Abstract
Objectives
To test the hypothesis that a nocturnal decrease of secretion of inflammation markers and catecholamines would be associated with mood and stress variables even after controlling for objective sleep variables.
Methods
A total of 130 healthy volunteers participated in this study, spending 2 nights in the Gillin Laboratory of Sleep and Chronobiology at the University of California, San Diego, General Clinical Research Center. Blood samples were obtained before sleep (10:30 PM) and after awakening (6:30 AM) on the first day, and these samples were assayed for inflammatory biomarkers and catecholamines. On the second night, polysomnographic records were scored for objective sleep variables, e.g., total sleep time and wake after sleep onset. Self-rating scales for mood, stress, depression, and daily hassles were administered the second day.
Results
The nocturnal decrease in interleukin-6 was smaller in people who reported more negative mood or fatigue and greater in those who reported more uplift events (e.g., with Profile of Mood States fatigue rp = −.25 to −.30). People with high stress or high depression levels had smaller nocturnal decreases of epinephrine. That relationship was even stronger when partial correlations were used to control for morning level and sleep variables. The associations between nocturnal changes of C-reactive protein, soluble tumor necrosis factor-receptor I, and norepinephrine with psychological states were nonremarkable.
Conclusions
The analyses of nocturnal change scores (difference scores) add substantial information compared with the traditional analyses of morning levels of immune variables and catecholamines alone. Subjective well-being is significantly associated with a greater nocturnal decrease of interleukin-6 and epinephrine. More research on nocturnal adaptation processes is warranted.
Keywords: sleep, stress, mood, cytokines, sympathetic nervous system, chronobiology
INTRODUCTION
Day to night variation in physiological processes is extensive. The amplitude of these circadian rhythms is considered to indicate health, and long-term disruption of these rhythms correlates with disease states (1). The origins of such variation may stem from so-called internal body clocks that are even demonstrated on a single cell level (1), or from changes in activity, such as bed rest. Because, in general, nocturnal rest reduces physiological arousal after daytime stress experiences, one might expect that hormones sensitive to stress would be lower first thing in the morning hours compared with the previous evening hours. One way of examining this topic is to contrast the change in hormone level from evening to morning. A large delta (Δ) (evening value minus morning value) would indicate high nocturnal decrease of its concentration. Although the meaning of humoral nocturnal declines is poorly understood, more research has been published dealing with nocturnal declines in blood pressure, where the degree of nocturnal dipping seems to be attenuated in people with more depressive symptoms or who experience more stressful daytime events (2,3). Attenuated nocturnal blood pressure dipping and heart rate dipping are associated with increased cardiovascular morbidity and mortality (4–6).
Similarly, the diurnal changes in cortisol, for example, are well known, and numerous associations have been reported between elevated early morning cortisol levels and depressive symptoms, stressful life events, and social adversity (7–11). Curiously, psychosocial determinants of nocturnal recovery of immune variables and indices of sympathetic nervous system activity have not typically been investigated. Therefore, we wanted to analyze whether nocturnal changes of immune and sympathetic nervous system parameters were associated with psychosocial variables reflecting well-being or adversity.
Overactivation of the immune system and increased secretion of immune parameters (e.g., interleukin [IL]-6, IL-1, tumor necrosis factor [TNF]-α) is associated with negative mood (12–14). Several proinflammatory cytokines show increased concentrations in depression (15,16). Increased morning cortisol and serum IL-6 levels predict the development of posttraumatic stress disorder in children and adolescents (17). If morning IL-6 concentrations are lower in healthy individuals than in patients with posttraumatic stress disorder, this could partly reflect different overnight decreases of IL-6. Such observations could imply that difference scores of bedtime versus next morning concentrations of immune parameters should correlate with aspects of adversity or well-being.
Sleep itself can be considered as a potential link between psychological well-being and nocturnal immune changes. For instance, increased morning IL-6 levels have been associated with sleeping problems, such as greater wake after sleep onset (WASO) (18). Burgos et al. (19) investigated 11 patients with primary insomnia and found higher nocturnal IL-6 excretion in the insomniacs as compared with participants without sleeping problems. Perry et al. (20) confirmed diurnal variations of cytokines and postulated a similar overnight variation of IL-6 compared with cortisol with a rise in the early morning hours. Moreover, an association of nocturnal wake time with 24-hour IL-6 concentrations was also confirmed (21). The same group reported nocturnal variations not only for IL-6 but also for TNF-α (22). In addition, daytime napping after a night of sleep loss results in decreases in cortisol and IL-6 (23). If sleeping patterns of patients with obstructive sleep apnea syndrome are normalized using continuous positive airway pressure, serum IL-6 levels are significantly reduced (24). All of these results suggest that restful sleep might be associated with a reduction of proinflammatory cytokines. Because sleep disturbance and negative mood can affect one another, it remains unclear whether negative mood is directly related with nocturnal immune changes, or whether such an association is explained by disturbed sleep parameters. With polysomnographic (PSG) monitoring, it is possible to determine if the Δ hormone value is more closely associated with the stressful daytime experiences or with disruptions of sleep itself.
