Abstract
Purpose
Men with apparently localized prostate cancer often relapse years after radical prostatectomy (RP). We sought to determine if epithelial-like cells identified from bone marrow (BM) in patients after RP (commonly called disseminated tumor cells, DTC) were associated with biochemical recurrence (BR).
Experimental Design
We obtained BM aspirates from 569 men prior to RP and from 34 healthy men with PSA<2.5 ng/ml to establish a comparison group. Additionally, an analytic cohort consisting of 98 patients after RP with no evidence of disease (NED) was established to evaluate the relationship between DTC and BR. Epithelial cells in the BM were detected by magnetic bead enrichment with antibodies to CD45 and CD61 (negative selection) followed by antibodies to human epithelial antigen (positive selection) and confirmation with FITC-labeled anti-BerEP4 antibody.
Results
DTC were present in 72% (408/569) of patients prior to RP. There was no correlation with pathologic stage, Gleason grade, or pre-operative PSA. Three of 34 controls (8.8%) had DTC present. In patients NED post-RP, DTC were present in 56/98 (57%). DTC were detected in 12/14 (86%) NED patients post-RP who subsequently suffered BR. Presence of DTC in NED patients was an independent predictor of recurrence (HR 6.9, CI 1.03–45.9).
Conclusions
Approximately 70% of men undergoing RP had DTC detected in their BM prior to surgery, suggesting that these cells escape early in the disease. Though pre-operative DTC status does not correlate with pathologic risk factors, persistence of DTC after RP in NED patients was an independent predictor of recurrence.
Keywords: Disseminated tumor cells, circulating tumor cells, prostate cancer, tumor markers, dormancy
INTRODUCTION
While over 90% of prostate cancer (PCa) is considered localized at the time of diagnosis, the rate of biochemical (PSA) recurrence after radical prostatectomy (RP) is 20–30%. Additionally, though PSA elevations occur most often in the five years following RP, they may occur as long as ten to fifteen years after surgery (1, 2). Furthermore, the mean time from PSA recurrence to clinical metastasis is 8 years (1). These facts suggest the persistence of tumor cells in a state of either complete or near dormancy prior to metastatic progression.
Persistence of cancer cells within the vasculature of breast cancer patients years after treatment and without evidence of disease has been well documented with a variety of techniques, including flow cytometry, immunohistochemistry, and immunomagnetic selection (3, 4). Breast cancer cells appear to disseminate early from the primary tumor and undergo a distinct evolutionary process (5). Some of these cells are capable of remaining dormant and may be responsible for delayed metastatic progression (6).
We hypothesize that a similar process of tumor dormancy occurs in patients with PCa. Numerous studies have shown the ability to detect tumor cells in the blood (circulating tumor cells, CTC) and bone marrow (disseminated tumor cells, DTC) of PCa patients, however their significance has not been determined (7–11 reviewed in 12). As in breast cancer, there is evidence that prostate cancer cells disseminate early on from the primary tumor. Using comparative genomic hybridization (CGH), Klein et al. (5) found significant heterogeneity in DTC within individual patients with prostate or breast cancer. These genetic disparities may indicate early divergence of the DTC and separate evolution. However, unlike breast cancer, in which large prospective trials have consistently demonstrated a prognostic impact of these cells (13–15), evidence for a correlation between disease progression and the presence of circulating or disseminated tumor cells in PCa is limited to small pilot studies (7, 16). Furthermore, there is little data on the prognostic significance of these cells after RP (16, 17). Therefore, the importance of these cells in PCa remains unclear, and the possibility that they may represent dormant tumor cells has not been well elucidated.
Prostate cancer metastases occur predominantly in the bone, and we have refined a sensitive assay that enriches and identifies DTC from the bone marrow (BM) of men with PCa (8, 10). We measured the presence of DTC before and after RP to determine if these data would be clinically relevant and lend insight into the metastatic process. The purpose of this study was to describe the prevalence of pre-RP and post-RP DTC, and to determine if the presence of post-RP DTC is associated with biochemical recurrence.
METHODS
Patient Selection
Between 1/1/2002 and 12/31/2006, BM aspirates from PCa patients were prospectively collected for the purpose of identifying important molecular markers in DTC and evaluating whether these cells are correlated with clinical outcomes. Here, in order to assess the clinical importance of these cells, we retrospectively assembled a cohort of 631 consecutive patients with prostate cancer and no other known prior or simultaneous epithelial malignancies who underwent satisfactory BM aspiration. Aspirates were obtained from 569 patients at the time of RP and 98 patients with no evidence of disease (NED) after RP. Forty-nine patients were present in both groups. All patients who underwent RP at the authors’ institution and all those seen in follow-up after RP were asked to participate. Samples from NED patients were drawn at least 3 months after RP, and in eight men with more than one sample after RP only their first sample was included in order to standardize the analysis.
