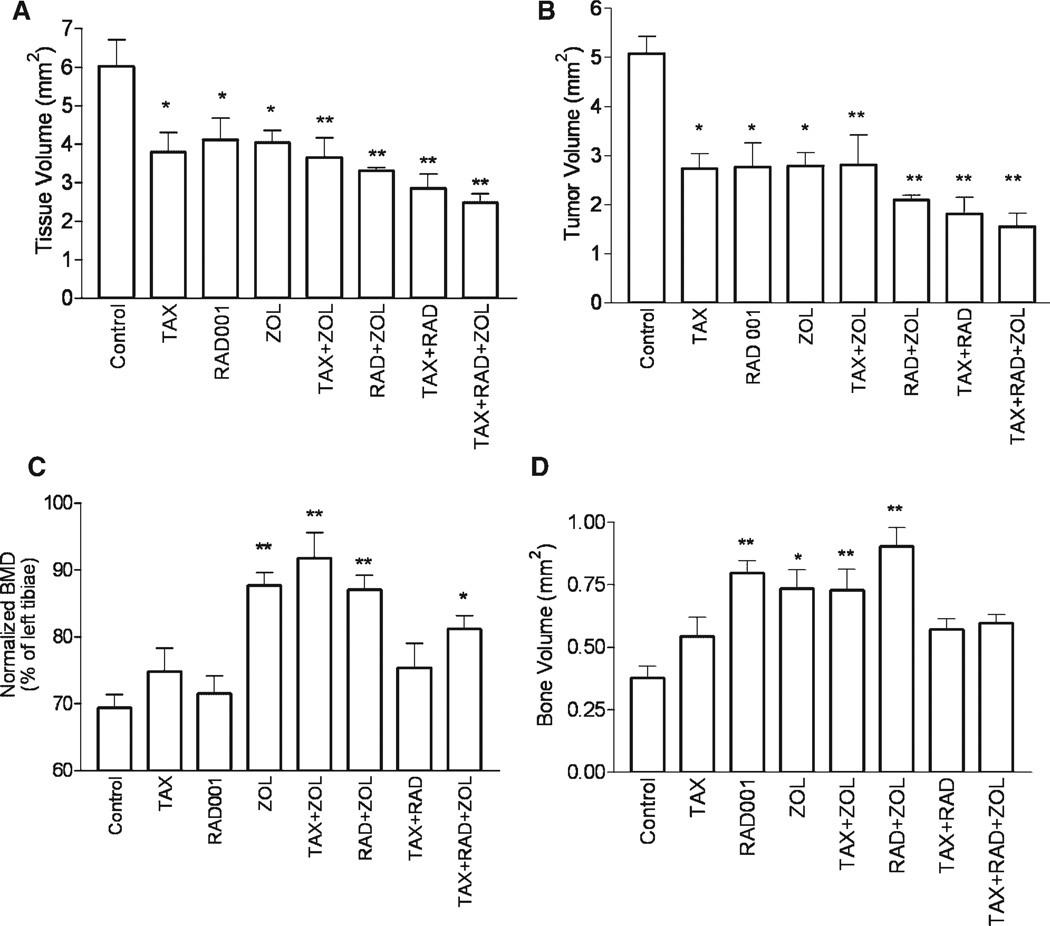
Serum PSA levels are frequently used to monitor tumor response to therapy, however serum PSA is not always concordant with tumor volume, particularly in advanced disease. Therefore, to further define the effects of the treatments on tumor growth, we performed histomorphometrical analysis. Our results show that tibiae with untreated C4-2 tumors are larger than those of the treated animals as a result of the tumor growth. This expansion of the tumored tibiae was inhibited in all treatment groups. Compared to untreated mice, tissue volume was decreased by 24–37% with monotherapy (P < 0.05), 39–53% with dual therapy (P < 0.01), and 59% (P < 0.01) with all three agents (Fig. 3A) as determined by ANOVA. These data suggest that RAD001, TAX, ZOL, and their combinations inhibit the bone expansion caused by tumor growth. Further analysis showed that tumor volume decreased by 48% with each single agent treatment (P < 0.01 for each; Fig. 3B), and decreases of 47–66% were detected with dual agent treatment (P < 0.01 for each). The combination of all three drugs resulted in a 71% decrease in tumor volume versus the control group (P < 0.01). Comparing the effect of RAD001 as a monotherapy to its use in combination with TAX and/or ZOL showed a trend toward a greater decrease in tumor volume with combination therapy. There was a decrease of 59.5 ± 6.1% (P = 0.09) with the addition of TAX, 68.7 ± 3.4% (P = 0.12) with ZOL, and 50.1 ± 8.9% (P = 0.042) when all three drugs were used. Our results also revealed a significant positive correlation between TuV and TV (R = 0.95, P < 0.0001), confirming our conclusion that tumor growth expands the tibiae. To normalize for changes in tissue volume, we also analyzed the ratio of tumor volume to tissue volume (%TuV/TV) in each treatment group. One-way ANOVA showed significant decreases with the treatments (P = 0.041) with a significant decrease in %TuV/ TV in each of the RAD001 groups: [RAD001 alone: 77.5 ± 3.2% of the control; RAD001 + ZOL: 74.3 ± 3.4% of the control; TAX + RAD001: 74.3 ± 6.8% of the control; and TAX + RAD001 + ZOL: 71.9 ± 5.4% of the control (P < 0.05 for all)]. These data suggest that the decreases in total TuV are not simply related to the limitation of space caused by increases in BV related to the ZOL treatment, but are due to a direct effect of the drug and their combinations.
