Abstract
Temperament is usually considered biologically based and largely inherited, however the environment can shape the development of temperament. Allelic variation may confer differential sensitivity to early environment resulting in variations in temperament. Here we explore the relationship between measures of temperament in mothers and their first-born offspring and the role of genetic sensitivity in establishing the strength of these associations. Temperament ratings were conducted on 3-4 month old rhesus monkeys after a 25-hour biobehavioral assessment. Factor analysis revealed a four factor structure of temperament. Females assessed as infants have reproduced and their offspring have also been evaluated through the standardized testing paradigm. Canonical correlation analysis revealed statistically significant associations between factor scores of mothers and sons, but not mothers and daughters. Further, offspring possessing the high activity, “low risk”, alleles of the rhMAOA-LPR or rh5-HTTLPR showed statistically significant canonical correlations, whereas those possessing other alleles did not, suggesting differential genetic sensitivity to the normative early experience of maternal temperament.
Keywords: personality, nonhuman primate, intergenerational, Macaca mulatta, alleles, genetics, behavior, early experience, environment, animal, sensitivity
Introduction
Similarities in measures of temperament between mothers and offspring have traditionally been attributed to the cross-generational consistency in rearing environments and to the genes that are shared between mothers and offspring. Early environment plays a large role in shaping characteristics of temperament (Capitanio, Mason, Mendoza, Del Rosso, & Roberts, 2006; Francis & Meaney, 1999; Harlow, Dodsworth, & Harlow, 1965; Mason, 1960), and it is likely that offspring are reared in an environment similar to that of their mother’s rearing including similarities in the style of maternal behavior received (Champagne & Meaney, 2001; Fleming et al., 2002; Suomi, 1991). Temperament traits also show considerable heritability (h2 ranging from .20 to .60: see Fairbanks et al., 2004; Saudino, 2005), indicating that genetic differences are responsible for at least some proportion of individual differences in temperament. It follows that the genes shared between mothers and offspring increase the likelihood that they show similarities in characteristics of temperament. While measures of heritability focus on population-level analyses in order to establish the contribution of genetic factors as a whole to variability in temperament, other research investigates the role of variability in candidate genes in the development of individual differences in temperament (Barr et al., 2003; Gershenfeld & Paul, 1998). One exciting line of behavioral genetic research has begun to explore the way in which temperament outcomes are influenced by genetic polymorphisms that may predispose individuals to greater sensitivity or resiliency to environmental events (Belsky et al., 2009; Obradovic & Boyce, 2009). In the study presented here, we compare measures of temperament in mother and offspring rhesus monkeys (Macaca mulatta) and the role that two specific genetic polymorphisms play in cross-generational associations of temperament.
Rhesus monkeys are largely dependent on their mother at birth and spend most of their time in contact with or proximity to her during the first 3 months of life (Berman, 1980b). This close relationship between mothers and offspring allows for ample exposure to the mother’s temperament and the opportunity for the transmission of behavioral and psychological characteristics from mother to offspring (Suomi, 1991). Investigations of adult mothers and their infant offspring show modest correlations when assessing the same personality trait in both the mother and her offspring (Stevenson-Hinde & Simpson, 1981). Stronger correlations have been found between mother and offspring personality ratings across dimensions, but the structure of this relationship is dependent on offspring sex (Stevenson-Hinde & Simpson, 1981). Additionally, when temperament is measured at comparable ages (both assessed in early life) in both mothers and their offspring, there are cross-generational consistencies in patterns of behavior (Cohen, Kasen, Brook, & Hartmark, 1998; S. J. Suomi, 1987), indicating similarity in propensities to respond—temperament.
There is also a growing literature that suggests that allelic variation in genes associated with the monoamine system contribute to differential sensitivity to the influence of environmental events, including early social experience (Caspi et al., 2002; Karere et al., 2009). The monoaminergic system is often the target of investigation due to its well-established association with psychological processes (Cloninger, 1986; Higley et al., 1992; Manuck, Flory, Ferrell, Mann, & Muldoon, 2000; Zuckerman, 1985). Two functional polymorphisms that have been shown to moderate the effects of the environment are located in the promoter regions of the monoamine oxidase-A gene (MAOA-LPR) and the serotonin transporter gene (5-HTTLPR) (Newman et al., 2005; Sjoberg et al., 2007). Individuals possessing the allelic variant that confers low functional activity for either of these genes are predisposed to greater sensitivity to the effects of early adverse experience and the effects of this genetic sensitivity appear to be cumulative (Kinnally et al., 2010; Reif et al., 2007). Thus far, research has primarily investigated the role of these polymorphisms on psychological and behavioral outcomes after major perturbations in early experience (Champoux et al., 2002; e.g. maltreatment in humans: Kim-Cohen et al., 2006; motherless rearing in monkeys: Newman, et al., 2005). However, less dramatic differences in environmental experience may also result in variations in psychological and behavioral outcomes, and the effects of such experience have the potential to be moderated by genotype. Here, we examine one aspect of normative, non-adverse early experience by investigating the relationship between mother and offspring temperament, including the role of genetic sensitivity to the effects of early experience conferred by the MAOA-LPR and 5-HTTLPR in rhesus monkeys.
In humans, an X-linked untranslated variable number tandem repeat (uVNTR) in the promoter region of the gene that codes for the MAOA enzyme (MAOA-uVNTR) consists of 2-6 copies of a 30-basepair (bp) repeated sequence; alleles with 3.5 or 4 copies are transcribed 2-10 times more efficiently (“high activity”) than those with 3 or 5 copies of the repeat (“low activity”; Sabol, Hu, & Hamer, 1998). An 18-bp variable number tandem repeat region orthologous to the human MAOA-uVNTR (rhMAOA-LPR) has been found in rhesus monkeys and consists of a 5, 6, or 7 repeat region that also confers differential transcriptional efficiencies (5 and 6 repeats have high transcriptional activity, 7 repeat has low activity) of the MAOA gene (Newman, et al., 2005). Monoamine oxidase A (MAOA) is an enzyme that catalyzes the oxidation of monoaminergic neurotransmitters dopamine (DA), norepinephrine (NE), and serotonin (5-HT) to inactive metabolites (homovanillic acid (HVA), 3-methoxy-4-hydroxyphenylglycol (MHPG), and 5-hydroxyindoleacetic acid (5-HIAA), respectively). Women with genotypes containing the high activity MAOA-uVNTR variant have higher levels of HVA, MHPG, and 5-HIAA than those with the low activity allele (Jonsson et al., 2000), suggesting that the higher activity form of the MAOA-uVNTR produces higher concentrations of the MAOA enzyme, resulting in higher amounts of degraded monoamine products. However, men with the high activity MAOA-uVNTR exhibit lower HVA (Ducci et al., 2006; Jonsson, et al., 2000) and higher 5-HIAA concentrations (Williams et al., 2003) than those with the low activity allele. These sex differences may be the result of the location of this gene on the X chromosome (Shih, Grimsby, Chen, & Zhu, 1993). Males are hemizygous for the MAOA-uVNTR allele, having only one version that is inherited from their mother, while females may be hetero- or homozygous. Evidence also suggests that the MAOA gene is one of the 15% of X-linked genes that escape inactivation in females (Carrel & Willard, 2005), therefore, females may experience a dosage difference in gene expression based on their combination of alleles (Ducci et al., 2008).
The different versions of the MAOA-uVNTR also confer differential sensitivity to early experiences; individuals possessing the low activity MAOA-uVNTR variant appear to be more vulnerable to the impacts of experience, whereas individuals displaying the high activity version are more resilient to the influence of early adversity. Individuals possessing the high activity MAOA-uVNTR and exposed to maltreatment in childhood are less likely to develop behaviors associated with antisocial personality disorder than those with the low activity version who also experienced early adversity (Caspi, et al., 2002; Ducci, et al., 2008). Additionally, of infant rhesus monkeys reared by their mother, no behavioral differences were reported for animals possessing low- versus high-activity rhMAOA-LPR genotypes, but only if animals were reared in complex environments; in socially impoverished environments, those with the low activity version displayed more behaviors reflective of anxiety (Karere, et al., 2009). These gene x environment (G x E) effects may be dependent on timing (Huizinga et al., 2006) and context (Karere, et al., 2009; Widom & Brzustowicz, 2006) of early adversity.