Men were considered to be NED following RP if they had a serum PSA level less than 0.4 ng/ml. Patients with PSA ≥0.4 ng/ml and patients who had undergone salvage radiation treatment were considered to have recurrent disease. This PSA cutoff was selected due to the large percentage of patients with PSA between 0.1 and 0.4 ng/ml who never undergo further PSA progression and therefore do not have a definitive recurrence of their prostate cancer (18, 19). For example, in a cohort of 773 men with PSA between 0.20 and 0.29 ng/ml after RP, the rate of further PSA rise or treatment reported by Amling et al. (18) was only 50%. Thirty-four men with PSA <2.5 ng/ml, negative DRE, and no evidence of any malignancy were studied as controls. All specimens were obtained after informed consent and collected using protocols approved by the Institutional Review Board and Human Subject Division at the University of Washington.
Collection of Samples
Ten ml of BM was aspirated from the iliac crest into a 30 ml syringe containing 10 ml of 6% sodium citrate. In samples obtained from patients just prior to RP, bilateral aspirates were obtained and combined for a total of 20 ml of BM. Samples from patients prior to RP and from controls were obtained under general anesthesia and from the anterior iliac crest. Post-prostatectomy samples were obtained using local anesthesia and taken from the posterior iliac crest. Processing of samples commenced within 1 hour and was completed within 4 hours.
Cell Enrichment
Cell enrichment and isolation was performed as previously described (10). Briefly, BM aspirates were placed over a 15 ml volume of Ficoll-Isopaque 1.077 g/ml (Accurate Chemical, Westbury, NY). Centrifugation subsequently yielded a mononuclear cell layer containing DTC, if present. The MACS system for immunomagnetic selection (Miltenyi Biotec, Auburn, CA) was then employed. Anti-CD45 and anti-CD61 antibodies were used for negative selection, targeting leukocytes, megakaryocytes, and platelets. Positive selection was then performed with immunomagnetic beads coated with anti-human epithelial antigen (HEA) antibodies.
Identification of Disseminated Tumor Cells
For identification and isolation of DTC, the enriched population was subjected to immunostaining with fluorescein isothiocyanate (FITC) labeled anti-BerEP4 antibodies (Dako, Carpinteria, CA) which bind a different epitope on HEA than the anti-HEA antibody used for positive selection. A phycoerythrin (PE) conjugated anti-CD45 antibody was also added for identification of leukocytes. The cells were kept on ice and viewed under fluorescent light using an inverted microscope. Patients were considered DTC-positive if they had one or more cells staining BerEP4 positive and CD-45 negative. This cut-point of one cell was defined a priori as we hypothesized that a single detectable cell may carry significance for tumor recurrence.
Statistical Methods
Descriptive statistics were used to compare demographic and disease characteristics of patients with and without DTC. Univariate comparisons were tested using the Pearson chi-square test. Kaplan-Meier methods were used to compare the unadjusted survival of patients with and without DTC. Cox proportional hazards regression was used to compare survival of patients with and without DTC after adjusting for demographic and disease characteristics. Age (categorized), race (White, African-American), preoperative PSA (continuous), pathologic stage (organ confined, non-organ confined), pathologic grade (Gleason 5–6, Gleason 7, Gleason 8–10), and margin status (positive, negative) were selected a priori as covariates for the multivariate regression model.
Because time between RP and BM aspiration was not standardized, two separate models were considered. In the first model, time under observation started at the date of RP. In the second model, time under observation started at the date of BM aspiration post-surgery. Patients who did not exhibit a PSA recurrence were censored at the date of last follow-up. Proportional hazards assumptions were tested with Schoenfeld residuals. Stata version 9.2 (StataCorp, College Station, TX) was used for the statistical analyses.
RESULTS
DTC prior to prostatectomy
Prior to RP, 408/569 patients (72%) had one or more DTC present (Table 1). The median age of this group was 63 years (range, 40 to 81 years). In the control group of men with PSA <2.5 ng/ml, 3/34 (8.8%) had cells present in their BM (χ2, p<0.01). The median age of the controls was 50 years (range, 24 to 81 years). Of the subset of controls greater than 40 years old (median 62 years), 1/20 (5%) were positive. The three positive controls were followed for a median of 48 months (range 13 to 48 months), and none have developed any evidence of malignancy. Pathologic characteristics of the pre-RP cohort are shown in Table 1. The median age at RP for this group was 60 years (range, 35 to 78 years). There was no correlation between the presence of DTC and any of the following variables: pre-operative PSA, Gleason grade, pathologic stage, or tumor volume. The absence of correlation persisted even when patients were stratified by number of DTC detected (i.e. ≥5 or ≥100 cells per sample; data not shown).
Table 1.