Fig. 3.
Effects of RAD001, TAX, and ZOL alone and in combination on tumor and bone. Bone histomorphometry was used to evaluate the effects of the treatment on tissue volume (TV) and tumor volume (TuV). Analyses were performed on longitudinal sections of tibiae (n = 5 per group). BMD and BHM analyses were used to evaluate the effects of the treatments on bone. Tissue volume (A), and tumor volume (B) were significantly decreased in all treatment groups versus the control group. The largest decreases in TV and TuV were observed with all three agents in combination. Comparison of BMD normalized to contralateral (non-tumored) tibiae for each treatment. C4-2 cells cause decreases in BMD. Treatment with low doses of ZOL alone or in combination preserved bone (C). Treatments with ZOL generally caused increases in BV. RAD001 treatment increased BV, but the combination of three agents did not (D). Results are plotted as mean ± SEM. Significant differences versus controls are denoted by: *P < 0.05, **P < 0.01.
Effects of RAD001, TAX, and ZOL on Bone With C4-2 Tumors
Growth of CaP in the bone causes dysregulation of bone remodeling, increasing the rates of both bone formation and osteolysis. We investigated the effects of combination therapy on the bone in the presence of CaP cells, employing multiple modalities, including BMD, BHM, and µCT analyses. C4-2 tumor growth caused decreases in BMD (69.4 ± 2.0% of contralateral tibiae, P < 0.0001) that were similar to our published results [26]. Although TAX and RAD001 decreased serum PSA levels and tumor volume, they did not prevent changes in BMD (Fig. 3C). Overall, the normalized BMD of tumored tibiae in groups that did not receive ZOL did not differ significantly from controls (P = 0.44). Treatment with ZOL alone or in combination resulted in higher BMD of tumored tibiae versus untreated C4-2 tibiae (124 ± 2.1%, range 117–132%, P < 0.05). No significant differences were observed between the four ZOL groups. Bone volume of tumored tibiae as determined by BHM analysis was increased in the RAD001 group (210.0 ± 13.1%, P < 0.01), the ZOL group (193.4 ± 20.2%, P < 0.05), and the combination groups of TAX + ZOL (192.1 ± 22.0%, P < 0.01) and RAD001 + ZOL (238.2 ± 19.3%, P < 0.01) versus control tibiae with untreated C4-2 tumors. Interestingly, the bone volume of tibiae of animals treated with all three agents was not significantly different from that of untreated C4-2 tibiae (Fig. 3D).
