Abstract
Introduction
Dementia caregivers have an increased risk of cardiovascular disease (CVD), and it is possible that metabolic disturbances contribute to this risk. Regular physical exercise reduces cardiometabolic risk, but caregivers may have less opportunity to engage in such activity. We hypothesized that regular physical activity would moderate cardiometabolic risk in dementia caregivers.
Methods
115 Alzheimer’s caregivers and 54 non-caregiving controls were assessed for medical history and health habits. Physical activity was defined as the number of days per week participants performed light (score 0–4), moderate (score 0–4), or vigorous (score 0–4) exercise (total score 0–12). A cardiometabolic risk score was calculated by adding standardized z-scores of five metabolic syndrome (MetS) components: body mass index, triglycerides, high-density lipoprotein cholesterol, systolic blood pressure and glucose.
Results
Caregivers were less physically active than non-caregivers (5.1±3.0 vs. 6.3±2.7, p=0.008). A significant caregiver status-by-physical activity interaction was found for the standardized cardiometabolic risk score controlling for gender, age, education, smoking, alcohol consumption, health problems, cholesterol-lowering medication, negative affect, role overload, and fasting state (p=0.035). Among participants with low levels of physical activity, caregivers had greater cardiometabolic risk score than non-caregivers (0.58±0.31 vs. −1.23±0.54, p=0.017); no group difference emerged in participants with high levels of physical activity (p=0.81).
Conclusions
Cardiometabolic risk was particularly high in caregivers reporting reduced level of regular physical activity. Intervention studies aimed at increasing physical activity in caregivers seem warranted to examine whether that would possibly lower cardiometabolic risk to the level of non-caregivers.
Keywords: Alzheimer’s disease, cardiovascular disease, exercise, metabolic syndrome, psychological stress
INTRODUCTION
The chronic stress of providing care to a disabled or ill spouse (e.g. with Alzheimer’s disease) has been associated with an increased risk of physical diseases, particularly cardiovascular disease (CVD) (36). Compared to non-caregiving individuals, caregivers are more likely to develop arterial hypertension (31) and incident CHD (22,35), and to also have an elevated Framingham CHD risk score (38). Moreover, distress with dementia caregiving was a significant predictor of time to CVD diagnosis (24).
The metabolic syndrome (MetS) is an insulin-resistant state that aggregates several modifiable risk factors of CVD such as obesity, elevated lipids, glucose, and blood pressure (BP) (11). Different definitions exist and the distinct clinical value is a matter of debate, as, for example, the CVD risk associated with the syndrome appears to be no greater than the sum of its parts (16). Nevertheless, the MetS is a strong predictor of CHD (10). Chronic psychosocial stress like stressful life events (28), low socioeconomic status (23), and job stress (5) increase the risk of developing MetS. The risk of having MetS also increases with greater amount of psychosocial stress and psychological distress in the elderly (37). Consistent with this research, one pathway through which chronic dementia caregiving stress might contribute to atherosclerosis and the clinical manifestation of CHD is by a relation with the MetS (35).
Low levels of regular physical activity (i.e. low energy expenditure beyond resting expenditure) is a major contributing factor to the pandemic increase in the prevalence of MetS in modernized western societies (16). Regular moderate-intensity physical activity of 30 minutes per day has become one recommended life-style intervention for the prevention and clinical management of cardiometabolic risk factors and MetS in order to ultimately reduce CVD morbidity and mortality (11,32). The burden of caregiving has been associated with reduced level of physical activity because strained caregivers have little time to engage in exercise (2,30). In addition to sedentary behavior, caregiver burden-related psychological distress may lead to risky health habits such as excessive alcohol consumption and smoking (36).
Although the literature is limited and restricted to samples of children and young adults, research suggests that physical activity might moderate the relation between chronic psychosocial stress and cardiometabolic risk (14). One study reported an interaction between physical activity levels and personal/community stress predicting adiposity measures after controlling for sociodemographic risk factors and parental smoking (41). In another study daily life stress interacted with physical activity in predicting a metabolic risk score (which was defined as waist circumference, BP, glycosylated hemoglobin, and high-density lipoprotein (HDL) cholesterol) (13).
We tested the hypothesis that the level of regular physical activity would moderate the relationship between caregiver status and cardiometabolic risk. Specifically, we predicted that caregivers with high physical activity would endorse similar cardiometabolic risk as non-caregivers, whereas caregivers with low physical activity would show greater cardiometabolic risk than non-caregivers. This might indicate that high level of physical activity “buffers” cardiometabolic risk in caregivers to a level seen in non-caregivers, in which case there would be important clinical implications in terms of tailored interventions aimed at reducing CVD risk in caregivers.
METHODS
Study Participants
We used cross-sectional data obtained at study entry from participants in the University of California San Diego (UCSD) “Alzheimer’s Caregiver Study”, which is a longitudinal study on effects of dementia caregiving stress on health. Inclusion criteria were being ≥55 years old, married, and dwelling in the community with a spouse. Exclusion criteria were presence of any major illnesses (e.g., cancer), severe hypertension (BP>200/120mmHg), or treatment with oral anticoagulants, non-selective beta blockers, or steroids. Caregivers had to provide primary care for a spouse with a physician-based diagnosis of Alzheimer’s disease. Non-caregivers were not excluded for life stress and recruited with regard to demographic characteristics to yield a gender- and age-equated comparison group. Participants were recruited through referrals from the UCSD Alzheimer’s Disease Research center, community support groups and agencies serving caregivers, local senior citizen health fairs, and referrals from other participants. All participants provided written consent to the study protocol, which was approved by the UCSD Institutional Review Board. We screened a total of 295 participants for eligibility to this project. Of these, 24 were not interested in participating and 180 were eligible and willing to participate. The remaining 91 participants did not meet eligibility criteria for our study. Reasons for ineligibility were: a) caregiver was providing care to a non-spouse (e.g., parent; n=18); b) participant was too young (n=1); c) spouse was no longer living (e.g., prior caregiver but spouse passed away; n=23); d) participant did not reside with a spouse (e.g., Alzheimer’s disease patient was in long-term care; divorced/non-married; n=27); e) participant had an exclusion medical condition (e.g., cancer requiring chemotherapy; n=11); f) participant did not speak English (n=2); g) caregiver of non-Alzheimer’s disease patient (e.g., Parkinson’s disease; n=6); h) taking medications that affect biomarkers (n=2); and i) participant did not reside in our catchment area (n=1). Out of the total enrollment of 180 participants (121 caregivers, 59 non-caregiving controls), we report on 115 caregivers and 54 non-caregivers with complete data for the present investigation.