DTC in patients prior to RP stratified by clinicopathological variables. Controls were men without malignancy and with PSA <2.5 ng/ml. Four patients had T0 disease. All p values are χ2.
| Characteristic | Overall
|
DTC
|
p | |
|---|---|---|---|---|
| Yes
|
No
|
|||
| n (%) | n (%) | n (%) | ||
| Controls | 34 | 3 (9) | 31 (91) | |
| All Pre-RP | 569 | 408 (72) | 161 (28) | <0.001 |
| Pathologic stage | ||||
| pT2 | 461 (81) | 331 (72) | 130 (28) | |
| pT3-4, N0 | 72 (13) | 49 (68) | 23 (32) | |
| pTany, N+ | 32 (6) | 25 (78) | 7 (22) | 0.57 |
| Gleason grade | ||||
| 4–6 | 235 (41) | 181 (77) | 54 (23) | |
| 7 | 286 (50) | 194 (68) | 92 (32) | |
| 8–10 | 44 (8) | 30 (68) | 14 (32) | 0.06 |
| PSA (ng/ml) | ||||
| <4 | 110 (19) | 83 (75) | 27 (25) | |
| 4–10 | 360 (63) | 253 (70) | 107 (30) | |
| 10–20 | 79 (14) | 60 (76) | 19 (24) | |
| >20 | 20 (4) | 12 (60) | 8 (40) | 0.37 |
| Tumor volume (cc) | ||||
| 0.01–0.5 | 95 (17) | 72 (76) | 23 (24) | |
| 0.6–2.0 | 217 (38) | 161 (74) | 56 (26) | |
| 2.1–5.0 | 190 (33) | 123 (65) | 67 (35) | |
| >5.0 | 63 (11) | 49 (78) | 14 (22) | 0.07 |
Abbreviations: DTC, disseminated tumor cells; PSA, prostate specific antigen
DTC in men NED after RP
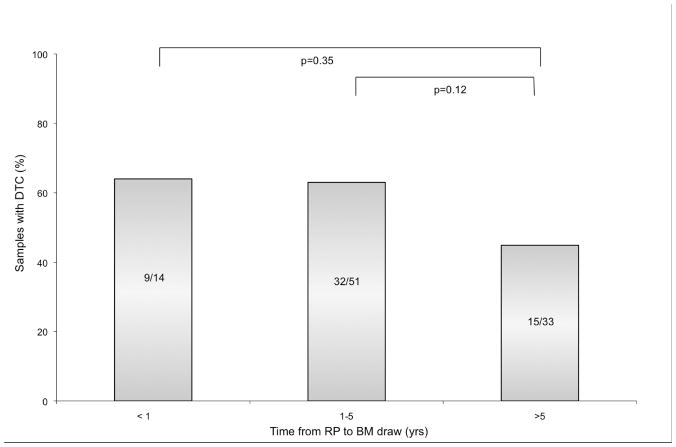
Follow-up BM aspirates were performed in 98 patients at a median of 20 months after RP (range, 3 to 216 months). Median PSA follow-up was 42 months (range 13 to 228 months) from the time of RP and 13 months (range 1 to 58 months) from the time of BM aspiration. Table 2 displays the characteristics of NED patients after RP stratified by presence of DTC. Overall, 57% of patients classified as NED after RP had DTC present, which was significantly different than the rate of detection prior to RP (p=0.004). The rate of detection of DTC was similar at <1 year from RP (9/14, 64%) and 1–5 years (32/51, 63%; Figure 1). At >5 yrs, detection was slightly lower (15/33, 45%), however, this difference was not statistically significant compared with detection at ≤ 5 years (χ2, p=0.10). Additionally, of the 83 men with PSA <0.1 ng/ml at the time of BM aspiration, 48 (58%) had DTC present. DTC were detected in 2 men 12 years post-RP, neither of whom have recurred to date.
Table 2.
DTC in patients NED after RP stratified by clinicopathological variables. Complete staging information was unavailable from 2 patients. All p values are χ2.
| Characteristic | Overall
|
DTC
|
p | |
|---|---|---|---|---|
| Yes
|
No
|
|||
| n (%) | n (%) | n (%) | ||
| No. of patients | 98 | 56 (57) | 42 (43) | |
| Pre-operative PSA | ||||
| <4 | 12 (12) | 6 (50) | 6 (50) | |
| 4–10 | 69 (70) | 43 (62) | 26 (38) | |
| 10–20 | 11 (11) | 5 (45) | 6 (55) | |
| >20 | 6 (6) | 2 (33) | 4 (67) | 0.39 |
| Pathologic stage | ||||
| T2 | 84 (86) | 48 (57) | 36 (43) | |
| T3-4 | 11 (11) | 8 (73) | 3 (27) | |
| Tany, N+ | 2 (22) | 0 (0) | 2 (100) | 0.15 |
| Gleason grade | ||||
| 4–6 | 70 (71) | 42 (60) | 28 (40) | |
| 7 | 23 (23) | 13 (57) | 10 (43) | |
| 8–10 | 3 (3) | 1 (33) | 2 (67) | 0.64 |
| Surgical margin | ||||
| Positive | 35 (36) | 21 (60) | 14 (40) | |
| Negative | 61 (62) | 35 (57) | 26 (43) | 0.8 |
| Age | ||||
| 40–50 | 11 (11) | 6 (55) | 5 (45) | |
| 51–60 | 40 (41) | 27 (68) | 13 (33) | |
| >60 | 47 (48) | 23 (49) | 24 (51) | 0.22 |
| Race | ||||
| African-American | 6 (6) | 3 (50) | 3 (50) | |
| White | 92 (94) | 53 (58) | 39 (42) | 0.72 |
Abbreviations: DTC, disseminated tumor cells; PSA, prostate specific antigen
Figure 1.