Whereas several studies have investigated either evening or morning levels of immune variables, the overnight change in levels has seldom been investigated. We postulate that it is not only the level per se but the nocturnal decrease from evening to morning scores that is associated with sleep quality and psychological states. We analyzed covariates of nocturnal changes of immune variables and catecholamines, and we hypothesized that nocturnal reductions of the concentration of these biological parameters would be associated with psychological states. To analyze whether nocturnal difference scores add information to the analysis of morning levels, these associations were controlled for morning levels of the corresponding biological variables. Moreover, potential associations of nocturnal decrease of immune parameters with psychological states were controlled for objective sleep variables to analyze whether they could be explained by sleep problems that frequently coexist with depressed mood.
METHODS
As part of a larger study on health of African Americans and white Americans, this study examined 130 healthy controls, who were recruited between 2006 and 2009 using advertisements and announcements. Mean age was 35 years, and 43% were female. The sample was roughly evenly divided between white and African Americans (Table 1). Paid volunteers stayed in the Gillin Laboratory for Sleep and Chronobiology at the University of California, San Diego General Clinical Research Center for 2 nights for sleep monitoring, psychological testing, and blood sampling. Exclusion criteria were: current heart disease other than hypertension, serious pulmonary disease, history of psychosis, current alcohol or drug abuse, moderate or heavy smoking (>10 cigarettes/day), increased caffeine intake (>600 mg/day), hormone therapies including contraceptives, pregnancy, and medication use other than antihypertensives. If participants were taking antihypertensive medication, they were tapered off with the permission of their prescribing physician and underwent a 3-week washout period before study participation. The study was approved by the University of California, San Diego Institutional Review Board.
TABLE 1.
Sociodemographic and Clinical Sample Characteristics
| Valid n | n (%) or Mean | SD | |
|---|---|---|---|
| % female | 130 | 56 (43%) | |
| Hispanics | 130 | 8 (6%) | |
| African Americans | 130 | 60 (46%) | |
| White Americans | 130 | 62 (48%) | |
| Employed | 130 | 120 (92%) | |
| Age | 130 | 34.9 | 9.6 |
| BMI (self-report) | 129 | 25.6 | 3.87 |
| CHUS Hassle Frequency | 120 | 25.0 | 11.12 |
| CHUS Hassle Severity | 119 | 1.50 | .37 |
| CHUS Uplift Frequency | 118 | 33.2 | 10.30 |
| CHUS Uplift Severity | 117 | 1.86 | .39 |
| PSS10 Total Score | 122 | 13.1 | 6.5 |
| MFSIsf Total | 118 | 3.35 | 16.4 |
| POMS Tension | 121 | 4.59 | 4.5 |
| POMS Depression | 121 | 3.94 | 5.5 |
| POMS Anger | 120 | 3.73 | 4.6 |
| POMS Vigor | 121 | 12.35 | 5.3 |
| POMS Fatigue | 121 | 4.56 | 4.5 |
| POMS Confusion | 121 | 3.39 | 3.8 |
| POMS Total | 120 | 7.84 | 22.0 |
| CES-D Depression Score | 117 | 11.24 | 8.98 |
| PSQI Sleep Duration | 120 | .72 | .93 |
| PSQI Global Score | 120 | 5.30 | 3.15 |
| Total sleep time (min) | 124 | 394.1 | 54.43 |
| Wake after sleep onset (min) | 124 | 24.27 | 22.61 |
| AHI | 124 | 10.00 | 20.44 |
| REM Sleep (min) | 124 | 87.3 | 28.1 |
| Stage 1 sleep (min) | 124 | 24.2 | 17.1 |
| Stage 2 sleep (min) | 124 | 201.9 | 45.5 |
| Stage 3 sleep (min) | 124 | 30.3 | 14.8 |
| Stage 4 sleep (min) | 124 | 50.4 | 34.8 |
| CRP 10:30 PM Bedtime (mg/L) | 122 | 1.03 | 1.32 |
| CRP 6:30 AM Awake (mg/L) | 125 | 1.07 | 1.41 |
| ΔCRP (mg/L) | 122 | .048 | .27 |
| IL-6 10:30 PM Bedtime (pg/mL) | 118 | 6.40 | 9.45 |