Measures
Participants were interviewed in their homes by a single interviewer with a Bachelor’s Degree in Psychology. Interviews consisted of questionnaires assessing demographics, mood states, stress/stressors, health status, and current medication. For all interviews, the interviewer read an introduction to the questionnaire (e.g., “The next questions pertain to your moods over the past week. We’d like you to tell us how often you experienced the mood using the following scale”). Upon completing the introduction to the questionnaire, the interviewer would present a response card with available responses to the question(s) at hand. The interviewer would then read each question out loud for the participant and record the participant’s response on the form. If participants were confused or uncertain about a particular question, the interviewer would clarify using a standardized response. Most typically this involved participants reporting two responses (e.g., “somewhere between 2 and 3”), to which the interviewer would respond, “Which answer seems most true during the past ____ week(s)?”
Demographic factors
We collected information on gender, age, and years of education for socioeconomic status.
Smoking status
Smoking status was assessed by the question: “Have you previously been a smoker and quit permanently?” and defined in terms of ever (i.e., former plus current) smoker (n=73) versus never smoker (n=96). Only one participant was a current smoker.
Alcohol consumption
The amount of alcohol consumption was assessed using the following two questions: a) “During the past 30 days, on how many days did you have at least one drink containing alcohol (not include a few sips of wine for religious purposes)?” Scores were 0 (0 days), 1 (1 or 2 days), 2 (3–5 days), 3 (6–9 days), 4 (10–19 days), 5 (20–29 days), and 6 (all 30 days); b) “On the days you drank alcohol, how many drinks did you usually drink? Scores were 0 (none), 1 (< 1 drink), 2 (1 drink), 3 (2 drinks), 4 (3 drinks), 5 (4 drinks), 6 (5 or more drinks). We multiplied the scores of questions a) and b) to obtain a total score of alcohol consumption.
Physical activity
Participants were administered the Rapid Assessment of Physical Activity (RAPA) scale which had been validated in older adults (mean age 73 years) (33). There were high correlations between the RAPA scores and other ratings of physical activities. For example, the RAPA was highly correlated the Community Healthy Activities Model Program for Seniors (CHAMPS) moderate caloric expenditure (r = .54) and showed good sensitivity (81%), positive predictive value (77%), and negative predictive value (75%) in predicting moderate or strenuous physical activities as indicated by metabolic equivalent values of 3.0 or higher (33). For the assessment of physical activity, participants were asked the questions “In a typical week, how often do you do light/moderate/strenuous exercises?” Typical examples given to participants were for light activities stretching or vacuum cleaning (i.e., heart beats slightly faster than normal), for moderate activities fast walking or strength training (i.e., heart beats faster than normal), and for strenuous activities jogging or badminton (i.e., heart rate increases a lot). For each of the three exercise intensity categories, participants rated the frequency as follows (score points): 0 days (0), 1 or 2 days (1), 3 or 4 days (2), 5 or 6 days (3), every day (4) yielding a total score for regular physical activity ranging from 0 to 12.
Number of health problems
Participants were asked the question “Do you currently have, or has a doctor told you that you have, any of the following health problems?” and provided a list with 18 items (e.g., arthritis, heart disease, problems with your kidneys). Positive items were added to one number of health problems.
Negative affect
We used the negative affect subscale of the Positive and Negative Affect Scale comprising 10 items (e.g., nervous, irritable, hostile). Participants were asked to rate the extent to which they experienced each mood over the “past few weeks” on a 5-point scale (1=very slightly or not at all, 5= extremely) yielding a total score between 10 and 50 (40).
Role overload
We used Pearlin’s Role Overload scale to form an index of participants’ perception about feeling overwhelmed and exhausted by responsibilities (e.g., “You work hard but never seem to make any progress”) (27). Responses are rated on a 4-point Likert scale (0=not at all, 1=somewhat, 2=quite a bit, 3=completely) yield a total score between 0 and 12.
Duration of care
Participants were asked to provide the approximate date their spouse was diagnosed with Alzheimer’s disease. We then calculated the duration of care from that date to the interview date.
Clinical dementia rating scale
The severity of the spouse’s dementia was assessed with the Clinical Dementia Rating (CDR) scale, which provides a global rating of dementia by integrating six behavioral and cognitive domains: memory, orientation, judgment and problem-solving, community affairs, home and hobbies, and personal care (15). Each domain is rated along five levels of impairment: 0=no dementia, 0.5=questionable dementia, 1=mild dementia, 2=moderate dementia, and 3=severe dementia. Our trained interviewer asked participants to rate a series of memory and behavioral symptoms they observe in their spouses and then calculated an overall CDR score based upon published formulae (26).