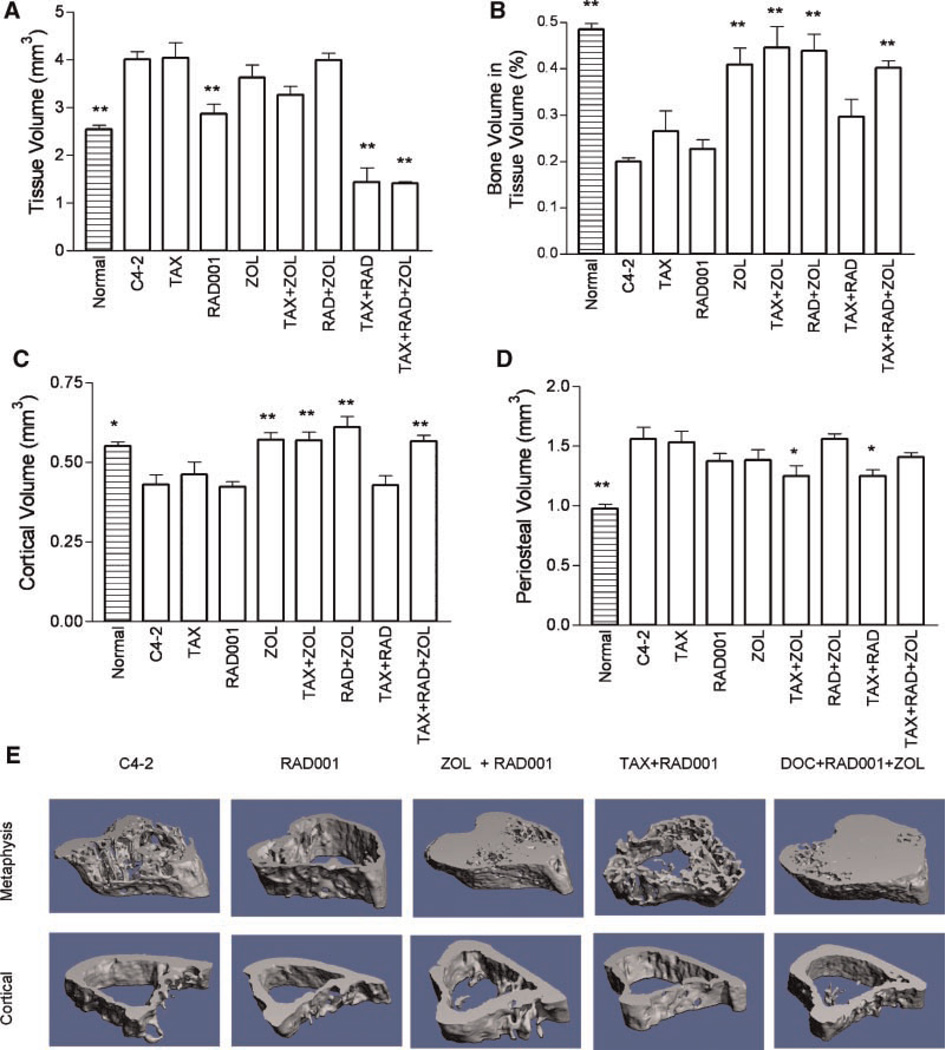
µCT analysis provides a three-dimensional view of the bone which we used to further evaluate effects of the treatments on the bone and the relationship between tissue and bone volumes. This analysis showed that growth of C4-2 cells expands the bone, increasing TV to 159.6 ± 6.5% (P < 0.001) of that of normal tibia. Treatment with RAD001, TAX + RAD001, or TAX + RAD001 + ZOL inhibited C4-2-induced expansion of tibiae in the metaphyseal region to 67.0 ± 2.5%, 35.8 ± 7.6%, and 35.2 ± 0.9% of the TV of C4-2 tibiae (Fig. 4A). There were also significant differences between the RAD001-monotherapy group and the RAD001 combinations: RAD001 + ZOL, 39.9 ± 4.9% of the RAD001 monotherapy; RAD001 + TAX, 49.9 ± 10.6%; and RAD001 + TAX + ZOL, 49.2 ± 1.2% (all P < 0.001). In contrast to TV, C4-2 growth in untreated mice resulted in a decrease in BV (71.3 ± 2.6%, P = 0.0024) versus normal tibiae. Only ZOL alone or in dual combinations resulted in increased BV. The BV/TV ratio was increased in all groups treated with ZOL (Fig. 4B). Analysis of cortical volume at the mid-diaphysis revealed similar findings (Fig. 4C). Periosteal growth occurs in some advanced CaP metastases [37]. In our model C4-2 cells cause increases in the periosteal bone volume relative to normal tibiae (159.5 ± 10.1%, P = 0.002). Treatment with TAX + ZOL and TAX + RAD001 interfered with the periosteal stimulation and decreased periosteal volume to ~80% of the tibiae with untreated C4-2 tumors (Fig. 4E). There is discordance between BHM and µCT results in evaluating BV in RAD001 monotherapy group. We hypothesize that this is perhaps because µCT evaluates 3D, while BHM is limited to the mid-tibia section. Moreover, µCT evaluation of the trabecular bone was used only in the region adjacent to the growth plate, while BHM analysis was performed on whole tibia sections, encompassing more tissue.
Fig. 4.