The 5-HTTLPR in humans is a 44-bp insertion/deletion polymorphism in the promoter region of the gene that codes for the serotonin transporter, which is a protein that allows for the reuptake of serotonin from the synapse into the presynaptic neuron (Heils et al., 1996). A 21-bp ortholog of this polymorphic region (rh5-HTTLPR) is also present in rhesus monkeys (Lesch et al., 1997). Individuals with the insertion region are considered to have the long version of this allele, whereas those without the insertion are characterized as possessing the short allele. Two versions of this allele confer differences in functional activity of gene expression: the short allele confers lower transcriptional efficiency (“low activity”) of the serotonin transporter gene in comparison to the long allele (“high activity”; Heils, et al., 1996). Individuals possessing the high activity version of this polymorphism have higher levels of 5-HIAA (Williams et al., 2003), in comparison to those with the low activity version. Similar to the MAOA-uVNTR, individuals with the form of the allele that confers higher transcriptional efficiency appear to be less severely affected by early adversity: individuals who have experienced childhood maltreatment and possess the low activity allele are more likely to develop depression than those with the high activity version of the allele (Caspi, Sugden, et al., 2003).
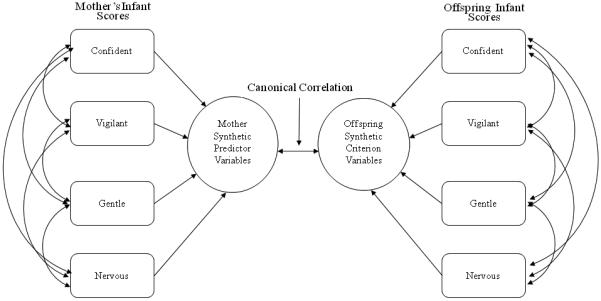
Here we extend previous findings by determining the strength of the association between mother and offspring temperament factor scores when assessed through the same testing paradigm when both mothers and offspring were infants. In order to assess the overall strength and structure of the relationship between mother and offspring temperament, we used a statistical technique underutilized in personality research: canonical correlation. Whereas correlations and multiple regressions are informative in assessing the relationship between two variables or between a dependent variable and a set of independent variables, canonical correlation analysis allows us to explore relationships between two sets of variables (Fig. 1), enabling evaluation of the strength and structure of the relationship between mother and offspring temperament factor scores. Additionally, we evaluate the role of sensitivity conferred by offspring rhMAOA-LPR and rh5-HTTLPR genotype to the strength of associations between mother and offspring temperament factor scores. We hypothesize that individuals with the low activity “sensitive” versions of the rhMAOA-LPR and rh5-HTTLPR will be more sensitive to maternal influences in their early environment and will therefore exhibit stronger canonical correlations between mother and offspring temperament, compared to infants possessing the high activity versions.
Figure 1.
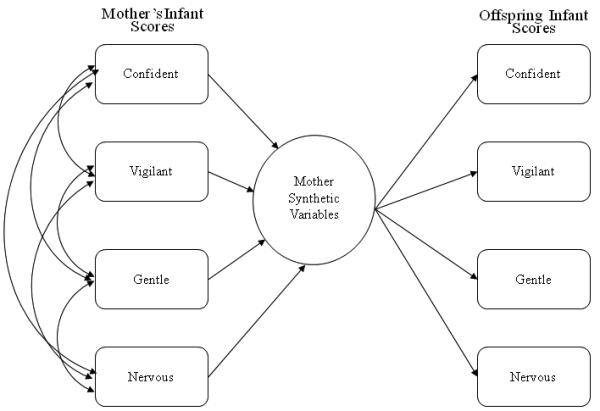
a. Multiple Regression Model
b. Canonical Correlation Model
Methods
Subjects
Subjects were 179 infant (92 males) rhesus monkeys (Macaca mulatta) between 90 and 125 days old (mean age = 106.96 days) at the time of the assessment (see below). Subjects included here were the biological offspring of primiparous mothers, who themselves had been assessed through the same testing procedure when they were infants (between 90 and 124 days old, mean age = 108.15 days). All individuals lived in socially complex half-acre enclosures consisting of 100-150 animals of mixed age- and sex-classes of close kin, distant kin, and non-kin at the California National Primate Research Center (CNPRC). A commercial diet (Outdoor Monkey Lab Diet, PMI Nutrition Int’l, Brentwood, Missouri) was provided twice daily, fruit and vegetable supplements were provided twice weekly, and water was available ad libitum.
Subject Relocation
Five to eight mother-infant pairs at a time were captured from their half-acre enclosures, and were separated from each other for a 25-hr period. Infants were housed in standard laboratory cages (60cm × 65cm × 79cm, Lab Products, Inc., Maywood, NJ), with all five to eight members of a given cohort housed in the same room throughout the 25-hr period. At the conclusion of the 25-hour testing period, infants were reunited with their mother and were housed together for one hour before being returned to their outdoor enclosures.
Infant Biobehavioral Assessment
All subjects were part of an ongoing biobehavioral assessment program at the CNPRC (Capitanio, Mendoza, Mason, & Maninger, 2005; Golub, Hogrefe, Widaman, & Capitanio, 2009; Hinde & Capitanio, 2010). Over the 25-hr. testing period, behavioral data were collected in a variety of standardized testing situations designed to assess responsiveness to the separation and relocation, recognition memory, responses to social stimuli, contact with novel objects, and behavioral response to graded conditions of challenge (see details in Golub et al., 2009).
Temperament Ratings
At the conclusion of the 25-hr. testing period, technicians who performed the testing rated each infant on 16 trait adjectives describing characteristics of temperament (see Table 3 in Golub, et al., 2009; Hinde & Capitanio, 2010). Infant temperament ratings were conducted in order to obtain an overall “thumbnail” portrait of the animal’s functioning during the entire Infant Biobehavioral Assessment period. Details of the rating procedure have been presented (Sullivan et al. 2010). Briefly, ratings were made using a seven point Likert scale. Agreement between independent observers (a difference of no more than 1 scale point) for each trait was significantly greater than chance using chi-square (p<0.000001). Ratings on each adjective were z-scored across all subjects within a given birth year. Exploratory and confirmatory factor analyses of temperament adjective ratings for 1294 infants revealed a four factor structure which satisfied fit statistics for exploratory and confirmatory models (for details see Golub, et al., 2009). Factors were named for the trait adjective with the highest positive loading: Confident (active, bold, confident, curious, playful), Gentle (calm, curious, flexible, gentle), Vigilant (vigilant, not depressed, not tense, not timid), and Nervous (fearful, nervous, timid, not calm, not confident) . The trait adjectives preceded by the word “not” reflect a negative loading in the factor analysis. A composite score for each factor was created by adding the z-scores of each trait rating adjective (or the reverse-coded z-score for negative loadings). These scores were then standardized within each factor scale and z-scores were created. Cronbach’s alpha (scale reliability) values ranged from 0.6 to 0.9.
Table 3.
Full model canonical correlations
| N | Wilks’s λ | F | p-value | Function 1 RC2 |
Function 2 RC2 |
Function 3 RC2 |
Function 4 RC2 |
|
|---|---|---|---|---|---|---|---|---|
| All Offspring | 179 | .786 | 2.66 | <.001 | .170 | .032 | .021 | .001 |
| Males | 92 | .617 | 2.75 | <.001 | .282 | .127 | .014 | .001 |
| Females | 87 | .761 | 1.39 | .146 | .177 | .047 | .029 | .000 |
| rhMAOA-LPR | ||||||||
| Low Activity | 53 | .608 | 1.53 | .098 | .301 | .094 | .037 | .003 |
| Heterozygous | 41 | .552 | 1.36 | .176 | .280 | .223 | .013 | .000 |
| High Activity | 85 | .551 | 3.14 | < .001 | .269 | .136 | .092 | .041 |
| rh5-HTTLPR | ||||||||
| Low Activity | 78 | .833 | .824 | .658 | .131 | .025 | .010 | .006 |
| High activity | 101 | .652 | 2.65 | .001 | .224 | .119 | .037 | .010 |
Note. Squared canonical correlations (RC2) over 15% were considered for interpretation.
Blood Collection
As part of the ongoing Infant Biobehavioral Assessment, four blood samples (0.5 to 1.0 ml) were collected from infants during the 25-hour maternal separation and relocation to a novel environment. Blood was collected via femoral venipuncture at approximately 1100 h and 1600 h on the first day of separation and at 0830 h and 0900 h on the second day of assessment. Blood was immediately centrifuged at 4° C, and plasma was removed. The remaining buffy coat and red blood cells were refrigerated at 4° C until DNA was isolated.