Definition of Cardiometabolic Risk Factors
Our definition of cardiometabolic risk factors defining a clinical diagnosis of MetS was based on guidelines suggested by the Adult Treatment Panel III (ATP III) according to which any three of the following must be present (11): elevated waist circumference, elevated triglycerides, reduced HDL cholesterol, elevated BP, and elevated fasting glucose. Because we did not measure waist circumference and fasting state was not a prerequisite in order to not interfere with caregiver daily routine, our definition of cardiometabolic risk factors partially deviated from ATP III definitions (Table 1). We defined elevated body weight per body mass index (BMI) ≥ 30kg/m2 (8) and conservatively set cut offs for high triglycerides at ≥ 200 mg/dl (39), and for high glucose at ≥ 140 mg/dl and/or having diabetes per the assessment of health problems (8). All participants had blood draws between 10:00–10:45 a.m. We did not standardize the length of time since breakfast in participants who did not fast but covaried our analysis for fasting state (yes/no).
Table 1.
Definition of Cardiometabolic Risk Factors in the Present Study
| Factor | Defining level |
|---|---|
| 1. Obesity | Body mass index ≥ 30 kg/m2 |
| 2. Elevated triglycerides | ≥200 mg/dl (non-fasting) |
| 3. Reduced high-density lipoprotein cholesterol | <50 mg/dl in women <40 mg/dl in men |
| 4. Elevated blood pressure | Systolic blood pressure >130 mmHg and/or diastolic blood pressure >85 mmHg and/or taking blood pressure lowering medications |
| 5. Elevated blood glucose | Glucose ≥ 140 mg/dl (non-fasting) and/or positive history of diabetes |
The presence of three or more of these factors defines the metabolic syndrome
We asked participants for their weight and height to calculate the BMI. Using a non-invasive Microlife Blood Pressure monitor, three BP measurements were collected by the research nurse over a 15-minute resting period (5 participants missed one reading and one participant missed two readings). The average was computed and taken as the participant’s mean systolic and diastolic resting BP. Prescribed BP medications were noted (e.g., diuretics, calcium-channel blockers). Plasma triglycerides and HDL cholesterol were determined by standard methodology at the clinical chemistry laboratories at the UCSD medical center. Participants taking any cholesterol lowering drug (n=77) did not differ in frequency of elevated triglyceride levels (p=0.16) and reduced HDL-cholesterol levels (p=0.17) from participants not taking cholesterol lowering medications. However, continuously scaled levels of HDL-cholesterol were relatively lower in those taking cholesterol-lowering medication (49.3±18.8 mg/dl vs. 55.0±15.7 mg/dl, p=0.017), whereas triglyceride levels showed no group difference (114.6±54.8 mg/dl vs. 115.8±71.0 mg/dl, p=0.36). Therefore, we covaried our analysis for intake of any cholesterol- lowering medication (yes/no).
Our primary outcome was a continuously scaled cardiometabolic risk score to account for the somewhat arbitrary definition of MetS factors across different definitions and because CVD risk shows a partly gradual relationship with, for instance, BP and glucose (17). For this purpose, we added standardized z-scores of BMI, triglycerides, HDL cholesterol (inverse polarity), glucose, and systolic BP. Systolic BP was preferred because it confers greater CVD risk than diastolic BP in individuals over 50 years of age (6). Our secondary outcome variable was the mean of the absolute number of cardiometabolic risk factors defined in Table 1.
Statistical Analysis
Data were analyzed using PASW 18.0 statistical software package (SPSS Inc., Chicago, IL) with two-tailed significance level of p<0.05. Prior to beginning our study, we determined that we would have 88.2% power to detect a medium effect size (d=0.50) using a t-test with a 2-tailed alpha of 0.05 and with 120 caregivers and 60 non-caregiving controls. Years of education, alcohol consumption and physical activity scores, number of health problems, negative affect scores, role overload scores, years of caregiving, and CDR scores did all not show a Gaussian distribution. Because of highly skewed variables, three groups (tertiles) were formed for education level [1) 5–13 years, n=46; 2) 14–16 years, n=68; 3) 17–27 years, n=55] and alcohol consumption [1) abstainers: score = 0, n=56; 2) mild consumption: score 1–8, n=65; 3) moderate consumption: score 9–30, n=48] to be used in statistical analysis. Group comparisons used Mann-Whitney U test for continuous variables. Pearson chi-square test and Fisher’s exact test, where appropriate, were conducted for categorical measures. Spearman correlation coefficients were calculated to estimate bivariate association.
We employed hierarchical linear regression analysis, using forced entry, to test the a priori defined main hypothesis that the caregiver status-by-exercise interaction would significantly be linked to cardiometabolic risk independent of covariates (primary outcome: standardized cardiometabolic risk score; secondary outcome: number of cardiometabolic risk factors). We did not consider diagnosis of MetS (i.e., 3 or more risk components) as an outcome because with 33 participants having MetS and the number of covariates (n=13) the model would be overfitted and thus unstable. In addition to fasting state and cholesterol-lowering medication as delineated above, we controlled our analysis for gender, age, socioeconomic status, smoking, alcohol consumption, psychological distress, and number of health problems, because all of these have been associated with cardiometabolic risk factors (11,18,23,36). Because the duration and magnitude of exposure to caregiving stress would be expected to impact cardiometabolic risk accumulation, we further controlled in the group of caregivers for years of caregiving and dementia severity in the care recipient.
In case of a significant interaction we applied the Holmbeck method to test whether the level of regular physical activity (i.e. high vs. low physical activity levels) would moderate the relationship between caregiver status and cardiometabolic risk (12). To reduce problems resulting from multicollinearity, all independent variables were centered to the mean (19). Categorical variables were defined as +0.5 (e.g., caregivers) versus −0.5 (e.g., non-caregivers). Collinearity between predictors was tested and found to be tolerable in all analyses. Multivariate normality of data distribution was assured using leverage analysis and Cook’s distance. Effect sizes were expressed as adjusted R2 and Cohen’s d (7).