Micro-CT analyses. Analysis of the response of the bone to treatment was performed for trabecular bone (metaphysis) and cortical bone. A: Tissue volume at the metaphysis. B: Ratio of bone volume/tissue volume at the metaphysis. C: Cortical bone volume. D: Periosteal volume. Results are plotted as mean ± SEM. Significant differences versus untreated C4-2 xenografts are denoted by:*P < 0.05, **P < 0.01. Also shown for reference in these figures are the parameters of normal untreated tibiae. E: Representative examples of metaphyseal and cortical bone from groups treated with RAD001. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Effects of RAD001 on Body Weight
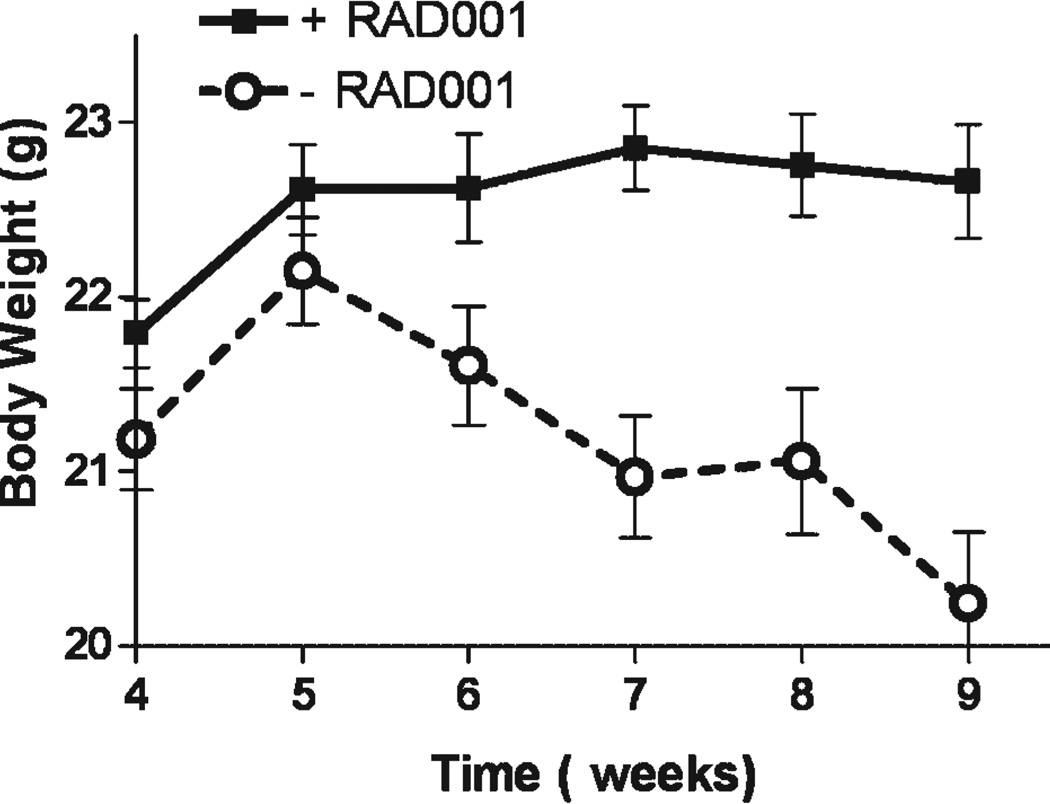
Because metastatic CaP is frequently associated with cachexia, and weight loss is also a side effect of some treatments, we evaluated the effect of the treatments on body weight. C4-2 cells grown in the bone environment caused decreases in body weight. The average weight at the end of the study was 91.0 ± 2.2% (P = 0.004) of the weight of the animals 1 week after the beginning of the treatment—the time point of greatest body weight. Interestingly, our results show that RAD001 as a monotherapy or in combination interfered with loss of body weight caused by tumor growth in the bone. Animals receiving RAD001 did not lose weight between 1 week after the beginning of treatment and the end of the study (99.3 ± 0.8%, P = 0.38), while animals not receiving RAD001 exhibited weight loss during the same period (94.1 ± 1.1%, P < 0.0001). There was a 10.7 ± 1.5% difference in sacrifice body weight between groups with and without RAD001 (P < 0.001) (Fig. 5). To further investigate this effect of RAD001 we examined total lean body mass as determined from BMD measurements of the whole animals. This analysis showed a significantly greater lean body mass in all animals treated with RAD001 (15.8 ± 0.2 g) versus all animals not receiving RAD001 (14.8 ± 0.3 g, P = 0.008).
Fig. 5.
RAD001 effects on bodyweight. Treatment with RAD001 inhibited weight loss caused by C4-2 growth in bone. For this analysis, animals were grouped based on whether or not they received RAD001. Total bodyweight was maintained in mice receiving RAD001, while animals with C4-2 tumors not treated with RAD001 experienced loss of body weight. There was a 10.7 ± 1.5% difference in final body weight between groups with and without RAD001 treatment (P < 0.001). Results are plotted as mean ± SEM.