Genotyping
Genomic DNA was isolated using a Qiagen DNeasy Tissue kit (Valencia, CA) or a modified salting out method (Miller, Dykes, & Polesky, 1988). MAOA and 5-HTT length polymorphic region genotyping was performed on an ABI 377 DNA Analyzer using a fluorescent technology (Applied Biosystems, ABI, Foster City, CA). The MAOA primers (forward, 5′ CAG AAA CAT GAG CAC AAA CG 3′ and reverse, 5′ TAC GAG GTG TCG TCC AAG TT 3′) were designed from the published sequence of the rhesus MAOA-LPR (GenBank Accession number AJ544234), as were the rh5-HTTLPR primers (GenBank Accession number AF285761). Each 15 μl reaction contained 1.5 uL of DNA, 0.5 uL of each primer (labeled forward primer for rhMAOA-LPR, labeled reverse primer for rh5-HTTLPR), 1.5 uL 1X polymerase chain reaction (PCR) buffer and 0.9 uL MgCl2, 0.3 uL dNTP and 0.1 uL of MasterAmp Taq DNA polymerase. PCR was performed on a GeneAmp 9700 thermal cycler (Perkins Elmer, Foster City, CA) with the following profile; 1) initial denaturation for 5 min at 95° C, 2) 35 cycles of 94° C at 1 min, 55° C at 1 min and 72° C at 1 min, and 3) a final primer extension at 72° C for 10 min. Genotypes were determined using STRand software with respect to the GeneScan 350 ROX size-standard. Allelic sizes and identity were confirmed by direct sequencing on an ABI 377 DNA Analyzer using BigDye Terminator Sequencing chemistry v3.1 (ABI) as described elsewhere (Lyons et al., 2004). Genotypes were determined based on previous reports of putative activity of gene expression. For rhMAOA-LPR, 5 or 6-repeat hemizygous males, 5/6 heterozygous females, and 5/5 and 6/6 homozygous females were categorized as “high activity”; 5/7 and 6/7 heterozygous females were categorized as “heterozygous activity”; and 7-repeat hemizygous males or 7/7 homozygous females were considered “low activity” (Newman et al., 2005). For 5-HTTLPR, those homozygous for the long version (l/l) were categorized as “high activity” and those homozygous for the short version (s/s) or heterozygous (l/s) were considered “low activity”.
Data Analysis
Analyses of variance were conducted to assess differences in offspring temperament factor scores. One-way ANOVAs were conducted for offspring sex. Separate two-way ANOVAs were conducted for males and females to account for differences in the presence of heterozygosity in the rhMAOA-LPR activity in females, but not males. For females, 2 × 3 ANOVAs included rh5-HTTLPR (high and low activity) and rhMAOA-LPR (high, heterozygous, and low activity) functional genotypes. For males, 2 × 2 ANOVAs were conducted that included rh5-HTTLPR (high and low activity) and rhMAOA-LPR (high and low activity) functional genotypes.
Bivariate correlations were conducted to compare the same temperament factors between mothers and offspring. The significance of the difference between correlations was computed as described in Blalock (1972). Due to the naturally complex relationship between temperament factors, intercorrelations were assessed through the multivariate technique of canonical correlation analysis (CCA). CCA is similar to multiple regression (Fig. 1A), although it differs in that there are several variables on each side of the equation, rather than just a single predicted dependent variable (Fig. 1B; (Tabachnick & Fidell, 2000). In canonical correlation, each set of variables (e.g., mothers’ four temperament scores, obtained when they were infants) is combined to produce a synthetic variable that has the highest correlation with a synthetic variable on the other side of the equation (e.g., infants’ four temperament scores). There are as many of these canonical functions as there are variables in the smaller of the two variable sets, with the first function providing the strongest correlation between variable sets and each remaining orthogonal function partitioning the remaining residual variance. The statistical significance of the full canonical model evaluates the variance shared between all canonical functions. The canonical correlation coefficients (RC) are the Pearson r relationships between two synthetic variables for each function, but due to standardized weights in linear equations can only range from 0 to 1, and can therefore be interpreted in a manner analogous to the multiple R in regression (Sherry & Henson, 2005). The squared canonical correlation (RC2) represents the proportion of variance shared by the two synthetic variables. Therefore, only functions that explain a reasonable amount of variance are interpreted, here the cut-off was 15%. The individual contributions of variables (in this case, temperament factor scores) to the synthetic variable, taking into consideration shared variance between variables, can be interpreted in a way analogous to the use of beta weights in multiple regression. Here, canonical correlations were performed between the set of four infant temperament factor scores predicted by the set of their mothers’ four infant temperament factor scores in order to evaluate the shared relationship between the two variable sets.
Results
Effects of genotypes and sex on temperament factor scores
Males’ and females’ temperament factor scores did not differ (p > .05). For males, 2 × 2 ANOVAs by genotype (rhMAOA-LPR x rh5-HTTLPR) revealed no statistically significant differences in temperament factor scores (p > .05). For females, 2 × 3 ANOVA for genotype revealed a main effect of rhMAOA-LPR for only the Nervous temperament factor, F(5, 81) = 2.359, p = .047: females with the low activity version of the rhMAOA-LPR had scores on the nervous factor that were higher than those with either high or high/low activity. Genotype x genotype interactions were not statistically significant for this temperament factor, nor were main effects or genotype x genotype interactions for the other three temperament factors scores (p > .05).
Similarity in temperament scores between mothers and offspring
Offspring temperament factor scores were correlated with those scores of their mother for three of the four temperament factors (Table 1): scores on Confident, Vigilant, and Gentle were significantly correlated between mothers and offspring, while scores on Nervous were not. When correlations were calculated separately for mothers with their male offspring and mothers with their female offspring, within factor correlations were statistically significant for the Confident, Vigilant, and Gentle factors for mothers and offspring of either sex (Table 2). Correlation coefficients for mother and sons did not differ significantly from those between mothers and daughters (p > .05). As can be seen in Tables 1 and 2, there were also statistically significant between-factor correlation coefficients for mothers and offspring.
Table 1.
Zero-order correlation matrix of mother-offspring temperament factor scores – full sample.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Mother Confident | 1.00 | |||||||
| 2. Mother Vigilant | .630** | 1.00 | ||||||
| 3. Mother Gentle | .644* | .544** | 1.00 | |||||
| 4. Mother Nervous | −.559** | −.635** | −.681** | 1.00 | ||||
| 5. Offspring Confident | .319** | .289** | .303** | −.206** | 1.00 | |||
| 6. Offspring Vigilant | .303** | .318** | .318** | −.238** | .845** | 1.00 | ||
| 7. Offspring Gentle | .270** | .207** | .315** | −.162* | .579** | .555** | 1.00 | |
| 8. Offspring Nervous | −.142 | −.170* | −.256** | .134 | −.445** | −.523** | −.754** | 1.00 |
Note.
p < .05
p < .01.
Table 2.
Zero-order correlation matrix of temperament factor scores – males (below diagonal) and females (above diagonal).
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|
| 1. Mother Confident | 1.00 | .552** | .608** | −.488** | .211* | .163 | .115 | −.039 |
| 2. Mother Vigilant | .691** | 1.00 | .491** | −.645** | .302** | .220* | .194 | −.102 |
| 3. Mother Gentle | .674** | .585** | 1.00 | −.657** | .323** | .290** | .320** | −.265* |
| 4. Mother Nervous | −.619** | −.628** | −.701** | 1.00 | −.280** | −.285** | −.223* | .129 |
| 5. Offspring Confident | .405** | .283** | .292** | −.162 | 1.00 | .805** | .473** | −.281** |
| 6. Offspring Vigilant | .414** | .393** | .346** | −.214* | .872** | 1.00 | .525** | −.463** |
| 7. Offspring Gentle | .390** | .216* | .312** | −.112 | .669**, | .591** | 1.00 | −.757** |
| 8. Offspring Nervous | −.233* | −.226* | −.248* | .130 | −.602** | −.597** | −.753** | 1.00 |
Note. Correlations for male offspring are shown below the diagonal, those for female offspring above the diagonal.
p < .05
p < .01.