RESULTS
Participant Characteristics
Table 2 shows the demographic and medical characteristics, health-related behaviors, and cardiometabolic risk indices in the 115 caregivers and 54 non-caregiving controls. Caregivers had been providing 4.14±3.45 years (range 0.50–16.76) of care to their spouses whose dementia was severe in 7 cases (6%), moderate in 60 cases (52%), mild in 42 cases (37%), and questionable in 6 cases (5%). The latter cases were kept in the analysis because all caregivers’ spouses were diagnosed with Alzheimer dementia based on comprehensive physician work-up, whereas the caregiver interview focuses primarily on daily functioning of the patient. Relative to non-caregivers, caregivers were physically less active (particularly with regard to moderately intense activities), reported more health problems, greater negative affect and greater role overload.
Table 2.
Characteristics of 169 Study Participants
| Variable | Caregivers(n=115) | Non-caregivers(n=54) | P-value |
|---|---|---|---|
| Female gender (%) | 69.6 | 66.7 | 0.705 |
|
| |||
| Age (years) | 73.8±8.2 | 74.4±6.3 | 0.977 |
|
| |||
| Education (years) | 15.2±3.1 | 15.7±3.2 | 0.142 |
|
| |||
| Total physical activity (score) | 5.14±2.97 | 6.33±2.66 | 0.008 |
| Light activity (score) | 2.97±1.40 | 3.39±1.14 | 0.069 |
| Moderate activity (score) | 1.62±1.53 | 2.19±1.30 | 0.014 |
| Strenuous activity (score) | 0.55±1.10 | 0.76±1.21 | 0.158 |
|
| |||
| Ever smoker (%) | 46.1 | 37.0 | 0.268 |
|
| |||
| Alcohol consumption (score) | 5.62±5.83 | 5.91±6.17 | 0.712 |
|
| |||
| Number of health problems | 3.40±1.86 | 2.78±1.57 | 0.029 |
|
| |||
| Any cholesterol-lowering medications (%) | 47.0 | 42.6 | 0.595 |
|
| |||
| Negative affect (score) | 17.8±6.1 | 13.6±5.3 | <0.001 |
|
| |||
| Role overload (score) | 5.17±3.20 | 1.44±1.99 | <0.001 |
|
| |||
| Fasting state (%) | 14.8 | 20.4 | 0.362 |
|
| |||
| Body mass index (kg/m2) | 26.6±4.9 | 26.3±6.2 | 0.264 |
|
| |||
| Triglycerides (mg/dl) | 120.2±67.9 | 104.7±53.6 | 0.262 |
|
| |||
| High-density lipoprotein cholesterol (mg/dl) | 51.9±16.0 | 53.5±16.0 | 0.564 |
|
| |||
| Systolic blood pressure (mmHg) | 134.8±15.4 | 132.4±14.2 | 0.309 |
|
| |||
| Diastolic blood pressure (mmHg) | 75.9±8.7 | 73.2±10.4 | 0.102 |
|
| |||
| Any blood pressure lowering medications (%) | 60.1 | 55.6 | 0.512 |
|
| |||
| Glucose (mg/dl) | 105.0±44.4 | 94.2±20.1 | 0.293 |
|
| |||
| History of diabetes (%) | 12.2 | 3.7 | 0.096 |
|
| |||
| Standardized cardiometabolic risk score (primary outcome) | 0.27±2.99 | −0.57±2.99 | 0.059 |
|
| |||
| Number of cardiometabolic risk factors (secondary outcome) | 1.74±1.12 | 1.31±1.02 | 0.017 |
| Obesity (%) | 22.6 | 16.7 | 0.374 |
| Elevated triglycerides (%) | 15.7 | 3.7 | 0.038 |
| Reduced high-density lipoprotein-cholesterol (%) | 39.1 | 33.3 | 0.467 |
| Elevated blood pressure (%) | 77.4 | 70.4 | 0.325 |
| Elevated blood glucose (%) | 19.1 | 7.4 | 0.066 |
|
| |||
| Metabolic Syndrome (%) | 22.6 | 13.0 | 0.140 |
Values are given as means±SD or percentages
In terms of cardiometabolic risk indices, caregivers had a greater standardized cardiometabolic risk score (primary outcome) and also 74% greater prevalence of MetS (i.e., 3 or more of MetS factors present) than non-caregivers, but these differences did not reach statistical significance. Caregivers had significantly more cardiometabolic risk factors than non-caregivers (secondary outcome). In absolute numbers, all MetS factors were more prevalent in caregivers than in non-caregivers and the prevalence of elevated triglycerides was significantly different between groups.
Bivariate Associations with the Standardized Cardiometabolic Risk Score
Across all participants, the standardized cardiometabolic risk score was higher in men than in women (1.23±3.09 vs. −0.56±2.81, p=0.001) and in ever smokers than in never smokers (0.63±3.25 vs. −0.48±2.73, p=0.016). Greater standardized cardiometabolic risk score was also associated with fewer years of education (r=−0.25, p=0.001), reduced physical activity (r=−0.35, p<0.001), reduced alcohol intake (r=−0.17, p=0.025), and more health problems (r=0.18, p=0.018). There were no significant associations between the standardized cardiometabolic risk score and age (p=0.47), intake of cholesterol-lowering medication (p=0.13), negative affect (p=0.46), role overload (p=0.83), and fasting state (p=0.23). The standardized cardiometabolic risk score and the number of cardiometabolic risk factors correlated with each other, explaining 67% of the mutual variance (r=0.82, p<0.001).
Multivariate Model for Predictors of Cardiometabolic Risk
Standardized Cardiometabolic Risk Score
Table 3 shows the multivariate model in which the interaction between caregiver status and physical activity was significantly associated with the standardized cardiometabolic risk score (primary outcome) independent of covariates (gender, age, education, smoking, alcohol consumption, health problems, cholesterol-lowering medication, negative affect, role overload, fasting state). In addition to the interaction term, male gender, fewer years of education, and less alcohol consumption also emerged as independent correlates of a greater standardized cardiometabolic risk score. Post hoc analysis for the three exercise intensity categories showed that the interaction between caregiver status and moderate physical activity scores significantly predicted the standardized cardiometabolic risk score (B=−0.90±0.32, p=0.006); however, interactions between caregiver status and scores for light (B=0.01±0.37, p=0.97) and strenuous (B=−0.62±0.38, p=0.11) physical activity did not.