Presence of DTC in patients NED after RP at <1, 1–5, and >5 years from RP to BM draw.
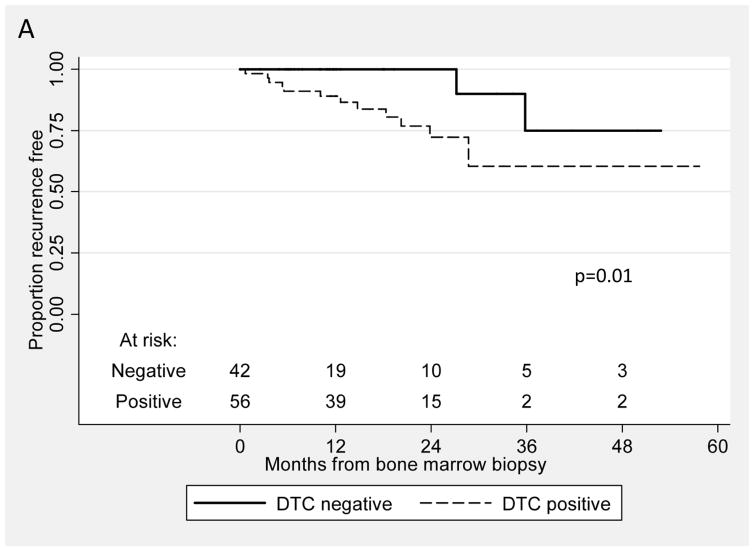
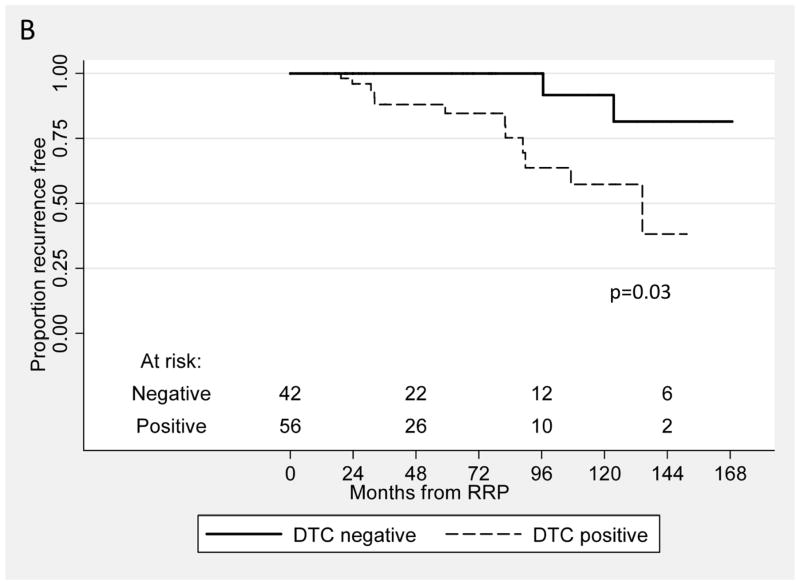
Fourteen patients (14%) in the NED cohort have recurred after BM aspiration to date, and twelve of these 14 patients (86%) had DTC present after RP. In comparison, DTC were detected in 44/84 (52%) of patients who have not recurred to date (χ2, p=0.02). As seen in Table 3, in the multivariate analysis and in the model using time from RP, DTC was an independent predictor for recurrence: men with DTC present after RP had a significantly greater risk of recurrence than men without these cells present (hazard ratio 6.87, CI 1.03 to 45.87). Figure 2A shows the Kaplan-Meier recurrence-free survival curve from the time of RP for patients with and without DTC (log rank, p=0.01). Similarly, in the second model, when time under observation started as the time of BM aspiration (Table 3), detection of DTC was associated with a hazard ratio of 4.20 (CI 0.79 to 22.39). The Kaplan-Meier curve for this analysis is shown in Figure 2B (log rank, p=0.03).
Table 3.
Multivariate Cox regression analysis controlling for PSA, Gleason Grade, pathologic stage, surgical margin, age, and race in patients NED after RP. All p values from Wald test.
| Variable | Time from RP
|
Time from BM
|
||
|---|---|---|---|---|
| Hazard ratio (95% CI) | p | Hazard ratio (95% CI) | p | |
| DTC | 6.87 (1.03–45.87) | 0.047 | 4.20 (0.79–22.39) | 0.09 |
| PSA | 1.00 (0.86–1.16) | 0.97 | 1.04 (0.91–1.81) | 0.91 |
| Grade | ||||
| 4–6 | 1.0 (referent) | 1.0 (referent) | ||
| 7 | 4.7 (1.20–18.49) | 0.03 | 4.14 (1.15–14.97) | 0.03 |
| 8–10 | 8.23 (0.58–116.20) | 0.12 | 5.45 (0.39–76.17) | 0.21 |
| Stage | ||||
| T2 | 1.0 (referent) | 1.0 (referent) | ||
| T3-4 or N+ | 2.17 (0.62–7.63) | 0.23 | 2.26 (0.64–7.95) | 0.2 |
| Margin (+) | 1.6 (0.43–6.0) | 0.49 | 1.89 (0.55–6.52) | 0.31 |
| Age | ||||
| 40–50 | 1.0 (referent) | 1.0 (referent) | ||
| 51–60 | 1.08 (0.21–5.64) | 0.93 | 0.99 (0.18–5.31) | 0.99 |
| >60 | 1.25 (0.20–7.65) | 0.81 | 0.80 (0.13–5.06) | 0.82 |
| Race | 2.55 (0.21–31.25) | 0.47 | 0.74 (0.07–7.01) | 0.79 |
Abbreviations: DTC, disseminated tumor cells; PSA, prostate specific antigen
Figure 2.