DISCUSSION
Our studies yielded several novel findings: (1) RAD001 slowed the growth of C4-2 CaP cells in bone; (2) RAD001 inhibition of tumor growth was enhanced by administration of low doses of TAX and ZOL, with a trend toward decreased tumor volume as more agents were added; and (3) RAD001 maintained body weight in tumor-bearing animals.
Our results in this preclinical setting suggest that RAD001 is an effective treatment for advanced CaP in the bone as measured by all parameters related to tumor growth. The growth-inhibitory activity of RAD001 was significant even at the low dose of 2.5 mg/kg, as shown by marked decreases in both serum PSA levels and tumor volume. BHM and µCT results also indicated inhibition by RAD001 of tumor-related tibial expansion. TAX is currently the only drug that yields a survival benefit in patients with advanced CaP, but in our studies, low doses of RAD001 demonstrated similar inhibitory effects on tumor growth in the bone and serum PSA levels. Inhibition of the mTOR pathway may be of particular importance for CaP bone metastasis, since mTOR is a downstream effector of type I insulin-like growth factor (IGF-1) which is present at high levels in the bone environment. These findings are of special significance because most patients with advanced CaP have bone metastases, and these are associated with significant morbidity and mortality.
It is growing increasingly apparent with the ongoing development of targeted signal transduction therapies that cancer cells readily acquire resistance to these agents when administered as monotherapies. We have recently reported that the combination of ZOL with TAX was more effective than either drug alone in inhibiting growth of CaP in the bone [38], and early clinical studies of this combination have been encouraging [39]. As a logical next step, we have undertaken to add RAD001 to the combination, since this drug affects tumors by an entirely different mechanism. At this point there are few preclinical studies in CaP evaluating mTOR inhibitors in combination with other therapeutics. The mTOR inhibitors rapamycin and CCI-779 in combination with doxorubicin, mitoxantrone, docetaxel, and receptor tyrosine kinase inhibitors have yielded mixed results in studies with CaP cell lines and/or xenografts [9,13,40]. In our studies, we failed to detect synergistic effects with combination therapies, but a trend toward more pronounced inhibition was observed as more agents were added. Administration of all three agents in combination resulted in significantly greater inhibition of tumor growth than treatment with RAD001 alone.
Serum PSA is widely used in the clinical setting to evaluate the response of prostate cancer to treatment. In this study, the drug combinations did not lead to lower PSA levels at sacrifice than treatments with single agents, despite the fact that smaller tumor volumes were observed. While the mechanisms that underlie the decoupling of PSA and tumor volume in bone metastases await elucidation, we and others have previously observed similar discrepancies [26]. These observations coincide with clinical studies involving patients with bone metastases and illustrate the limitations of PSA as a surrogate marker of response in advanced patients.
Growth of CaP tumors in the bone causes dysregulation of bone remodeling. CaP bone metastases are usually of osteoblastic character, but increased bone lysis is also associated with advanced CaP. Our studies and others have shown that ZOL inhibits growth of CaP tumors in the bone while increasing bone volume. In the present study, only groups treated with ZOL exhibited significant increases in bone volume by BHM and µCT analyses. Interestingly, despite the observed inhibition of tumor growth by RAD001 and TAX, we did not observe inhibition of the osteolysis caused by the growth of these cells in the bone. We hypothesize that the perturbations in bone remodeling due to the presence of CaP cells occurred early in the course of the experiments, and therefore could not be affected by treatments that began subsequent to tumor establishment in the bone.
Advanced CaP is associated with cachexia, which is a serious complication of many cancers [41]. Our results show that growth of C4-2 tumor cells in the bone results in decreased body weight of tumor-bearing animals, and that treatment with RAD001 results in maintenance of body weight and lean body mass. The Akt/mTOR pathway has been shown to play a role in anorexia, including, possibly, tumor-related cachexia [42]. Leptin and leucin stimulate hypothalamic mTOR and decrease food intake and body weight in rats. These effects can be inhibited by administration of rapamycin. Although no impact of mTOR inhibitors on tumor cachexia has yet been demonstrated, the data presented, coupled with a sound biological rationale, suggest the need to pursue this possibility in further studies.