Table 3.
Multivariate Model for the Standardized Cardiometabolic Risk Score
| Entered variables | Unstandardized coefficient B | Std. Error | P-value |
|---|---|---|---|
| Constant | 0.23 | 0.30 | 0.434 |
| Gender (male = −0.5) | −2.00 | 0.44 | <0.001 |
| Age (years) | −0.02 | 0.03 | 0.392 |
| Education (years) | −0.80 | 0.28 | 0.004 |
| Ever smoker (yes = +0.5) | 0.77 | 0.41 | 0.067 |
| Alcohol consumption(score) | −0.71 | 0.27 | 0.009 |
| Number of health problems | 0.02 | 0.12 | 0.841 |
| Cholesterol-lowering medication (yes = +0.5) | 0.47 | 0.42 | 0.258 |
| Negative affect score | −0.05 | 0.04 | 0.232 |
| Role overload score | −0.09 | 0.08 | 0.241 |
| Fasting state (yes = +0.5) | −0.17 | 0.55 | 0.754 |
| Physical activity score | −0.27 | 0.08 | 0.001 |
| Caregiver status(caregiver = +0.5) | 0.83 | 0.51 | 0.108 |
| Caregiver status-by-physical activity interaction | −0.33 | 0.16 | 0.035 |
The entire model explained 30.7% of the variance (adjusted R2=0.307) in cardiometabolic risk (F13.155=6.71, p<0.001).
Number of Cardiometabolic Risk Factors
The above analyses were rerun with the same set of covariates but with the number of cardiometabolic risk factors as the dependent variable (secondary outcome). As was seen in the primary analysis, the caregiver status-by-physical activity interaction was significantly associated with the number of cardiometabolic risk factors independent of covariates (B=−0.14±0.06, p=0.017). The model accounted for 27.9% of the variance (adjusted R2=0.279) in cardiometabolic risk (F13,155=5.99, p<0.001). In addition to the interaction term, male gender (p=0.001), fewer years of education (p=0.002), and less alcohol consumption (p=0.006) were also independent predictors of the number of cardiometabolic risk factors. Post hoc analysis for the three exercise intensity categories showed that the interaction between caregiver status and moderate physical activity scores significantly predicted the number of cardiometabolic risk factors (B=−0.29±0.12, p=0.017). As was seen in the primary analysis, interactions between caregiver status and scores for light (B=−0.11±0.14, p=0.41) and strenuous (B=−0.22±0.14, p=0.12) physical activity did not significantly predict the number of cardiometabolic risk factors.
Analysis of the Interaction Between Caregiver Status and Physical Activity for Cardiometabolic Risk
Standardized Cardiometabolic Risk Score
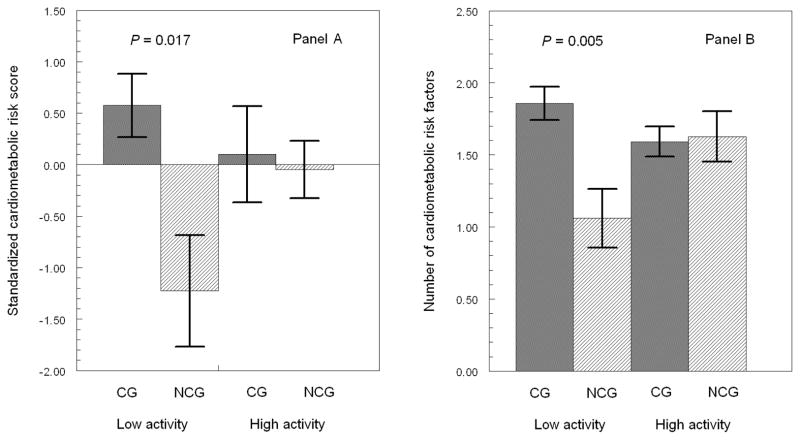
Figure 1 (Panel A) shows that caregivers with low physical activity had a significantly greater cardiometabolic risk score than non-caregivers with the same low physical activity level (p=0.017; Cohen’s d=0.50), whereas the standardized cardiometabolic risk score was similar in caregivers and non-caregivers with high level of physical activity (p=0.81). Post hoc analysis showed that caregivers with low levels of moderate physical activity had a significantly higher (mean±SEM) cardiometabolic risk score than non-caregivers with the same low level of moderate physical activity (0.69±0.31 vs. −1.47±0.55, p=0.005; Cohen’s d=0.58). In contrast, the cardiometabolic risk score was not significantly different between caregivers and non-caregivers with the same high level of moderate physical activity (−0.16±0.29 vs. 0.34±0.48, p=0.44).
Figure 1. Regular Physical Activity and Cardiometabolic Risk Indices.
Error bar graphs (mean±SEM) show significantly greater standardized cardiometabolic risk score (A) and also greater number of cardiometabolic risk factors (B) in 115 caregivers (CG) and 54 non-caregivers (NCG) with the same level of low physical activity; CG and NCG with the same level of high physical activity have similar cardiometabolic risk indices. All analyses controlled for gender, age, education, smoking, alcohol consumption, health problems, cholesterol-lowering medication, negative affect, role overload, and fasting state.