Kaplan-Meier recurrence-free survival curves from the time of RP (A) and from the time of BM aspirate (B) for patients with and without DTC (log rank, p=0.01 and 0.03, respectively).
We also looked separately at the 49 patients who had BM aspirates both at the time of RP and in follow-up when NED. Two patients were negative in both samples, 3 patients were negative at RP and positive in follow-up, 17 patients had DTC detected at RP and none when NED, and 27 patients had DTC in both samples. Recurrences have occurred in 1/3 (33%) who changed from negative to positive and in 4/27 (15%) with DTC in both samples. No patients in the other groups have recurred.
DISCUSSION
The objective of this study was to describe the prevalence of DTC in patients with PCa prior to RP and to assess the relationship between risk of PCa recurrence and presence of disseminated prostate cancer cells after RP. We presented three major findings: First, DTC were present in over 70% of patients with PCa prior to RP—a significantly larger proportion than most previous reports (20–23). The high rate of dissemination prior to treatment may explain the apparent lack of correlation between disseminated prostate cells and tumor pathology and is suggestive of early shedding of prostate cancer cells that have the capacity to reside within the BM. Second, DTC were detected in 57% of patients NED after RP, including 45% (15/33) of patients more than five years removed from surgery. This has implications in terms of tumor dormancy and delayed recurrence. Finally, NED patients with DTC present after RP had a nearly 7-fold increased risk of recurrence compared to those patients without DTC. This is the first report of an association between DTC and biochemical recurrence in men without evidence of disease after RP.
Early dissemination of cancer cells regardless of stage, grade, or tumor volume is supported by molecular evidence demonstrating significant heterogeneity among DTC and between DTC and their primary tumors (4, 5). Furthermore, a comparison of genetic patterns in patients with multifocal PCa and circulating tumor cells primary. suggested that a single focus—often the smallest focus and as small as 0.2 cm3—may have been the most likely source of CTC in these patients (24). This concurs with our data showing a high rate of DTC detection prior to RP even in low-risk patients.
We also have completed an initial comprehensive study on genomic aberrations in the DTC population using array CGH (25). Using as few as 10 pooled DTC from 48 patients prior to RP and 11 patients with advanced disease we made several notable observations. Epithelial cells derived from the bone marrow aspirates of patients prior to RP had significantly more genomic deviations than normal cells (14 vs 4, p = 0.005), implying again that these are tumor cells. These genomic alterations were similar to, but not identical with, those of the primary tumor. There was also a progressive evolution in genomic changes between those DTC analyzed from patients pre-RP and those from patients with advanced disease. Additionally, the genomic aCGH profile of the DTC from advanced stage patients were more similar to those of metastases than to those of primary tumors, with commonly expressed gains in 8q and losses in 8p12-23, 10q26, 13q and 16q21. These molecular investigations provide strong justification for the further characterization of these shed tumor cells with the expectation that such studies will provide insight to help stratify patients prior to treatment, select the most appropriate treatment, and facilitate novel therapeutic strategies.
To date, there are few published studies evaluating the significance of CTC and DTC in PCa patients after RP. One report evaluated 50 post-prostatectomy patients by peripheral blood RT-PCR, detecting CTC in 47% of patients with a rising PSA versus only 3% without a rising PSA at the time of blood draw (26) Tombal et al. (27) evaluated 55 men with biochemical recurrence after RP and detected a trend towards a shorter doubling time in men with CTC compared to those without CTC. Only one recent study has tested for tumor cells in the BM after therapy, finding that the presence of DTC ≥2 years after definitive radiotherapy was associated with some decrease in progression-free survival (28).
Given that studies after primary therapy in breast cancer have shown an independent prognostic value for CTC and DTC, (15, 29, 30) there is a clear need to identify their role in PCa. It is also important to determine, on a biologic level, what mechanisms enable PCa to recur after many years without detection. Our data indicate that, while many patients with DTC prior to RP no longer have detectable DTC after surgery, a large proportion of NED patients harbor tumor cells in their BM long after RP. Additionally, those patients with persistent DTC after RP have a significantly higher risk of biochemical recurrence than those without DTC. These results suggest that tumor dormancy plays a prominent role in PCa recurrence after definitive therapy. Murine models of dormancy have been induced through immunization with tumor markers, and the existence of dormant states due to a lack of angiogenesis has been well documented (31, 32). We suggest that tumor cells in the BM of patients who are clinically disease-free several years after RP are, in fact, dormant tumor cells that may eventually lead to recurrence (33). It remains unknown whether all DTC or a subset of these cells with specific phenotypic characteristics are capable of forming metastases.