CONCLUSIONS
In summary, our data show that RAD001 inhibits the growth of CaP in the bone environment at a low dose of 2.5 mg/kg. Moreover, RAD001 combinations with TAX, ZOL, or both exhibit trends toward greater inhibition of tumor growth than any of these agents alone. Inclusion of ZOL in the combination also prevented increased osteolysis and bone loss associated with the growth of these tumors in the bone. Additionally, RAD001 prevented weight loss associated with growth of C4-2 tumors in the bone. Further prospective studies are warranted to determine whether these results are reproducible in other in vivo models. These preclinical observations support the use of RAD001 in the clinical setting, both on its own and in combination with other agents, in the treatment of advanced CaP.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Michael Corey for editorial assistance. RAD001 and zoledronic acid were kindly provided by Novartis Institute for Bio-Medical Research, Oncology, Basel, Switzerland. The reagents for determination of PSA levels were provided by Abbott Laboratories, Abbott Park, IL. This research was supported by the Pacific Northwest Prostate Cancer SPORE P50 CA097186 Pilot Award.
REFERENCES
- 1.Vignot S, Faivre S, Aguirre D, Raymond E. mTOR-targeted therapy of cancer with rapamycin derivatives. Ann Oncol. 2005;16:525–537. doi: 10.1093/annonc/mdi113. [DOI] [PubMed] [Google Scholar]
- 2.Vlietstra RJ, van Alewijk DC, Hermans KG, van Steenbrugge GJ, Trapman J. Frequent inactivation of PTEN in prostate cancer cell lines and xenografts. Cancer Res. 1998;58:2720–2723. [PubMed] [Google Scholar]
- 3.Heaney RP. The bone-remodeling transient: Implications for the interpretation of clinical studies of bone mass change. J Bone Miner Res. 1994;9:1515–1523. doi: 10.1002/jbmr.5650091003. [DOI] [PubMed] [Google Scholar]
- 4.Koksal IT, Dirice E, Yasar D, Sanlioglu AD, Ciftcioglu A, Gulkesen KH, Ozes NO, Baykara M, Luleci G, Sanlioglu S. The assessment of PTEN tumor suppressor gene in combination with Gleason scoring and serum PSA to evaluate progression of prostate carcinoma. Urol Oncol. 2004;22:307–312. doi: 10.1016/j.urolonc.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Dreher T, Zentgraf H, Abel U, Kappeler A, Michel MS, Bleyl U, Grobholz R. Reduction of PTEN and p27kip1 expression correlates with tumor grade in prostate cancer. Analysis in radical prostatectomy specimens and needle biopsies. Virchows Arch. 2004;444:509–517. doi: 10.1007/s00428-004-1004-6. [DOI] [PubMed] [Google Scholar]
- 6.Hermans KG, van Alewijk DC, Veltman JA, van Weerden W, Van Kessel AG, Trapman J. Loss of a small region around the PTEN locus is a major chromosome 10 alteration in prostate cancer xenografts and cell lines. Genes Chromosomes Cancer. 2004;39:171–184. doi: 10.1002/gcc.10311. [DOI] [PubMed] [Google Scholar]
- 7.Deocampo ND, Huang H, Tindall DJ. The role of PTEN in the progression and survival of prostate cancer. Minerva Endocrinol. 2003;28:145–153. [PubMed] [Google Scholar]
- 8.Wang S, Gao J, Lei Q, Rozengurt N, Pritchard C, Jiao J, Thomas GV, Li G, Roy-Burman P, Nelson PS, Liu X, Wu H. Prostate-specific deletion of the murine Pten tumor suppressor gene leads to metastatic prostate cancer. Cancer Cell. 2003;4:209–221. doi: 10.1016/s1535-6108(03)00215-0. [DOI] [PubMed] [Google Scholar]
- 9.Wu L, Birle DC, Tannock IF. Effects of the mammalian target of rapamycin inhibitor CCI-779 used alone or with chemotherapy on human prostate cancer cells and xenografts. Cancer Res. 2005;65:2825–2831. doi: 10.1158/0008-5472.CAN-04-3137. [DOI] [PubMed] [Google Scholar]
- 10.Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R, Frost P, Gibbons JJ, Wu H, Sawyers CL. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castedo M, Ferri KF, Kroemer G. Mammalian target of rapamycin (mTOR): Pro- and anti-apoptotic. Cell Death Differ. 2002;9:99–100. doi: 10.1038/sj.cdd.4400978. [DOI] [PubMed] [Google Scholar]