Number of Cardiometabolic Risk Factors
Figure 1 (Panel B) shows that caregivers with low physical activity showed a greater number of cardiometabolic risk factors than non-caregivers (p=0.005; Cohen’s d =0.58). However, at high levels of physical activity, caregivers and non-caregivers did not significantly differ in the number of cardiometabolic risk factors (p=0.88). In post hoc analysis caregivers with low levels of moderate physical activity had a significantly higher (mean±SEM) number of cardiometabolic risk factors than non- caregivers with the same low level of moderate physical activity (1.86±0.12 vs. 1.07±0.21, p=0.006; Cohen’s d=0.57). In contrast, the cardiometabolic risk score was not significantly different between caregivers and non-caregivers with the same high level of moderate physical activity (1.58±0.11 vs. 1.65±0.18, p=0.78).
Do Specific Aspects of Caregiver Stress Account for the Relationship Between Physical Activity and Cardiometabolic Risk?
We tested whether the duration and magnitude of exposure to caregiving stress would better account for the inverse relationship between physical activity (total score) and cardiometabolic risk in caregivers. All analyses were adjusted for gender, age, education, smoking, alcohol consumption, health problems, cholesterol-lowering medication, negative affect, role overload, and fasting state. However, when entered additionally and separately, years of caregiving (p-values >0.33) and dementia severity of the spouse (p-values >0.88), were not related to either cardiometabolic risk index, which, in turn, maintained their significant relationship with physical activity (all p-values <0.001).
DISCUSSION
Dementia caregiving stress is now an acknowledged natural model of the human stress experience to investigate physical adversities imposed by chronic psychosocial stress (36). We found that, relative to age- and gender-equated non-caregiving controls, Alzheimer’s caregivers perceived significantly more role overload in meeting everyday responsibilities and greater psychological distress in the form of negative affect. This demonstrates that caregivers felt on average indeed more stressed than their non-caregiving counterparts.
We corroborated previous studies showing that Alzheimer’s caregivers, when compared to non-caregivers, are physically less active (2,30)and show greater cardiometabolic risk (35), as was evidenced in our study by factors defining MetS. Low statistical power may be an explanation for why the 74% higher prevalence of the MetS in caregivers than non-caregivers was not significant. Our main finding was that the level of physical activity moderated the relationship between caregiver status and cardiometabolic risk. Specifically, caregivers reporting low level of physical activity had significantly greater standardized cardiometabolic risk score than non-caregivers with the same low physical activity level. In contrast, when caregivers reported high level of physical activity, they had a similar cardiometabolic risk score as non-caregivers with the same high level of physical activity. These results suggest that high level of physical activity might buffer cardiometabolic risk in caregivers to a level seen in non-caregivers. In other words, as was shown for younger populations (13,41), chronically stressed elderly might overcome cardiometabolic peril if they are regular exercisers.
Given that the literature is somewhat ambiguous in terms of cut points defining cardiometabolic risk factors (11,16), we computed two different indices of cardiometabolic risk. Our primary outcome measure employed a continuous score of standardized MetS factors. Our secondary outcome merely counted the number of factors defining MetS. We found an association between low level of regular physical activity and high cardiometabolic risk in caregivers regardless of whether cardiometabolic risk factors were operationalized as clinical categories (e.g. high versus normal BP) or as continuously scaled measures across a range of normal, subclinical, and clinical values. The latter comes as no surprise given that, for instance, the gradual relation between systolic BP and CVD risk starts at a level as low as 115 mmHg, which is far below the cut point of 140 mmHg defining stage 1 hypertension (6).
We found the moderating effect of physical activity on cardiometabolic risk in caregivers to be independent of a range of sociodemographic and life style factors, which are commonly treated as potentially confounding variables when studying MetS as a risk factor of CVD (10). Consistent with the literature (36), relative to non-caregivers, caregivers had more health problems, which comprised myocardial infarction, chronic heart failure, angina, and other heart diseases. Adjustment for these was important to account for the possibility that caregivers’ high cardiometabolic risk simply reflects poor physical and cardiovascular health, respectively. A previous study found that Alzheimer’s caregivers with CHD had higher MetS levels (i.e., sum of z-scores of BP, lipids, insulin, glucose, and BMI) than non-caregivers with CHD; however, no such difference was observed between caregivers and non-caregivers free of CHD (34). That study further showed that poor health habits (composite score of poor diet and sedentary behavior) moderated the relationship between MetS levels and CHD risk in caregivers (34). We did not consider dietary habits, though the findings from that and from our study together support a model whereby the chronic stress of dementia caregiving may interact with poor health habits to trigger increase in cardiometabolic risk. This increased risk may in turn translate into clinically significant CHD subsequently.
Not all studies find caregivers are less physically active than non-caregivers (25,29), likely because the lack of opportunity to engage in such activity may depend on the severity of stress exposure and perception (2,30). We therefore considered in our analyses years of caregiving, dementia severity of the care recipient, psychological distress in the form of negative affect, and perceived role overload as control variables. However, we found that the relation between reduced level of physical activity and higher cardiometabolic risk in caregivers was not better explained by caregiver burden and distress. Dementia severity of the spouse and years of caregiving did also not attenuate the significance of the inverse association between physical activity and cardiometabolic risk in caregivers. The latter suggests that cardiometabolic risk in caregivers was not simply a consequence of years of lack of physical activity. However, it is possible that the average stress exposure and perception in our caregivers did not reach an intensity that evidently affected regular physical activity level, as, for instance, only 6% of spouses suffered severe dementia.
The difference in cardiometabolic risk between caregivers and non-caregivers with low physical activity showed a moderate effect suggesting that it is clinically important. Cardiometabolic risk is reduced by long-term exercise training (3)and interventions to increase regular physical activity in dementia caregivers reduced caregiver distress significantly (4). Moderate intensity, home-based physical activity telephone interventions were shown to be feasible means to increase total weekly physical activity levels and self-efficacy in Alzheimer’s caregivers (9). In our caregivers, moderate physical activities seemed also more important than light and strenuous activities in contributing cardiometabolic benefit. Therefore, caregivers with low levels of physical activity might particularly profit from moderately intense physical activities (e.g. fast walking) intervention as a way of lowering their cardiometabolic risk.