The observations from our NED cohort must be tempered by the study size of 98 patients as well as the need for longer follow-up given the delayed nature of biochemical recurrence after RP. There have been only 14 instances of biochemical failure in this cohort, and more events will be required in order to confirm the findings observed to date. Furthermore, it is possible that the low number of events resulted in an imprecise estimate of the influence of stage, grade, pre-operative PSA, and margin status as predictors of recurrence in the multivariate analysis. Another limitation of this analysis is the varying time from surgery to BM aspiration; ideally, BM aspirates would have been acquired at a standard interval, although we found that the detection of DTC in these specimens changed very little with time. Additionally, in order to help account for the varying intervals, we employed both a time from RP model and a time from BM aspirate model for the multivariate analysis with similar estimates of risk. Differences in the statistical significance between these two models are likely related to the differences in median follow-up time (42 vs. 13 months).
There are significant technical barriers to identifying individual tumor cells from a BM aspirate containing approximately 107 cells. We utilize a multi-step enrichment technique with both positive and negative selection, using both epithelial and leukocyte staining markers in order to identify only those cells likely to be DTC. While a semi-automated method of CTC detection has been successfully developed (34), the quantity and variety of cells in the BM poses a greater technical challenge for the detection of DTC. Additionally, the significance of the three “false positives” in the control group remains unclear. Some have suggested that EpCAM may be detected on normal hematopoetic cells (35), however we identified no morphologic features that distinguished positive-staining cells in controls from those seen in PCa patients. Whether the detection of DTC in controls accurately reflects the error rate of the DTC identification technique or signals an undetected epithelial malignancy will take further study and molecular characterization. Furthermore, while others have used cutoffs of 5 or more cells to define samples as positive, we sought to maximize sensitivity of the assay by utilizing a single DTC cutoff. A higher cell cutoff would have decreased the “false positive” rate, however, the correlation between DTC and biochemical recurrence seen here supports the single cell definition. As other investigators have concluded, since identification of a single DTC may have clinical importance, this is the ideal definition of a positive sample (36).
The present data indicate that at least some DTC may be dormant tumor cells capable of eventually proliferating and leading to tumor recurrence. While some DTC-positive patients have not recurred, all but two patients who recurred had DTC detected while still NED in our definition. Those NED patients with DTC appeared to be at a significantly increased risk of recurrence. This investigation was performed as a retrospective analysis of prospectively collected data and therefore a separate prospective study will be necessary before any recommendations can be made regarding routine analysis of BM after prostatectomy. A full understanding of these cells will require molecular techniques such as cDNA microarrays and comparative genomic hybridization, and these analyses are ongoing. Further research may help elucidate potential intrinsic and extrinsic factors responsible for enabling a dormant state in these cells and allowing some to proliferate successfully after years of dormancy. Finally, identification of clinically relevant molecular markers on these cells may allow more sensitive cell detection, better prognostication, and, potentially, targets for future therapies.
Statement of clinical relevance.
Tumor progression following radical prostatectomy occurs in 10–30% of patients and can begin years after surgery. Even though pathological stage, Gleason Sum, surgical margin status, and PSA levels are useful prognosticators, the prediction of whether an individual patient will relapse is an inexact science. We hypothesized that disease progression frequently results from prostate cancer cells disseminated prior to removal of the primary tumor and that persistence of these cells after surgery predicts future recurrence. The data from this study, as well as from those who have studied breast cancer, clearly demonstrates that dissemination can begin at an early stage when the tumor is small. There is little data regarding the detection of disseminated tumor cells in patients after radical prostatectomy. Here, we demonstrate that the presence of these cells in patients with no evidence of disease after prostatectomy is an independent predictor of recurrence. This not only lends insight into the biology of prostate cancer progression, but provides a potential mechanism for identifying patients at risk of recurrence. Further work will be needed to determine whether these at-risk patients would benefit from early treatment and whether disseminated tumor cell detection could be used to monitor treatment efficacy in these patients.
Acknowledgments
The authors wish to thank Martin Kinnunen and Bryce Lakely who have dedicated several years to perfecting the techniques discussed here as well as to gathering laboratory data. We also acknowledge the financial support from our prior NIDDK George M. O’Brien grant, the Department of Defense Prostate Cancer Research Program grant (#17-03-2-0033), the NCI NW SPORE Prostate Cancer Grant (#CA97186), the Department of Veteran’s Affairs (RLV is a Research Career Scientist), and the Richard M. Lucas Foundation.