| IL-6 6:30 AM Awake (pg/mL) | 112 | 5.07 | 7.31 |
| Δ IL-6 (pg/mL) | 112 | 1.31 | 11.65 |
| sTNF-RI 10:30 PM Bedtime (pg/mL) | 105 | 939.8 | 284.3 |
| sTNF-RI 6:30 AM Awake (pg/mL) | 107 | 1026.0 | 346.5 |
| Δ sTNF-RI (pg/mL) | 105 | −89.36 | 228.03 |
| Epinephrine 10:30 PM Bedtime (pg/mL) | 91 | 38.09 | 41.8 |
| Epinephrine 6:30 AM Awake (pg/mL) | 85 | 25.43 | 13.1 |
| Δ Epinephrine (pg/mL) | 84 | 13.47 | 43.2 |
| Norepinephrine 10:30 PM Bedtime (pg/mL) | 95 | 444.5 | 216.8 |
| Norepinephrine 6:30 AM Awake (pg/mL) | 89 | 369.5 | 144.2 |
| Δ Norepinephrine (pg/mL) | 89 | 76.39 | 176.7 |
SD = standard deviation; BMI = body mass index; CHUS = Combined Hassles and Uplift Scale; PSS10 = Perceived Stress Scale; MFSIsf = Multidimensional Fatigue Symptom Inventory-Short Form; POMS = Profile of Mood States; CES-D = Center for Epidemiological Studies Depression Scale; PSQI = Pittsburgh Sleep Quality Index; AHI = Apnea-Hypopnea Index; REM = rapid eye movement; CRP = C-reactive protein; IL = interleukin; sTNF-RI = soluble TNF-receptor I.
Procedure
After prescreening, participants were invited to come to the Gillin Laboratory for Sleep and Chronobiology at the University of California, San Diego General Clinical Research Center at 8:30 PM. They were hooked-up for an acclimation night of sleep monitoring, and an evening blood sample was taken at 10:30 PM before lights were turned off for sleeping. The morning blood sample was taken after wakening the subject at 6:30 AM the next day independently of sleep stage. Both blood samples were taken from subjects while they were recumbent in bed. During the next night, sleep was monitored with PSG. Time with lights off was identical between the first and second nights. The sleep rooms were 180 ft (2) sound-shielded rooms with a wall port for equipment cables. Sleep data acquisition was performed with PSG system (A10, Embla Systems Inc., Broomfield, Colorado) and included electroencephalography (C4/C3), electrooculography, chin electromyography, leg electromyography, airflow with an oronasal thermal sensor, airflow with an air pressure transducer, snore events with a piezo snore sensor, respiratory effort with piezo thoracic and abdominal belts, oxygen saturation with a pulse oximeter sensor, and body position with a position indicator. Recordings were scored by a trained sleep technician according to criteria from Rechtschaffen and Kales (25). From the PSG, we computed total sleep time (TST), WASO, and the Apnea-Hypopnea Index (AHI). TST was calculated as the total minutes from sleep onset to wake-up and includes time spent in Stages 1 through 4 of sleep and rapid eye movement. WASO was considered to be the number of minutes spent awake after initiating sleep. Apneas were defined as decrements in airflow of ≥90% from baseline for ≥10 seconds. Hypopneas were defined as decrements in airflow of ≥50% but <90% from baseline for ≥10 seconds. Significant oxyhemoglobin desaturations were defined as transient drops in oxyhemoglobin saturation by ≥3% from baseline. The total number of apneas and hypopneas per hour of sleep were calculated to yield the AHI. Further sleep variables are presented in Table 1.
There is a trade-off between response burden on subjects and optimal data integrity for testing hypotheses. We used a two-night protocol to be able to recruit a broad cross-section of subjects who were working. A longer protocol would have increased response burden. We separated the sleep recording night from the blood sampling night because of the effect of blood sampling on sleep parameters. The second night followed immediately to the first night. Activities between the two nights were ad libidum to allow the continuation of everyday activities.