The findings of our study need to be interpreted within the frame of the study design and characteristics of participants. Women were overrepresented, but the link between caregiver burden, MetS, and CVD risk seems particularly prominent in male dementia caregivers (35). Our participants were in good health such that their overall physical activity level may not be representative for populations of less mobile elderly and those with poorer health status. The cross-sectional design does not allow one to draw causal inferences. Although we controlled for a range of confounders that may affect physical activity level, we cannot rule out the possibility that cardiometabolic burden impacted, at least in part, the level of physical activity. Particularly excess weight can be both a cause and a consequence of low physical activity. Since BMI is part of the definition of MetS, we could not factor out BMI as a contributor to low levels of physical activity. However, this seemed not to contribute to the observed group difference because physical activity and BMI showed similarly inverse correlations in caregivers (r=−0.44, p<0.001) and controls (r=−0.30, p=0.030). In persons 70 years and older, measured and self-reported weight show high correlation, but self-report of height has its limitations in that the BMI was one unit lower when computed with self-reported height than when calculated from measured height (21). Whether the relationships would hold if cardiometabolic risk indices were defined differently by, for instance, considering waist circumference and fasting lipid and glucose measures, remains unresolved. Although we controlled for fasting state, different timing of blood sampling relative to the postprandial breakfast might also have influenced elevations in triglycerides and glucose. Whether patients with a prior diagnosis of diabetes should be included in analysis of MetS is controversial. However, ATP III definition (applied in the present study) does not exclude hyperglycemia in the diabetes range and World Health Organization definition even considers patients with type 2 diabetes for the diagnosis of MetS (1).
In sum, our study shows that cardiometabolic risk seems particularly high in caregivers who report reduced level of regular physical activity. Interventions studies aimed at increasing physical activity level in caregivers seem thus warranted. These might be a feasible means to possibly reduce cardiometabolic risk and the associated CVD morbidity and mortality in Alzheimer’s caregivers to a level seen in their non-caregiving counterparts.
Acknowledgments
Funding Sources: This study was supported by NIH/NIA awards AG 15301 (to I.G.), AG 03090 (to B.M.), and AG 108415 (to S.A-I).
The authors are grateful to Susan Calleran and Christine Gonzaga for data collection. This study was supported by the National Institutes of Health/National Institute on Aging through award AG 15301 to Igor Grant. Additional support was provided through award AG 03090 to Brent Mausbach and AG 108415 to Sonia Ancoli-Israel. The results of the present study do not constitute endorsement by ACSM.
Footnotes
Conflict of Interest: None to declare.
References
- 1.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Burton LC, Newsom JT, Schulz R, et al. Preventive health behaviors among spousal caregivers. Prev Med. 1997;26:162–169. doi: 10.1006/pmed.1996.0129. [DOI] [PubMed] [Google Scholar]
- 3.Carroll S, Dudfield M. What is the relationship between exercise and metabolic abnormalities? A review of the metabolic syndrome. Sports Med. 2004;34:371–418. doi: 10.2165/00007256-200434060-00004. [DOI] [PubMed] [Google Scholar]
- 4.Castro CM, Wilcox S, O’Sullivan P, et al. An exercise program for women who are caring for relatives with dementia. Psychosom Med. 2002;64:458–468. doi: 10.1097/00006842-200205000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Chandola T, Brunner E, Marmot M. Chronic stress at work and the metabolic syndrome: prospective study. BMJ. 2006;332:521–525. doi: 10.1136/bmj.38693.435301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 7.Cohen J. Statistical power analysis for the behavioral sciences. 2. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. p. 567. [Google Scholar]
- 8.de Simone G, Olsen MH, Wachtell K, et al. Clusters of metabolic risk factors predict cardiovascular events in hypertension with target-organ damage: the LIFE study. J Hum Hypertens. 2007;21:625–632. doi: 10.1038/sj.jhh.1002203. [DOI] [PubMed] [Google Scholar]
- 9.Farran CJ, Staffileno BA, Gilley DW, et al. A Lifestyle physical activity intervention for caregivers of persons with Alzheimer’s disease. Am J Alzheimers Dis Other Demen. 2008;23:132–142. doi: 10.1177/1533317507312556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–1778. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 11.Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 12.Holmbeck GN. Post-hoc probing of significant moderational and mediational effects in studies of pediatric populations. J Pediatr Psychol. 2002;27:87–96. doi: 10.1093/jpepsy/27.1.87. [DOI] [PubMed] [Google Scholar]
- 13.Holmes ME, Eisenmann JC, Ekkekakis P, et al. Physical activity, stress, and metabolic risk score in 8- to 18-year-old boys. J Phys Act Health. 2008;5:294–307. doi: 10.1123/jpah.5.2.294. [DOI] [PubMed] [Google Scholar]
- 14.Holmes ME, Ekkekakis P, Eisenmann JC. The physical activity, stress and metabolic syndrome triangle: a guide to unfamiliar territory for the obesity researcher. Obes Rev. doi: 10.1111/j.1467-789X.2009.00680.x. (in press) [DOI] [PubMed] [Google Scholar]