- 12.Gao N, Zhang Z, Jiang BH, Shi X. Role of PI3K/AKT/mTOR signaling in the cell cycle progression of human prostate cancer. Biochem Biophys Res Commun. 2003;310:1124–1132. doi: 10.1016/j.bbrc.2003.09.132. [DOI] [PubMed] [Google Scholar]
- 13.Grunwald V, DeGraffenried L, Russel D, Friedrichs WE, Ray RB, Hidalgo M. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 2002;62:6141–6145. [PubMed] [Google Scholar]
- 14.van der Poel HG. Mammalian target of rapamycin and 3-phosphatidylinositol 3-kinase pathway inhibition enhances growth inhibition of transforming growth factor-beta1 in prostate cancer cells. J Urol. 2004;172:1333–1337. doi: 10.1097/01.ju.0000138829.97838.19. [DOI] [PubMed] [Google Scholar]
- 15.van der Poel HG, Hanrahan C, Zhong H, Simons JW. Rapamycin induces Smad activity in prostate cancer cell lines. Urol Res. 2003;30:380–386. doi: 10.1007/s00240-002-0282-1. [DOI] [PubMed] [Google Scholar]
- 16.Tolcher AW. Novel therapeutic molecular targets for prostate cancer: The mTOR signaling pathway and epidermal growth factor receptor. J Urol. 2004;171:S41–S43. doi: 10.1097/01.ju.0000108100.53239.b7. [DOI] [PubMed] [Google Scholar]
- 17.Lee JT, Jr, Steelman LS, McCubrey JA. Phosphatidylinositol 3′-kinase activation leads to multidrug resistance protein-1 expression and subsequent chemoresistance in advanced prostate cancer cells. Cancer Res. 2004;64:8397–8404. doi: 10.1158/0008-5472.CAN-04-1612. [DOI] [PubMed] [Google Scholar]
- 18.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR, Loda M, Lane HA, Sellers WR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 19.Boulay A, Zumstein-Mecker S, Stephan C, Beuvink I, Zilbermann F, Haller R, Tobler S, Heusser C, O'Reilly T, Stolz B, Marti A, Thomas G, Lane HA. Antitumor efficacy of intermittent treatment schedules with the rapamycin derivative RAD001 correlates with prolonged inactivation of ribosomal protein S6 kinase 1 in peripheral blood mononuclear cells. Cancer Res. 2004;64:252–261. doi: 10.1158/0008-5472.can-3554-2. [DOI] [PubMed] [Google Scholar]
- 20.Cho D, Signoretti S, Regan M, Mier JW, Atkins MB. The role of mammalian target of rapamycin inhibitors in the treatment of advanced renal cancer. Clin Cancer Res. 2007;13:758s–763s. doi: 10.1158/1078-0432.CCR-06-1986. [DOI] [PubMed] [Google Scholar]
- 21.McCarty MF. Targeting multiple signaling pathways as a strategy for managing prostate cancer: Multifocal signal modulation therapy. Integr Cancer Ther. 2004;3:349–380. doi: 10.1177/1534735404270757. [DOI] [PubMed] [Google Scholar]
- 22.Goudar RK, Shi Q, Hjelmeland MD, Keir ST, McLendon RE, Wikstrand CJ, Reese ED, Conrad CA, Traxler P, Lane HA, Reardon DA, Cavenee WK, Wang XF, Bigner DD, Friedman HS, Rich JN. Combination therapy of inhibitors of epidermal growth factor receptor/vascular endothelial growth factor receptor 2 (AEE788) and the mammalian target of rapamycin (RAD001) offers improved glioblastoma tumor growth inhibition. Mol Cancer Ther. 2005;4:101–112. [PubMed] [Google Scholar]
- 23.Petrylak DP, Tangen CM, Hussain MH, Lara PN, Jr, Jones JA, Taplin ME, Burch PA, Berry D, Moinpour C, Kohli M, Benson MC, Small EJ, Raghavan D, Crawford ED. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351:1513–1520. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 24.Tannock IF, de WR, Berry WR, Horti J, Pluzanska A, Chi KN, Oudard S, Theodore C, James ND, Turesson I, Rosenthal MA, Eisenberger MA. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–1512. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 25.Corey E, Brown LG, Quinn JE, Poot M, Roudier MP, Higano CS, Vessella RL. Zoledronic acid exhibits inhibitory effects on osteoblastic and osteolytic metastases of prostate cancer. Clin Cancer Res. 2003;9:295–306. [PubMed] [Google Scholar]
- 26.Quinn JE, Brown LG, Zhang J, Keller ET, Vessella RL, Corey E. Comparison of Fc-osteoprotegerin and zoledronic acid activities suggests that zoledronic acid inhibits prostate cancer in bone by indirect mechanisms. Prostate Cancer Prostatic Dis. 2005;8:253–259. doi: 10.1038/sj.pcan.4500815. [DOI] [PubMed] [Google Scholar]