Canonical Correlations
Canonical correlation using the mothers’ four temperament factor scores as predictors of their infants’ four temperament factor scores revealed a statistically significant full model, Wilks’s λ = .786, F(16, 520.00) = 2.66, p < .001 (Table 3, row 1). Because Wilks’s λ represents the variance unexplained by the model, 1 − λ yields the full model effect size in an r2 metric. Thus, for the set of four canonical functions, the r2 type effect size was .214, which is the proportion of variance shared between variable sets across all functions. The analysis revealed four functions with canonical correlations of .170, .032, .021, and .001 for each successive function; only Function 1 was considered noteworthy, contributing 17% of the variance. Table 4 (row 1) represents the standardized canonical function coefficients and structure coefficients for Function 1.
Table 4.
Canonical solutions for first canonical function of mother’s temperament factor scores predicting offspring temperament factor scores. A) Offspring temperament factor score contributions to the synthetic criterion variable; B) Mothers’ Temperament factor score contributions to the synthetic predictor variable.
| Offspring Temperament Factors |
Mothers’ Temperament Factors |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Confident | Vigilant | Gentle | Nervous | Confident | Vigilant | Gentle | Nervous | |||||||||
| Coef. | rs | Coef. | rs | Coef. | rs | Coef. | rs | Coef. | rs | Coef. | rs | Coef. | rs | Coef. | rs | |
| All Offspring | −.268 | −.895 | −.462 | −.896 | −.558 | −.823 | −.192 | .586 | −.432 | −.872 | −.363 | −.758 | −.617 | −.866 | −.335 | .557 |
|
| ||||||||||||||||
| Males | .020 | −.810 | −.644 | −.827 | −.891 | −.848 | −.545 | .499 | −.935 | −.901 | −.095 | −.596 | −.439 | −.677 | −.639 | .307 |
|
| ||||||||||||||||
| Females | −.869 | −.860 | .307 | −.704 | −.328 | −.807 | .312 | .654 | .289 | −.498 | −.541 | −.726 | −.956 | −.917 | −.197 | .640 |
|
| ||||||||||||||||
| rhMAOA-LPR Activity | ||||||||||||||||
|
| ||||||||||||||||
| Low Activity | .214 | −.664 | −.541 | −.825 | −1.184 | −.854 | −.724 | .437 | −.682 | −.935 | −.197 | −.846 | .270 | −.624 | .429 | .851 |
|
| ||||||||||||||||
| Heterozygous | 1.456 | .188 | −1.433 | −.407 | −.653 | −.351 | −.414 | .208 | .330 | .269 | .461 | −.035 | .383 | −.011 | 1.340 | .695 |
|
| ||||||||||||||||
| High Activity | .316 | −.700 | −.695 | −.688 | −1.109 | −.908 | −.478 | .552 | −.611 | −.694 | −.056 | −.377 | −.842 | −.647 | −.020 | −.020 |
|
| ||||||||||||||||
|
rh5-HTTLPR High Activity |
−.253 | −.893 | −.811 | −.948 | −.275 | −.567 | −.437 | .343 | −.645 | −.932 | −.449 | −.860 | .002 | −.676 | .610 | .610 |
Note. Solutions for first canonical functions contributing greater than 15% variance are included (see Table 3). Coef. = standardized canonical function Coefficient; rs = structure Coefficient. Structure Coefficients (rs) greater than |.45| are underlined. Coefficients providing primary contributions to synthetic variables are bolded.
For Function 1, the relevant criterion (offspring) variables were Vigilant, Confident, and Gentle, with Nervous making a secondary contribution to the synthetic criterion variable (Table 4a, row 1). This conclusion was supported by the large structure coefficients. These temperament factors also tended to have larger canonical function coefficients. A slight exception included the Confident and Nervous factors, which had relatively small function coefficients but large structure coefficients. This indicates multicollinearity with the other criterion variables. Furthermore, all of these variables’ structure coefficients had the same sign, indicating that they were all positively related. Regarding the predictor (mother’s) variable set in Function 1 (Table 4b, row 1), Gentle scores were also the primary contributors to the synthetic predictor variable, with Confident, Vigilant, and Nervous making secondary contributions after effects of multicollinearity were taken into consideration. Because the structure coefficients for all of the mothers’ temperament factor scores were negative, they were all positively related to the offspring temperament factors. Figure 2 presents scatterplots of mother and offspring temperament factor scores that were primary contributors to Function 1.
Figure 2.
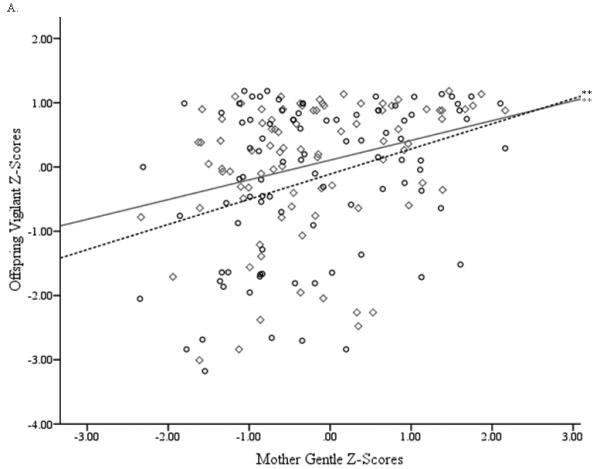
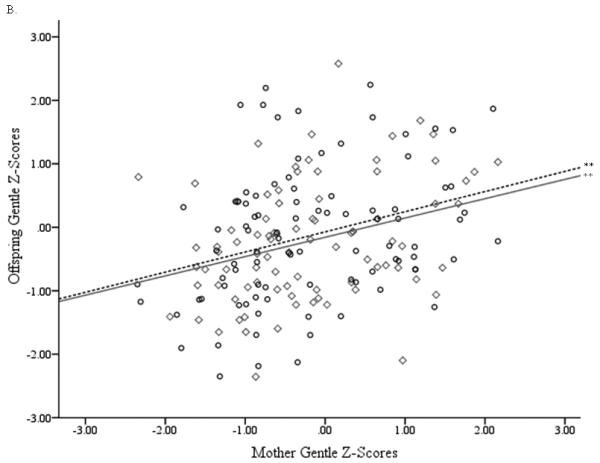
Scatterplots of mother and offspring temperament factor scores for primary contributors to whole sample canonical correlation analyses (males: ○, dashed line; females: ◇, solid line). (A) Mothers’ Gentle factor scores with offspring Vigilant factor scores, (B) Mothers’ Gentle factor score with offspring Gentle factor scores. **p > .01.
When analyses were conducted separately for male and female offspring, the full model across all functions was statistically significant for the relationship between temperament factor scores in males and their mothers, Wilks’s λ = .617, F (16, 256.26) = 2.75, p < .001 (Table 3, row 2), but not for females and their mothers, Wilks’s λ = .761, F(16, 238.93) = 1.39, p = .146 (Table 3, row 3). The effect size for males was 38.3% and for females was 23.9%, which was similar to that of the full sample (21.4%). The analyses for males yielded four functions with squared canonicals of .282, .127, .014, and .001 for each successive function. Given the r2 effects for each function, only the first function was considered significant in the context of this study, explaining 28.2% of the variance. Table 4a (row 2) presents the standardized canonical function coefficients and structure coefficients of Function 1 for males. The relevant criterion variables were Gentle, Vigilant, and Confident, with a secondary contribution from the Nervous factor. However, the Confident factor had a small function coefficient but large structure coefficients, indicating multicollinearity with the other criterion variables so was no longer considered a primary contributor. This function was predicted largely by the mothers’ Confident scores, with a secondary contribution by mothers’ Gentle scores (Table 4b, row 2). Mothers’ scores on the Vigilant factor also appeared to contribute to the predictor synthetic variable, however this variable also has a small function coefficient, so does not provide considerable additional variance to the model.
Canonical Correlations by Offspring Genotype
Canonical correlation analyses with mothers’ four temperament factors as predictors of the four temperament factors for offspring were found to be statistically significant for offspring with the high activity versions of rhMAOA-LPR or rh5-HTTLPR, but not for offspring with low activity alleles (Table 3, rows 6 and 8). For offspring with high activity rhMAOA-LPR alleles the full model canonical correlation explains 44.9% (1 − λ) of the variance between mother and offspring temperament factor scores. The first function contributed 26.9% of the variance for offspring with the high activity rhMAOA-LPR. For offspring with the high activity versions of the rhMAOA-LPR the relevant criterion variables were Gentle, Vigilant, and Confident, with a secondary contribution from the Nervous factor. However, the Confident factor had a small function coefficient but large structure coefficients, indicating multicollinearity with the other criterion variables so was not considered to be a primary contributor. The first function was largely predicted by mothers’ Confident and Gentle scores (Table 4b, row 6). The results for offspring possessing the low activity rhMAOA-LPR and heterozygous for the rhMAOA-LPR should be interpreted with caution, as sample sizes for these groups are at the lower end of the 5 to 10 subjects per variable ratio recommended by Thompson (1990) based on Monte Carlo replication calculations. Further, the effect sizes (1 − λ) for individuals possessing different version of the rhMAOA-LPR were relatively similar, with 45% of variance explained for offspring heterozygous or with the high activity allele and 39% accounted for in offspring with the low activity version (Table 3).