Abbreviations
- BM
Bone marrow
- CGH
Comparative genomic hybridization
- CTC
Circulating tumor cells
- DTC
Disseminated tumor cells
- NED
No evidence of disease
- PCa
Prostate cancer
- PSA
Prostate specific antigen
- RP
Radical prostatectomy
Footnotes
Disclosures: none
References
- 1.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281:1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 2.Amling CL, Blute ML, Bergstralh EJ, Seay TM, Slezak J, Zincke H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: continued risk of biochemical failure after 5 years. J Urol. 2000;164:101–5. [PubMed] [Google Scholar]
- 3.Meng SD, Tripathy D, Frenkel EP, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10:8152–62. doi: 10.1158/1078-0432.CCR-04-1110. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt-Kittler O, Ragg T, Daskalis A, et al. From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci USA. 2003;100:7737–42. doi: 10.1073/pnas.1331931100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein CA, Blankenstein JF, Schmidt-Kittler O, et al. Genetic heterogeneity of single disseminated tumour cells in minimal residual cancer. Lancet. 2002;360:683–9. doi: 10.1016/S0140-6736(02)09838-0. [DOI] [PubMed] [Google Scholar]
- 6.Klein C, Holzel D. Systemic cancer progression and tumor dormancy. Cell Cycle. 2006;5:1788–98. doi: 10.4161/cc.5.16.3097. [DOI] [PubMed] [Google Scholar]
- 7.Bianco FJ, Wood DP, Gomes de Oliveira J, Nemeth JA, Beamann AA, Cher ML. Proliferation of prostate cancer cells in the bone marrow predicts recurrence in patients with localized prostate cancer. Prostate. 2001;49:235–42. doi: 10.1002/pros.10018. [DOI] [PubMed] [Google Scholar]
- 8.Pfitzenmaier J, Ellis WJ, Hawley S, et al. The detection and isolation of prostate-specific antigen positive epithelial cells by enrichment: a comparison to standard prostate-specific antigen reverse transcriptase polymerase chain reaction and its clinical relevance in prostate cancer. Urol Oncol. 2007;25:214–20. doi: 10.1016/j.urolonc.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 9.Brandt B, Junker R, Griwatz C, et al. Isolation of prostate-derived single cells and cell clusters from human peripheral blood. Cancer Res. 1996;56:4556–61. [PubMed] [Google Scholar]
- 10.Ellis WJ, Pfitzenmaier J, Colli J, Arfman E, Lange PH, Vessella RL. Detection and isolation of prostate cancer cells from peripheral blood and bone marrow. Urology. 2003;61:277–81. doi: 10.1016/s0090-4295(02)02291-4. [DOI] [PubMed] [Google Scholar]
- 11.Gao CL, Rawal SK, Sun L, et al. Diagnostic potential of prostate-specific antigen expressing epithelial cells in blood of prostate cancer patients. Clin Cancer Res. 2003;9:2545–50. [PubMed] [Google Scholar]
- 12.Morgan T, Lange P, Vessella R. Detection and characterization of circulating and disseminated prostate cancer cells. Front Biosci. 2007;12:3000–9. doi: 10.2741/2290. [DOI] [PubMed] [Google Scholar]
- 13.Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351:781–91. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 14.Braun S, Vogl FD, Naume B, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. New England Journal of Medicine. 2005;353:793–802. doi: 10.1056/NEJMoa050434. [DOI] [PubMed] [Google Scholar]
- 15.Xenidis N, Perraki M, Kafousi M, et al. Predictive and Prognostic Value of Peripheral Blood Cytokeratin-19 mRNA-Positive Cells Detected by Real-Time Polymerase Chain Reaction in Node-Negative Breast Cancer Patients. J Clin Oncol. 2006;24:3756–62. doi: 10.1200/JCO.2005.04.5948. [DOI] [PubMed] [Google Scholar]
- 16.Moreno JG, Miller MC, Gross S, Allard WJ, Gomella LG, Terstappen LWMM. Circulating tumor cells predict survival in patients with metastatic prostate cancer. Urology. 2005;65:713–8. doi: 10.1016/j.urology.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Weckermann D, Wawroschek F, Krawckak G, Haude KH, Harzmann R. Does the immunocytochemical detection of epithelial cells in bone marrow (micrometastasis) influence the time to biochemical relapse after radical prostatectomy? Urol Res. 1999;27:285–90. [PubMed] [Google Scholar]
- 18.Amling CL, Bergstralh EJ, Blute ML, Slezak JM, Zincke H. Defining prostate specific antigen progression after radical prostatectomy: What is the most appropriate cut point? J Urol. 2001;165:1146–51. [PubMed] [Google Scholar]
- 19.Stephenson AJ, Kattan MW, Eastham JA, et al. Defining biochemical recurrence of prostate cancer after radical prostatectomy: A proposal for a standardized definition. J Urol. 2006;24:3973–8. doi: 10.1200/JCO.2005.04.0756. [DOI] [PubMed] [Google Scholar]