Participants were provided with questionnaires on admission and required to complete them before discharge. The following questionnaires were used: The Profile of Mood States (POMS) (26) to assess tension, depressed mood, anger, vigor, fatigue, confusion, as well as a total mood disturbance score; the Center for Epidemiological Studies Depression Scale (27); the Combined Hassles and Uplift Scale (28) to assess hassle frequency and severity, as well as uplifts frequency and severity; the Perceived Stress Scale (PSS10) revealing a general index for perceived stress (29); the total fatigue score derived from the short form of the Multidimensional Fatigue Symptom Inventory (MFSI) (30); and the global score of the Pittsburgh Sleep Quality Index (PSQI) (31).
Blood samples were obtained through an intravenous catheter to remove effects of pain caused by the needle stick. The intravenous catheter was inserted after admission around 4:30 PM. The bedtime draw was done around 10:30 PM and the waking draw was done around 7:30 AM the next morning. Participants were lying in their bed to avoid effects of movements or physical activities on immune biomarker levels and catecholamines. Plasma was stored at −80°C until assay. C-reactive protein (CRP) was assayed using the high sensitivity Denka-Seiken method (32). Intra-assay and interassay coefficients of variation were <3%; assay sensitivity was <0.05 mg/L. IL-6 and soluble TNF-receptor I (sTNF-RI) were determined by commercial ELISA (Quantikine, R&D Systems, Minneapolis, Minnesota). Intra-assay and interassay coefficients of variation were <5%; assay sensitivity was <0.72 pg/mL and <0.61 pg/mL for IL-6 and sTNF-RI, respectively. Catecholamines were determined using a COMT-based radioenzymatic assay (33). Intra-assay and interassay coefficients of variation were <7% and <11%, respectively.
All assays were run using samples that had not been previously freeze-thawed. To minimize intra-assay variance, both samples from each participant were analyzed within the same assay run.
Statistics
As a first step, the mean and standard deviation values of all variables were computed. All biological parameters of >3 standard deviation from the mean were considered to be outliers and excluded from the corresponding analysis. This was the case for ≤5%. All participants with CRP scores of >10 mg/L were also excluded because of potentially ongoing infectious diseases. For all biological parameters, difference scores of the evening minus morning concentrations were computed. Positive values indicate a nocturnal decrease of the variable. Because parametrical analyses in sample sizes >100 are quite robust even if normal distribution is not fully achieved, we did not log-transform these difference scores. Afterward, these biological change scores were correlated with the psychological variables.
If significant associations were found between biological change scores and psychological variables, we examined whether these variables shared variance that was not already included in associations of psychological variables with the biological baseline measure (morning concentrations). Therefore, these correlations were reanalyzed computing partial correlations correcting for morning levels of the biological variables. If these partial correlations were still significant, it implied that the nocturnal change scores included additional information compared with the morning levels of these variables.
Significant associations between psychological variables and biological change scores were corrected for objective sleep variables, again computing partial correlations and correcting for the influence of TST, WASO, and AHI. If the correlations still remained significant, it implied a direct association of psychological states with nocturnal biological change scores that was not mediated by objective sleep quality. Finally, if more than two psychological variables correlated with one biological change score, linear regression analysis was computed to control for interdependency of psychological variables. To visualize findings that are stable even after extracting the effect of sleep and morning variables, we occasionally dichotomized psychological variables (e.g., fatigue) and reported full data for biological nighttime and morning scores.
Adjusting for Multiple Testing
Interpreting significance levels from a large correlation matrix requires adjusting for multiple testing to correct for significant coefficients that occur just by chance. Because of the known conservative bias of the Bonferroni procedure (34), we used a modification of the Holm’s method (35) to correct for multiple testing in correlation matrices. Significant results are organized in increasing order according to their significance levels (or correlation coefficients). Then, it is defined how many significant correlations can be expected by chance due to multiple testing with α 5%, and the list of significant correlations is reduced according to this number, starting with the lowest coefficients that were significant on an individual level. This is still a conservative approach, as it assumes that significance levels of all coefficients are equal to 5%, whereas most are <5%. We report results with and without adjustment for multiple testing. Correlation coefficients with control variables (e.g., sleep parameters) were not adjusted for multiple testing, as it is more conservative not to exclude control variables prematurely.
RESULTS
Complete Data Sets
Due to the multiday assessments and occasional technical problems, missing values occurred. However, we obtained psychological tests on >90% of participants, sleep variables for 124 of 130 participants, IL-6 for 112 participants, CRP for 122, and sTNF-R1 for 105 of the 130 participants. Catecholamine data were received for 84 (Δ epinephrine) and 89 (Δ norepinephrine) participants (Table 1).