For offspring with high activity rh5-HTTLPR alleles the full model canonical correlation explained 34.8% (1 − λ) of the variance between mother and offspring temperament factor scores. This effect was significant (p = .001), unlike the effect for offspring with the low activity alleles (16.7%). For the high activity animals, the first function contributed 22.4% of the variance for offspring with the high activity rh5-HTTLPR. For these offspring, their temperament factor scores on the Vigilant and Confident factors provided considerable contribution to the criterion variable for Function 1, with Gentle factor scores providing secondary contributions (Table 4a, row 7). Both the Confident and Gentle factors had relatively small structure coefficients, however, indicating that they share variance with Vigilant temperament factor scores, so they were not considered primary contributors to the criterion variable. For Function 1, mothers’ scores on the Confident and Vigilant factors provided primary contribution to the predictor variable, with scores on the Gentle and Nervous factors providing secondary contributions. For this canonical correlation, the structure coefficients for mother’s scores on the Gentle factor were small, indicating that after variance among mothers’ temperament factors is accounted for, mothers’ Gentle factor scores did not contribute considerably to the solution (Table 4b, row 7). Furthermore, mothers’ Nervous factor scores were negatively associated with the other factors, indicated by the difference in structure coefficient signs between the Nervous and other factors. We were not able to conduct genotype x genotype or sex x genotype x genotype canonical correlations, due to small cell sizes for more rare genotypes and their combination.
Discussion
Mothers’ temperament factor scores were positively correlated with those of their offspring for the Confident, Vigilant, and Gentle factors. When assessing the combined effects of mothers’ four infant temperament factor scores as predictors of their offspring’s four infant temperament factor scores through the dimension reduction technique of canonical correlation, there were statistically significant associations between the temperament factor scores of mothers with their sons, but not for mothers and their daughters. Genotypes of alleles associated with differential sensitivity to early experience also played a role in the intergenerational relationships in temperament: offspring with the high activity version of either the rhMAOA-LPR or the rh5-HTTLPR (previously categorized as “low risk”) had temperament factor scores that were significantly predicted by their mothers’ infant temperament factor scores, while offspring with other alleles (low activity or heterozygous) did not.
This study supports previous findings of similarity in temperament between mothers and offspring. Results reveal cross-generational consistencies in temperament factor scores with correlation coefficients in the range of those previously reported (Cohen et al., 1998; Stevenson-Hinde and Simpson, 1981). Our results extend those studies, however, by demonstrating that when considered together, temperament factor scores of mothers predicted temperament factor scores of sons, but not daughters, with 14% more variance explained for sons compared to daughters. Sons’ Gentle and Vigilant temperament scores were predicted most strongly by their mother’s Confident scores. This indicates that mothers who, as infants, responded to a novel challenge in an active, unrestrained, curious, and playful manner had infant sons who responded to the same challenge with a calm, easy-going curiosity (Gentle) and with a relaxed, attentive (Vigilant) approach. This correspondence in temperament between mothers and sons may be related to the life-history of males. Male rhesus macaques emigrate from their natal group (Pusey & Packer, 1986) and attain rank in a new social group based partially on personality characteristics, whereas female rhesus monkeys remain in their natal troop and inherit rank from their mother (Berman, 1980a; Walters & Seyfarth, 1986). Therefore, the temperament of sons may be more sensitive to maternal influence earlier in life, in preparation for dispersal. The results presented here are in line with our previous studies that have revealed sex differences in the association between maternal indicators of environmental condition and offspring temperament, which has suggested that sons may be more responsive to maternal signals early in life (Sullivan, Hinde, Mendoza, & Capitanio, 2011).
We found it surprising that sons exhibited temperament characteristics that were associated with those of their mothers, but daughters did not. These results may have emerged due to the greater methodological control in comparison to previous studies. This study represents data from a large sample for a longitudinal cross-generational study in nonhuman primates, and unlike previous studies of nonhuman primate temperament, each subject was only represented once in an effort to maximize subject independence. The data collection methodology also differed considerably from previous studies: temperament factor scores were collected in infancy in both offspring and mothers and through a standardized assessment that was consistent across generations. Further, subjects were separated from their mother at the time of assessment; therefore infant behavior was not directly influenced by their mother’s behavior during the testing period and observed responses reflect those of the individual, rather than combined features of the mother-offspring dyad. Due to this maternal separation and relocation to a novel environment, temperament factor scores were reflective of patterns of responding during a situation of challenge, which presumably served to accentuate individual differences in temperament. Finally, it is also possible that daughters exhibit social dimensions of personality that are associated with characteristics of their mother, but that were not assessed here, rather than responsivity to novelty, which may be particularly important for sons. Future studies should assess similarity in mother-daughter temperament and personality from infancy into adulthood using standardized methodologies to test both mothers and their daughters at comparable stages of development.
Counter to our hypotheses, we found that individuals possessing the high activity versions of the rhMAOA-LPR or rh5-HTTLPR had temperament factor scores that were significantly associated with those of their mother. There were no statistically significant canonical correlations for those possessing the low activity version of either promoter polymorphism or females heterozygous for the rhMAOA-LPR. However, these results may have been due to limitations of sample size for the less common rhMAOA-LPR alleles; females heterozygous for rhMAOA-LPR displayed low, but statistically non-significant Wilks’s λ values that were nearly identical to those of individuals with the more common high activity alleles. For individuals heterozygous for rhMAOA-LPR or possessing the high activity alleles, Wilks’s λ values were slightly higher than for those for individuals with the low activity versions. This indicates that a larger proportion of the variance was explained by the models for those heterozygous or with high activity rhMAOA-LPR alleles, however analyses were only significant for those with the high activity version due to sample size. Due to this limitation, associations should be examined in future studies with larger sample sizes. For individuals possessing the high activity version of the rh5-HTTLPR, canonical correlations explained 34.8% of the variance in temperament factor scores, which was over twice that of individuals with the low activity version.
For those offspring possessing the high activity rhMAOA-LPR, maternal scores on the temperament factors Gentle and Confident positively and most strongly predicted scores of the Gentle and Vigilant factors. This was similar to the pattern for males (regardless of genotype), except for an additional contribution of the maternal Gentle factor. This was likely due to the inclusion of female offspring to the model, as the largest zero-order correlation between mothers and daughters was on the Gentle factor. For offspring possessing the high activity rh5-HTTLPR, maternal scores on the Confident and Vigilant factors most strongly predicted offspring’s Vigilant factor scores. These results are in support of previous studies investigating mother-offspring dyadic interactions contemporaneously. Mothers high in a factor similar to a combination of the Confident and Vigilant factors described here (Confident in Stevenson-Hinde & Simpson, 1981) show increased rates of rejection of sons (Stevenson-Hinde & Simpson, 1981), which are positively associated with increased rates of offspring exploration and curiosity (Simpson, 1985), components of the Gentle factors described here. Further, mothers high in a factor similar to our Gentle factor (reversed Excitability factor in Stevenson-Hinde & Simpson, 1981) showed fewer restrictive behaviors toward offspring which are associated with increased offspring curiosity and decreased cautiousness (Fairbanks & McGuire, 1988), daughters who are rated as higher in gentleness, and sons rated as higher in a factor similar to the Vigilant and Confident factors combined (Stevenson-Hinde & Simpson, 1981). Our results extend these findings by suggesting that mothers’ temperament, as assessed in infancy, influences adult maternal behavior, thereby shaping offspring temperament. The temperament ratings reported here reflect patterns of responding (Mendoza & Mason, 1989) that are likely consistent from infancy into adulthood (Caspi, Harrington, et al., 2003; Deal, Halverson Jr., Havill, & Martin, 2005; Suomi, 1987). Maternal personality is reflected in maternal behavior (Clark, Kochanska, & Ready, 2000; Elgar, Mills, McGrath, Waschbusch, & Brownridge, 2007; for review see Kagan & Snidman, 1999; Stevenson-Hinde & Simpson, 1981) which plays a large role in shaping offspring temperament (Olson, Bates, Sandy, & Schilling, 2002; Rosenblum & Paully, 1984; Stevenson-Hinde & Simpson, 1981). In addition to maternal contributions to offspring temperament, infants may encounter other environmental factors that contribute to variability in temperament, including the availability of resources and the larger social environment of the group (for review see Clarke & Boinski, 1995). However, in the captive colony examined here these factors remain largely consistent across generations. Therefore, environmental consistencies may account for similarities in measures of temperament between mothers and offspring, but they fail to explain differences in associations between mothers and offspring based on offspring sex and genotype.