- 20.Berg A, Berner A, Lilleby W, Fossa S, Nesland J, Kvalheim G. Impact of disseminated tumor cells in bone marrow at diagnosis in patients with nonmetastatic prostate cancer treated by definitive radiotherapy. Int J Cancer. 2007;120:1603–9. doi: 10.1002/ijc.22488. [DOI] [PubMed] [Google Scholar]
- 21.Thomas J, Gupta M, Grasso Y, et al. Preoperative Combined Nested Reverse Transcriptase Polymerase Chain Reaction for Prostate-Specific Antigen and Prostate-Specific Membrane Antigen Does Not Correlate With Pathologic Stage or Biochemical Failure in Patients With Localized Prostate Cancer Undergoing Radical Prostatectomy. J Clin Oncol. 2002;20:3213–8. doi: 10.1200/JCO.2002.11.097. [DOI] [PubMed] [Google Scholar]
- 22.Weckermann D, Muller P, Wawroschek F, Krawczak G, Reithmuller G, Schlimok G. Micrometastases of bone marrow in localized prostate cancer: correlation with established risk factors. J Clin Oncol. 1999;17:3438–43. doi: 10.1200/JCO.1999.17.11.3438. [DOI] [PubMed] [Google Scholar]
- 23.Wood DP, Banerjee M. Presence of circulating prostate cells in the bone marrow of patients undergoing radical prostatectomy is predictive of disease-free survival. J Clin Oncol. 1997;15:3451–7. doi: 10.1200/JCO.1997.15.12.3451. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt H, DeAngelis G, Eltze E, Gockel I, Semjonow A, Brandt B. Asynchronous Growth of Prostate Cancer Is Reflected by Circulating Tumor Cells Delivered from Distinct, Even Small Foci, Harboring Loss of Heterozygosity of the PTEN Gene. Cancer Res. 2006;66:8959–65. doi: 10.1158/0008-5472.CAN-06-1722. [DOI] [PubMed] [Google Scholar]
- 25.Holcomb IN, Grove DI, Kinnunen M, et al. Genomic alterations indicate tumor origin and varied metastatic potential of disseminated cells from prostate cancer patients. Cancer Res. 2008;68:1–10. doi: 10.1158/0008-5472.CAN-08-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millon R, Jacqmin D, Muller D, Guillot J, Eber M, Abecassis J. Detection of prostate-specific antigen or prostate-specific antigen-positive circulating cells in prostatic cancer patients: clinical implications. Eur Urol. 1999;36:278–85. doi: 10.1159/000020005. [DOI] [PubMed] [Google Scholar]
- 27.Tombal B, Van Cangh P, Loric S, Gala J. Prognostic value of circulating prostate cells in patients with a rising PSA after radical prostatectomy. Prostate. 2003;56:163–70. doi: 10.1002/pros.10237. [DOI] [PubMed] [Google Scholar]
- 28.Lilleby W, Nesland J, Fossa S, Torlakovic G, Waehre H, Kvalheim G. The prognostic impact of cytokeratin-positive cells in bone marrow of patients with localized prostate cancer. Int J Cancer. 2003;103:91–6. doi: 10.1002/ijc.10780. [DOI] [PubMed] [Google Scholar]
- 29.Braun S, Schlimok G, Heumos I, et al. erbB2 Overexpression on Occult Metastatic Cells in Bone Marrow Predicts Poor Clinical Outcome of Stage I–III Breast Cancer Patients. Cancer Res. 2001;61:1890–5. [PubMed] [Google Scholar]
- 30.Janni W, Hepp F, Rjosk D, et al. The fate and prognostic value of occult metastatic cells in the bone marrow of patients with breast carcinoma between primary treatment and recurrence. Cancer. 2001;92:46–53. doi: 10.1002/1097-0142(20010701)92:1<46::aid-cncr1290>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 31.Dyke R, McBride H, George A, Hamblin T, Stevenson F. Idiotypic vaccination against B-cell lymphoma leads to dormant tumour. Cell Immunol. 1991;132:70–83. doi: 10.1016/0008-8749(91)90007-x. [DOI] [PubMed] [Google Scholar]
- 32.Holmgren L, O’Reilly M, Folkman J. Dormancy of micrometastases: balanced proliferation and apoptosis in the presence of angiogenesis suppression. Nat Med. 1995;1:149–53. doi: 10.1038/nm0295-149. [DOI] [PubMed] [Google Scholar]
- 33.Wikman H, Vessella RL, Pantel K. Cancer micrometastasis and tumor dormancy. APMIS. 2008 doi: 10.1111/j.1600-0463.2008.01033.x. (in press) [DOI] [PubMed] [Google Scholar]
- 34.Allard WJ, Matera J, Miller MC, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10:6897–904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 35.Lammers R, Giesert C, Grunebach F, Marxer A, Vogel W, Buhring H. Monoclonal antibody 9C4 recognizes epithelial cellular adhesion molecule, a cell surface antigen expressed in early steps of erythropoiesis. Exp Hematol. 2002;30:537–45. doi: 10.1016/s0301-472x(02)00798-1. [DOI] [PubMed] [Google Scholar]
- 36.Tibbe A, Miller M, Terstappen L. Statistical considerations for enumeration of circulating tumor cells. Cytometry A. 2007;71:154–62. doi: 10.1002/cyto.a.20369. [DOI] [PubMed] [Google Scholar]