- 15.Hughes CP, Berg L, Danziger WL, et al. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. doi: 10.1192/bjp.140.6.566. [DOI] [PubMed] [Google Scholar]
- 16.Johnson LW, Weinstock RS. The metabolic syndrome: concepts and controversy. Mayo Clin Proc. 2006;81:1615–1620. doi: 10.4065/81.12.1615. [DOI] [PubMed] [Google Scholar]
- 17.Kahn R, Buse J, Ferrannini E, et al. The metabolic syndrome: time for a critical appraisal: joint statement from the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2005;28:2289–2304. doi: 10.2337/diacare.28.9.2289. [DOI] [PubMed] [Google Scholar]
- 18.Kloner RA, Rezkalla SH. To drink or not to drink? That is the question. Circulation. 2007;116:1306–1137. doi: 10.1161/CIRCULATIONAHA.106.678375. [DOI] [PubMed] [Google Scholar]
- 19.Kraemer HC, Morgan GA, Leech NL, et al. Measures of clinical significance. J Am Acad Child Adolesc Psychiatry. 2003;42:1524–1529. doi: 10.1097/00004583-200312000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Kraemer HC, Wilson GT, Fairburn CG, et al. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- 21.Kuczmarski MF, Kuczmarski RJ, Najjar M. Effects of age on validity of self-reported height, weight, and body mass index: findings from the Third National Health and Nutrition Examination Survey, 1988–1994. J Am Diet Assoc. 2001;101:28–34. doi: 10.1016/S0002-8223(01)00008-6. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Colditz GA, Berkman LF, et al. Caregiving and risk of coronary heart disease in U.S. women: a prospective study. Am J Prev Med. 2003;24:113–119. doi: 10.1016/s0749-3797(02)00582-2. [DOI] [PubMed] [Google Scholar]
- 23.Loucks EB, Magnusson KT, Cook S, et al. Socioeconomic position and the metabolic syndrome in early, middle, and late life: evidence from NHANES 1999–2002. Ann Epidemiol. 2007;17:782–790. doi: 10.1016/j.annepidem.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Mausbach BT, Patterson TL, Rabinowitz YG, et al. Depression and distress predict time to cardiovascular disease in dementia caregivers. Health Psychol. 2007;26:539–544. doi: 10.1037/0278-6133.26.5.539. [DOI] [PubMed] [Google Scholar]
- 25.McKibbin CL, Walsh W, Rinki M, et al. Lifestyle and health behaviors among female family dementia caregivers: a comparison of wives and daughters. Aging Ment Health. 1999;3:165–172. [Google Scholar]
- 26.Morris JC. The clinical dementia rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 27.Pearlin LI, Mullan JT, Semple SJ, et al. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30:583–589. doi: 10.1093/geront/30.5.583. [DOI] [PubMed] [Google Scholar]
- 28.Räikkönen K, Matthews KA, Kuller LH. Depressive symptoms and stressful life events predict metabolic syndrome among middle-aged women: a comparison of World Health Organization, Adult Treatment Panel III, and International Diabetes Foundation definitions. Diabetes Care. 2007;30:872–877. doi: 10.2337/dc06-1857. [DOI] [PubMed] [Google Scholar]
- 29.Scharlach E, Midanik LT, Runkle MC, et al. Health practices of adults with elder care responsibilities. Prev Med. 1997;26:155–161. doi: 10.1006/pmed.1996.0128. [DOI] [PubMed] [Google Scholar]
- 30.Schulz R, Newsom J, Mittelmark M, et al. Health effects of caregiving. The Caregiver Health Effects Study: An ancillary study of the Cardiovascular Health Study. Ann Behav Med. 1997;19:110–116. doi: 10.1007/BF02883327. [DOI] [PubMed] [Google Scholar]
- 31.Shaw WS, Patterson TL, Ziegler MG, et al. Accelerated risk of hypertensive blood pressure recordings among Alzheimer caregivers. J Psychosom Res. 1999;46:215–227. doi: 10.1016/s0022-3999(98)00084-1. [DOI] [PubMed] [Google Scholar]
- 32.Thompson PD, Buchner D, Pina IL, et al. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity) Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 33.Topolski TD, LoGerfo J, Patrick DL, et al. The Rapid Assessment of Physical Activity (RAPA) among older adults. Prev Chronic Dis. 2006;3:A118. [PMC free article] [PubMed] [Google Scholar]
- 34.Vitaliano PP, Scanlan JM, Siegler IC, et al. Coronary heart disease moderates the relationship of chronic stress with the metabolic syndrome. Health Psychol. 1998;17:520–529. doi: 10.1037//0278-6133.17.6.520. [DOI] [PubMed] [Google Scholar]
- 35.Vitaliano PP, Scanlan JM, Zhang J, et al. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosom Med. 2002;64:418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Vitaliano PP, Zhang J, Scanlan JM. Is caregiving hazardous to one’s physical health? A meta-analysis. Psychol Bull. 2003;129:946–972. doi: 10.1037/0033-2909.129.6.946. [DOI] [PubMed] [Google Scholar]
- 37.Vogelzangs N, Beekman AT, Kritchevsky SB, et al. Psychosocial risk factors and the metabolic syndrome in elderly persons: findings from the Health, Aging and Body Composition study. J Gerontol A Biol Sci Med Sci. 2007;62:563–569. doi: 10.1093/gerona/62.5.563. [DOI] [PubMed] [Google Scholar]
- 38.von Känel R, Mausbach BT, Patterson TL, et al. Increased Framingham Coronary Heart Disease Risk Score in dementia caregivers relative to non-caregiving controls. Gerontology. 2008;54:131–137. doi: 10.1159/000113649. [DOI] [PubMed] [Google Scholar]
- 39.Wannamethee SG, Shaper AG, Lennon L, et al. Metabolic syndrome vs Framingham Risk Score for prediction of coronary heart disease, stroke, and type 2 diabetes mellitus. Arch Intern Med. 2005;165:2644–2650. doi: 10.1001/archinte.165.22.2644. [DOI] [PubMed] [Google Scholar]
- 40.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J Pers Soc Psychol. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 41.Yin Z, Davis CL, Moore JB, et al. Physical activity buffers the effects of chronic stress on adiposity in youth. Ann Behav Med. 2005;29:29–36. doi: 10.1207/s15324796abm2901_5. [DOI] [PubMed] [Google Scholar]