While these results were not the consequence of main effects or interactions of sex and genotype, mother-offspring associations in temperament ratings may have been due to increased interactions between mothers and offspring. Males with high concentrations of 5-HIAA (associated with the high activity versions of both the rhMAOA-LPR and rh5-HTTLPR) spend more time in affiliative social interactions than those with low concentrations of 5-HIAA (Mehlman et al., 1995). This may influence the quality and quantity of maternal interactions and increase the opportunity for the inter-generational transmission of temperament. Moreover, associations reported here may be the result of genetic similarity between mothers and sons beyond the genotypes tested here.
Here, we report that individuals possessing the high activity versions of the rhMAOA-LPR and rh5-HTTLPR exhibit temperament traits that were significantly predicted by their mother’s infant temperament factor scores. This indicates that individuals with the “low risk” forms of these alleles, especially the rh5-HTTLPR, may be more sensitive to the early experience of their mother’s personality, resulting in increased associations between mother and offspring temperament. Conversely, it is possible that those with the low-activity, “high risk” versions of these alleles may be more sensitive to influences beyond the mother, resulting in temperament characteristics that are not as closely associated with those of their mother. Perhaps concepts of genetic vulnerability should be construed as sensitivity to different environmental cues (Ellis, Boyce, Belsky, Bakermans-Kranenburg, & van Ijzendoorn, 2011), rather than differential sensitivity to a single construct of “early experience”. For the most part, the early-life environments of mothers and offspring are largely similar and predictable. We propose that under normative conditions, those with “low risk” forms of these alleles will develop temperament traits in conjunction with those of their mother and these characteristics may allow the offspring to respond appropriately to normative demands of the environment. The “low risk” alleles associated with inter-generational stability should allow for adaptive advantages under consistent and predictable situations. And in fact, the high activity, “low risk”, alleles of both the rhMAOA-LPR and rh5-HTTLPR are more common than the “high risk” forms (Heils, et al., 1996; Sabol, et al., 1998; Wendland et al., 2006). In contrast, under extreme environmental conditions, individuals possessing the “high risk”, “sensitive” versions, whose temperament is not contingent upon that of the prior generation, may exhibit increased flexibility in their responses to extreme adversity or opportunity. Therefore, the high and low activity versions of these alleles may confer differential advantages that result in tradeoffs under different environmental conditions.
Taken together, our data suggest that males, and animals of either sex that possess the high activity versions of genes associated with plasticity, receive temperament factor ratings in response to novelty that are predicted by those of their mother. This variability in temperament may provide differential consequences throughout the lives of males, particularly during dispersal. Male emigration from their natal troop is, arguably, one of the most dangerous experiences that a monkey will encounter (Dittus, 1977). It is a time of unpredictable experiences which often results in injury, mortality, and loss of social status. Males that disperse earlier are more likely to survive than those who disperse later (Berard, 1993, 1999) and those possessing the low activity version of the rh5-HTTLPR disperse earlier than those with the high activity version (Trefilov, Berard, Krawczak, & Schmidtke, 2000). The flexibility conferred by the low activity versions of these alleles (and low concentrations of 5-HIAA; Mehlman, et al., 1995) may be advantageous to survival under situations of challenge. However, males with the high activity forms of these alleles exhibit increased social competence, which may result in increased opportunities upon immigration to a new troop.
These results may help explain the inconsistencies and difficulty in replication of previous studies. Like many other studies, we did not show differences in temperament factor scores based on infant sex or genotype (rhMAOA-LPR or rh5-HTTLPR)--except for females heterozygous for the rhMAOA-LPR who received lower ratings on the Nervous factor than females homozygous for the low activity version (these subjects are most often eliminated from analyses). We did, however, observe differential sensitivities based on sex x environment and genotype x environment interactions. These results emerged when comparing temperament in mothers and offspring, rather than mean-level differences in temperament scores. In this way, we were able to assess subtle differences in sensitivity to early experience that is within the normative range.
Finally, we acknowledge some limitations of our study and interpretation. Although our sample size is large for a nonhuman primate study of this kind, the canonical analyses for rhMAOA-LPR involved cell sizes that are close to the lower limit suggested for this statistical procedure (5 to 10 subjects per variable: Thompson, 1990, 1991). Additionally, cell sizes for rhMAOA-LPR genotypes were considerably uneven, likely resulting in non-significant results despite moderate effect sizes for groups with the less common alleles. Consequently, we urge caution in interpretation of these results. Furthermore, we reiterate that all of our mothers were primiparous; while the greater homogeneity of our sample may somewhat mitigate the first concern about cell size, it does limit the generalizability of our results. There is some evidence in the literature, for example, that primiparous mothers are more protective and less rejecting of offspring than are multiparous mothers (Brown & Dixson, 2000; Mitchell & Brandt, 1973; Schino, D’Amato, & Troisi, 1995; Seay, 1964). It is possible that the relationships that we found between mothers and sons, and between mothers and offspring of both sexes that possess the high activity versions of these genes, may be attenuated in later-born offspring. Finally, we acknowledge that fathers may also be contributors to intergenerational similarity in temperament. Because fathers contribute no paternal care in this species, their contribution is largely genetic (or epigenetic). Unfortunately, because males tend to reproduce at later ages than do females, we have a smaller sample of temperament-assessed fathers at this point in time; such an analysis is planned for the future, however.
Acknowledgments
We thank L. DelRosso, L. Calonder, G. Karere, and R. Grahn for contributions to this project. We also thank L. Lyons for the use of her laboratory for DNA isolation and genotyping. We would also like to thank two anonymous reviewers for their insightful comments that improved the quality of the paper. This research was supported by the American Society of Primatologists, NIH #RR019970 (JPC) and RR000169 to the California National Primate Research Center. All procedures were approved by the Institutional Animal Care and Use Committee at UC Davis and conducted in accordance with the laws of the United States of America.