- 27.Lee MV, Fong EM, Singer FR, Guenette RS. Bisphosphonate treatment inhibits the growth of prostate cancer cells. Cancer Res. 2001;61:2602–2608. [PubMed] [Google Scholar]
- 28.Saad F. Clinical benefit of zoledronic acid for the prevention of skeletal complications in advanced prostate cancer. Clin Prostate Cancer. 2005;4:31–37. doi: 10.3816/cgc.2005.n.009. [DOI] [PubMed] [Google Scholar]
- 29.Saad F. Zoledronic acid significantly reduces pathologic fractures in patients with advanced-stage prostate cancer metastatic to bone. Clin Prostate Cancer. 2002;1:145–152. doi: 10.3816/cgc.2002.n.016. [DOI] [PubMed] [Google Scholar]
- 30.Weinfurt KP, Anstrom KJ, Castel LD, Schulman KA, Saad F. Effect of zoledronic acid on pain associated with bone metastasis in patients with prostate cancer. Ann Oncol. 2006;17:986–989. doi: 10.1093/annonc/mdl041. [DOI] [PubMed] [Google Scholar]
- 31.Thalmann GN, Sikes RA, Wu TT, Degeorges A, Chang SM, Ozen M, Pathak S, Chung LW. LNCaP progression model of human prostate cancer: Androgen-independence and osseous metastasis. Prostate. 2000;44:91–103. doi: 10.1002/1097-0045(20000701)44:2<91::aid-pros1>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 32.Wu TT, Sikes RA, Cui Q, Thalmann GN, Kao C, Murphy CF, Yang H, Zhau HE, Balian G, Chung LW. Establishing human prostate cancer cell xenografts in bone: Induction of osteoblastic reaction by prostate-specific antigen-producing tumors in athymic and SCID/bg mice using LNCaP and lineage-derived metastatic sublines. Int J Cancer. 1998;77:887–894. doi: 10.1002/(sici)1097-0215(19980911)77:6<887::aid-ijc15>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 33.Corey E, Brown LG, Kiefer JA, Quinn JE, Pitts T, Blair JM, Vessela RL. Osteoprotegerin in prostate cancer bone metastasis. Cancer Res. 2005;65:1710–1718. doi: 10.1158/0008-5472.CAN-04-2033. [DOI] [PubMed] [Google Scholar]
- 34.Corey E, Quinn JE, Zhang J, Odman A, Brown LG, Keller ET, Vessella RL. Evaluation of effects of osteoprotegerin and zoledronic acid on C4-2 prostate cancer bone metastasis. Third North American Symposium on Skeletal Metastases 2002; Bethesda, MD. 2002. [Google Scholar]
- 35.Pfitzenmaier J, Quinn JE, Odman AM, Zhang J, Keller ET, Vessella RL, Corey E. Characterization of C4-2prostate cancer bone metastases and their response to castration. J Bone Miner Res. 2003;18:1882–1888. doi: 10.1359/jbmr.2003.18.10.1882. [DOI] [PubMed] [Google Scholar]
- 36.Corey E, Quinn JE, Bladou F, Brown LG, Roudier MP, Brown JM, Buhler KR, Vessella RL. Establishment and characterization of osseous prostate cancer models: Intra-tibial injection of human prostate cancer cells. Prostate. 2002;52:20–33. doi: 10.1002/pros.10091. [DOI] [PubMed] [Google Scholar]
- 37.Manning BD. Balancing Akt with S6K: Implications for both metabolic diseases and tumorigenesis. J Cell Biol. 2004;167:399–403. doi: 10.1083/jcb.200408161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brubaker KD, Brown LG, Vessella RL, Corey E. Administration of zoledronic acid enhances the effects of docetaxel on growth of prostate cancer in the bone environment. BMC Cancer. 2006;6:15. doi: 10.1186/1471-2407-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bertelli G, Heouaine A, Arena G, Botto A, Garrone O, Colantonio I, Occelli M, Fea E, Giubergia S, Merlano M. Weekly docetaxel and zoledronic acid every 4 weeks in hormone-refractory prostate cancer patients. Cancer Chemother Pharmacol. 2006;57:46–51. doi: 10.1007/s00280-005-0025-4. [DOI] [PubMed] [Google Scholar]
- 40.Masiello D, Mohi MG, McKnight NC, Smith B, Neel BG, Balk SP, Bubley GJ. Combining an mTOR antagonist and receptor tyrosine kinase inhibitors for the treatment of prostate cancer. Cancer Biol Ther. 2007;6:195–201. doi: 10.4161/cbt.6.2.3588. [DOI] [PubMed] [Google Scholar]
- 41.Argiles JM, Meijsing SH, Pallares-Trujillo J, Guirao X, Lopez-Soriano FJ. Cancer cachexia: A therapeutic approach. Med Res Rev. 2001;21:83–101. doi: 10.1002/1098-1128(200101)21:1<83::aid-med4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 42.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]