References
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, Goldman D, Suomi SJ, Higley JD. The utility of the non-human primate model for studying gene by environment interactions in behavioral research. Genes, Brain and Behavior. 2003;2(6):336–340. doi: 10.1046/j.1601-1848.2003.00051.x. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14(8):746–754. doi: 10.1038/mp.2009.44. doi: mp200944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard J. Male rank, reproductive behavior, and reproductive success in free-ranging rhesus macaques. Primates. 1993;34(4):481–489. [Google Scholar]
- Berard J. A four-year study of the association between male dominance rank, residency status, and reproductive activity in rhesus macaques (Macaca mulatta) Primates. 1999;40(1):159–175. doi: 10.1007/BF02557708. [DOI] [PubMed] [Google Scholar]
- Berman CM. Early agonistic experience and rank acquisition among free-ranging infant rhesus monkeys. International Journal of Primatology. 1980a;1(2):153–170. [Google Scholar]
- Berman CM. Mother-infant relationships among free-ranging rhesus monkeys on Cayo Santiago: A comparison with captive pairs. Anim. Behav. 1980b;28:860–873. [Google Scholar]
- Blalock HM. Social Statistics. McGraw-Hill; New York: 1972. [Google Scholar]
- Brown G, Dixson A. The development of behavioural sex differences in infant rhesus macaques (Macaca mulatta) Primates. 2000;41(1):63–77. doi: 10.1007/BF02557462. [DOI] [PubMed] [Google Scholar]
- Capitanio JP, Mason WA, Mendoza SP, Del Rosso LA, Roberts JA. Nursery rearing and biobehavioral organization. In: Sackett GP, Ruppenthal G, Elias K, editors. Nursery rearing of nonhuman primates in the 21st century. Springer; New York: 2006. pp. 191–213. [Google Scholar]
- Capitanio JP, Mendoza SP, Mason WA, Maninger N. Rearing environment and hypothalamic-pituitary-adrenal regulation in young rhesus monkeys (Macaca mulatta) Dev Psychobiol. 2005;46(4):318–330. doi: 10.1002/dev.20067. [DOI] [PubMed] [Google Scholar]
- Carrel L, Willard HF. X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature. 2005;434(7031):400–404. doi: 10.1038/nature03479. [DOI] [PubMed] [Google Scholar]
- Caspi A, Harrington H, Milne B, Amell JW, Theodore RF, Moffitt TE. Children’s behavioral styles at age 3 are linked to their adult personality traits at age 26. J Pers. 2003;71(4):495–513. doi: 10.1111/1467-6494.7104001. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301(5631):386–389. doi: 10.1126/science.1083968. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Champagne F, Meaney MJ. Like mother, like daughter: evidence for non-genomic transmission of parental behavior and stress responsivity. Prog Brain Res. 2001;133:287–302. doi: 10.1016/s0079-6123(01)33022-4. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Mol Psychiatry. 2002;7(10):1058–1063. doi: 10.1038/sj.mp.4001157. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Clark LA, Kochanska G, Ready R. Mothers’ personality and its interaction with child temperament as predictors of parenting behavior. J Pers Soc Psychol. 2000;79(2):274–285. doi: 10.1037//0022-3514.79.2.274. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Boinski S. Temperament in nonhuman primates. Am J Primatol. 1995;37:103–125. doi: 10.1002/ajp.1350370205. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. A unified biosocial theory of personality and its role in the development of anxiety states. Psychiatr Dev. 1986;4(3):167–226. [PubMed] [Google Scholar]
- Cohen P, Kasen S, Brook JS, Hartmark C. Behavior patterns of young children and their offspring: a two-generation study. Dev Psychol. 1998;34(6):1202–1208. [PubMed] [Google Scholar]
- Deal JE, Halverson CF, Jr., Havill V, Martin RP. Temperament factors as longitudinal predictors of young adult personality. 2005;51(3):315–334. [Google Scholar]
- Dittus WPJ. The Social Regulation of Population Density and Age-Sex Distribution in the Toque Monkey. Behaviour. 1977;63(3/4):281–322. [Google Scholar]
- Ducci F, Enoch MA, Hodgkinson C, Xu K, Catena M, Robin RW, Goldman D. Interaction between a functional MAOA locus and childhood sexual abuse predicts alcoholism and antisocial personality disorder in adult women. Mol Psychiatry. 2008:334–347. doi: 10.1038/sj.mp.4002034. [DOI] [PubMed] [Google Scholar]
- Ducci F, Newman TK, Funt S, Brown GL, Virkkunen M, Goldman D. A functional polymorphism in the MAOA gene promoter (MAOA-LPR) predicts central dopamine function and body mass index. Mol Psychiatry. 2006;11(9):858–866. doi: 10.1038/sj.mp.4001856. [DOI] [PubMed] [Google Scholar]
- Elgar FJ, Mills RS, McGrath PJ, Waschbusch DA, Brownridge DA. Maternal and paternal depressive symptoms and child maladjustment: the mediating role of parental behavior. J Abnorm Child Psychol. 2007;35(6):943–955. doi: 10.1007/s10802-007-9145-0. [DOI] [PubMed] [Google Scholar]
- Ellis BJ, Boyce WT, Belsky J, Bakermans-Kranenburg MJ, van Ijzendoorn MH. Differential susceptibility to the environment: An evolutionary-neurodevelopmental theory. Development and Psychopathology. 2011;23(01):7–28. doi: 10.1017/S0954579410000611. doi: doi:10.1017/S0954579410000611. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, McGuire MT. Long-term effects of early mothering behavior on responsiveness to the environment in vervet monkeys. Developmental Psychobiology. 1988;21(7):711–724. doi: 10.1002/dev.420210708. doi: 10.1002/dev.420210708. [DOI] [PubMed] [Google Scholar]
- Fairbanks LA, Newman TK, Bailey JN, Jorgensen MJ, Breidenthal SE, Ophoff RA, Comuzzie AG, Martin LJ, Rogers J. Genetic contributions to social impulsivity and aggressiveness in vervet monkeys. Biol Psychiatry. 2004;55(6):642–647. doi: 10.1016/j.biopsych.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Kraemer GW, Gonzalez A, Lovic V, Rees S, Melo A. Mothering begets mothering: the transmission of behavior and its neurobiology across generations. Pharmacol Biochem Behav. 2002;73(1):61–75. doi: 10.1016/s0091-3057(02)00793-1. doi: S0091305702007931. [DOI] [PubMed] [Google Scholar]
- Francis DD, Meaney MJ. Maternal care and the development of stress responses. Curr Opin Neurobiol. 1999;9(1):128–134. doi: 10.1016/s0959-4388(99)80016-6. [DOI] [PubMed] [Google Scholar]
- Gershenfeld HK, Paul SM. Towards a genetics of anxious temperament: from mice to men. Acta Psychiatrica Scandinavica. 1998;98:56–65. doi: 10.1111/j.1600-0447.1998.tb05968.x. doi: 10.1111/j.1600-0447.1998.tb05968.x. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Widaman KF, Capitanio JP. Iron deficiency anemia and affective response in rhesus monkey infants. Dev Psychobiol. 2009;51(1):47–59. doi: 10.1002/dev.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Dodsworth RO, Harlow MK. Total social isolation in monkeys. Proc Natl Acad Sci U S A. 1965;54(1):90–97. doi: 10.1073/pnas.54.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heils A, Teufel A, Petri S, Stöber G, Riederer P, Bengel D, Lesch KP. Allelic Variation of Human Serotonin Transporter Gene Expression. Journal of Neurochemistry. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Taub DM, Higley SB, Suomi SJ, Vickers JH, Linnoila M. Cerebrospinal fluid monoamine and adrenal correlates of aggression in free-ranging rhesus monkeys. Arch Gen Psychiatry. 1992;49(6):436–441. doi: 10.1001/archpsyc.1992.01820060016002. [DOI] [PubMed] [Google Scholar]
- Hinde K, Capitanio JP. Lactational programming? mother’s milk energy predicts infant behavior and temperament in rhesus macaques (Macaca mulatta) American Journal of Primatology. 2010;72(6):522–529. doi: 10.1002/ajp.20806. doi: 10.1002/ajp.20806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga D, Haberstick BC, Smolen A, Menard S, Young SE, Corley RP, Stallings MC, Grotpeter J, Hewitt JK. Childhood maltreatment, subsequent antisocial behavior, and the role of monoamine oxidase A genotype. Biol Psychiatry. 2006;60(7):677–683. doi: 10.1016/j.biopsych.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Jonsson EG, Norton N, Gustavsson JP, Oreland L, Owen MJ, Sedvall GC. A promoter polymorphism in the monoamine oxidase A gene and its relationships to monoamine metabolite concentrations in CSF of healthy volunteers. J Psychiatr Res. 2000;34(3):239–244. doi: 10.1016/s0022-3956(00)00013-3. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Early childhood predictors of adult anxiety disorders. Biol Psychiatry. 1999;46(11):1536–1541. doi: 10.1016/s0006-3223(99)00137-7. [DOI] [PubMed] [Google Scholar]
- Karere GM, Kinnally EK, Sanchez JN, Famula TR, Lyons LA, Capitanio JP. What is an “adverse” environment? Interactions of rearing experiences and MAOA genotype in rhesus monkeys. Biol Psychiatry. 2009;65(9):770–777. doi: 10.1016/j.biopsych.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, Moffitt TE. MAOA, maltreatment, and gene-environment interaction predicting children’s mental health: new evidence and a meta-analysis. Mol Psychiatry. 2006;11(10):903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Kinnally EL, Karere GM, Lyons LA, Mendoza SP, Mason WA, Capitanio JP. Serotonin pathway gene-gene and gene-environment interactions influence behavioral stress response in infant rhesus macaques. Dev Psychopathol. 2010;22(1):35–44. doi: 10.1017/S0954579409990241. doi: S0954579409990241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesch KP, Meyer J, Glatz K, Flügge G, Hinney A, Hebebrand J, Klauck SM, Poustka A, Poustka F, Bengel D, Mössner R, Riederer P, Heils A. The 5-HT transporter gene-linked polymorphic region (5-HTTLPR) in evolutionary perspective: Alternative biallelic variation in rhesus monkeys. Journal of Neural Transmission. 1997;104(11):1259–1266. doi: 10.1007/BF01294726. doi: 10.1007/bf01294726. [DOI] [PubMed] [Google Scholar]
- Lyons LA, Biller DS, Erdman CA, Lipinski MJ, Young AE, Roe BA, Qin B, Grahn RA. Feline polycystic kidney disease mutation identified in PKD1. J Am Soc Nephrol. 2004;15(10):2548–2555. doi: 10.1097/01.ASN.0000141776.38527.BB. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Res. 2000;95(1):9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Mason WA. The effects of social restriction on the behavior of rhesus monkeys. J Comp Phys Psychology. 1960;53:582–589. doi: 10.1037/h0045216. [DOI] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M. Correlation of CSF 5-HIAA concentration with sociality and the timing of emigration in free-ranging primates. Am J Psychiatry. 1995;152(6):907–913. doi: 10.1176/ajp.152.6.907. [DOI] [PubMed] [Google Scholar]
- Mendoza SP, Mason WA. Primate relationships: social dispositions and physiological responses. In: Seth PK, Seth S, editors. Perspectives in primate biology. Vol. 2. Today and tomorrow’s printers and publishers; New Delhi: 1989. pp. 129–143. [Google Scholar]
- Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16(3):1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GD, Brandt EM. Behavioral differences related to experience of mother and sex of infant in the rhesus monkey. Devl Psychol. 1973;3:149. [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, Suomi SJ, Higley JD, Lesch KP. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biol Psychiatry. 2005;57(2):167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Obradovic J, Boyce WT. Individual differences in behavioral, physiological, and genetic sensitivities to contexts: implications for development and adaptation. Dev Neurosci. 2009;31(4):300–308. doi: 10.1159/000216541. doi: 000216541. [DOI] [PubMed] [Google Scholar]
- Olson SL, Bates JE, Sandy JM, Schilling EM. Early developmental precursors of impulsive and inattentive behavior: from infancy to middle childhood. J Child Psychol Psychiatry. 2002;43(4):435–447. doi: 10.1111/1469-7610.00035. [DOI] [PubMed] [Google Scholar]
- Pusey AE, Packer C. Dispersal and Philoparty. In: Smuts B, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. The University of Chicago Press; Chicago: 1986. pp. 250–266. [Google Scholar]
- Reif A, Rosler M, Freitag CM, Schneider M, Eujen A, Kissling C, Wenzler D, Jacob CP, Retz-Junginger P, Thome J, Lesch KP, Retz W. Nature and nurture predispose to violent behavior: serotonergic genes and adverse childhood environment. Neuropsychopharmacology. 2007;32(11):2375–2383. doi: 10.1038/sj.npp.1301359. [DOI] [PubMed] [Google Scholar]
- Rosenblum LA, Paully GS. The Effects of Varying Environmental Demands on Maternal and Infant Behavior. Vol. 55. Blackwell Publishing on behalf of the Society for Research in Child Development; 1984. pp. 305–314. [PubMed] [Google Scholar]
- Sabol SZ, Hu S, Hamer D. A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet. 1998;103(3):273–279. doi: 10.1007/s004390050816. [DOI] [PubMed] [Google Scholar]
- Saudino KJ. Behavioral genetics and child temperament. J Dev Behav Pediatr. 2005;26(3):214–223. doi: 10.1097/00004703-200506000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schino G, D’Amato FR, Troisi A. Mother-infant relationships in Japanese macaques: sources of inter-individual variation. Animal Behaviour. 1995;49(1):151–158. [Google Scholar]
- Seay BM. Maternal behavior in primiparous and multiparous rhesus monkeys. Folia Primatologica. 1964;4:146–168. doi: 10.1159/000155049. [DOI] [PubMed] [Google Scholar]
- Sherry A, Henson RK. Conducting and interpreting canonical correlation analysis in personality research: a user-friendly primer. J Pers Assess. 2005;84(1):37–48. doi: 10.1207/s15327752jpa8401_09. doi: 10.1207/s15327752jpa8401_09. [DOI] [PubMed] [Google Scholar]
- Shih JC, Grimsby J, Chen K, Zhu QS. Structure and promoter organization of the human monoamine oxidase A and B genes. J Psychiatry Neurosci. 1993;18(1):25–32. [PMC free article] [PubMed] [Google Scholar]
- Simpson MJA. Effects of Early Experience on the Behaviour of Yearling Rhesus Monkeys (Macaca mulatta) in the Presence of a Strange Object: Classification and Correlation Approaches. Primates. 1985;26(1):57–72. [Google Scholar]
- Sjoberg RL, Nilsson KW, Wargelius HL, Leppert J, Lindstrom L, Oreland L. Adolescent girls and criminal activity: role of MAOA-LPR genotype and psychosocial factors. Am J Med Genet B Neuropsychiatr Genet. 2007;144(2):159–164. doi: 10.1002/ajmg.b.30360. [DOI] [PubMed] [Google Scholar]
- Stevenson-Hinde J, Simpson MJA. Mothers’ Characteristics, Interactions, and Infants’ Characteristics. Vol. 52. Blackwell Publishing on behalf of the Society for Research in Child Development; 1981. pp. 1246–1254. [PubMed] [Google Scholar]
- Sullivan EC, Hinde K, Mendoza SP, Capitanio JP. Cortisol concentrations in the milk of rhesus monkey mothers are associated with confident temperament in sons, but not daughters. Developmental Psychobiology. 2011:96–104. doi: 10.1002/dev.20483. doi: 10.1002/dev.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suomi . Genetic and maternal contributions to individual differences in rhesus monkey biobehavioral development. In: Krasnegor NA, Blass EM, Hofer MA, Smotherman WP, editors. Perinatal development: A psychobiological perspective. Academic Press; Orlando: 1987. pp. 397–419. [Google Scholar]
- Suomi SJ. Genetic and maternal contributions to individual differences in rhesus monkey biobehavioral development. In: Krasnagor NA, Blass EM, Hofer MA, Smotherman WP, editors. Perinatal development: A psychobiological perspective. Academic Press; New York: 1987. pp. 397–420. [Google Scholar]
- Suomi SJ. Uptight and laid-back monkeys: Individual differences in the response to social challenges. In: Brauth SE, Hall WS, Dooling RJ, editors. Plasticity of Development. MIT Press; Cambridge, MA: 1991. pp. 27–56. [Google Scholar]
- Tabachnick BG, Fidell LS. Using Multivariate Statistics. 4 ed. 2000. [Google Scholar]
- Thompson B. Finding a Correction for the Sampling Error in Multivariate Measures of Relationship: A Monte Carlo Study. Educational and Psychological Measurement. 1990;50(1):15–31. doi: 10.1177/0013164490501003. [Google Scholar]
- Thompson B. Invariance of Multivariate Results: A Monte Carlo Study of Canonical Function and Structure Coefficients. The Journal of Experimental Education. 1991;59(4):367–382. [Google Scholar]
- Trefilov A, Berard J, Krawczak M, Schmidtke J. Natal dispersal in rhesus macaques is related to serotonin transporter gene promoter variation. Behav Genet. 2000;30(4):295–301. doi: 10.1023/a:1026597300525. [DOI] [PubMed] [Google Scholar]
- Walters JR, Seyfarth RM. Conflict and Cooperation. In: Smuts B, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate Societies. The University of Chicago Press; Chicago: 1986. pp. 306–317. [Google Scholar]
- Wendland JR, Lesch KP, Newman TK, Timme A, Gachot-Neveu H, Thierry B, Suomi SJ. Differential functional variability of serotonin transporter and monoamine oxidase a genes in macaque species displaying contrasting levels of aggression-related behavior. Behav Genet. 2006;36(2):163–172. doi: 10.1007/s10519-005-9017-8. [DOI] [PubMed] [Google Scholar]
- Widom CS, Brzustowicz LM. MAOA and the “cycle of violence:” childhood abuse and neglect, MAOA genotype, and risk for violent and antisocial behavior. Biol Psychiatry. 2006;60(7):684–689. doi: 10.1016/j.biopsych.2006.03.039. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. Serotonin-related gene polymorphisms and central nervous system serotonin function. Neuropsychopharmacology. 2003;28(3):533–541. doi: 10.1038/sj.npp.1300054. doi: 10.1038/sj.npp.1300054. [DOI] [PubMed] [Google Scholar]
- Zuckerman M. Sensation seeking, mania, and monoamines. Neuropsychobiology. 1985;13(3):121–128. doi: 10.1159/000118174. [DOI] [PubMed] [Google Scholar]