Role of Sleep Variables
The objective PSG sleep variables TST, WASO, and AHI were not significantly associated with any of the biological change scores. All correlations of nocturnal changes of catecholamine with objective sleep variables were below r < .09 and nonsignificant. None of the overnight immune changes correlated significantly with objective sleep variables, with correlation coefficients ranging from .04 to .16. Analyzing associations with evening and morning concentrations separately, the only significant correlations were found for CRP, which was related to the AHI index (CRP 22:30 PM: r = .24; p < .01; CRP 6:30 AM: r = .18; p < .05).
For exploratory reasons, we also assessed subjective sleep duration (according to PSQI subscale) to extend potential findings on objective sleep variables. This variable showed a significant positive association with a nocturnal decrease of CRP (r = .22, p < .05) and a negative association with a nocturnal decrease of norepinephrine (r = −.26; p < .05).
The POMS fatigue score and the MFSI fatigue score were highly correlated (r = .75; p < .001). They were not significantly associated with objective sleep variables (all r = −.10).
Psychological Correlates of Nocturnal IL-6 Decrease
Table 2. shows that the nocturnal decrease of IL-6 was related to a variety of psychological dimensions. Seven of the 15 correlations in Table 2 were significant. Modified Holm adjustment for multiple testing denies two of them. The nocturnal decrease of IL-6 was related to positive daily events (uplift frequency, uplift severity), and negatively associated with feelings of tension and fatigue. Both fatigue scales (MFSI total fatigue score and POMS fatigue score) indicated less nocturnal decrease of IL-6 if participants reported more fatigue.
TABLE 2.
Associations of Biological Overnight Changes With Psychological Variables
| Subjective Variables | Δ IL-6 | Δ CRP | Δ sTNF-R1 | Δ Epinephrine | Δ Norepinephrine |
|---|---|---|---|---|---|
| CHUS Hassle Frequency | 0.140 | −0.079 | 0.032 | −0.167 | 0.073 |
| CHUS Hassle Severity | −0.055 | −0.025 | 0.066 | −0.109 | −0.003 |
| CHUS Uplift Frequency | 0.223* | −0.082 | 0.116 | −0.026 | −0.045 |
| CHUS Uplift Severity | (0.212*) | −0.130 | −0.058 | −0.026 | −0.153 |
| PSS10 Stress Score | −0.077 | (0.198*) | −0.035 | −0.241* | 0.065 |
| MFSIsf Fatigue Score | −0.268** | −0.062 | −0.159 | −0.226 | 0.022 |
| POMS Tension | −0.220* | −0.003 | −0.112 | −0.203 | −0.142 |
| POMS Depression | −0.122 | 0.025 | 0.009 | −0.103 | 0.028 |
| POMS Anger | −0.226* | 0.043 | −0.190 | −0.013 | −0.058 |
| POMS Vigor | 0.033 | −0.067 | 0.080 | 0.206 | −0.177 |
| POMS Fatigue | −0.255** | −0.029 | (−0.218*) | −0.115 | −0.103 |
| POMS Confusion | −0.142 | −0.001 | −0.051 | −0.189 | −0.054 |
| POMS Total | (−0.208*) | 0.025 | −0.132 | −0.171 | −0.023 |
| CES-D Depression Score | −0.002 | −0.025 | −0.004 | −0.285* | −0.082 |
| PSQI Global Score | −0.059 | 0.120 | −0.017 | −0.145 | −0.118 |
Numbers in parentheses are correlation coefficients that should not be interpreted as significant after applying the modified Holm’s method for multiple testing.
Text in bold represents significant bivariate correlation coefficients.
p < .05;
p < .01.
IL = interleukin; CRP = C-reactive protein; sTNF-RI = soluble TNF-receptor I; CHUS = Combined Hassles and Uplift Scale; PSS10 = Perceived Stress Scale; MFSIsf = Multidimensional Fatigue Symptom Inventory-Short Form; POMS = Profile of Mood States; CES-D = Center for Epidemiological Studies Depression Scale; PSQI = Pittsburgh Sleep Quality Index.
It could be hypothesized that the nocturnal decrease of IL-6 was a mere reflection of the general level of IL-6 concentrations, with elevated levels of IL-6 being related to more pronounced nocturnal decrease. Table 3 (third column) shows the partial correlations of all significant associations of ΔIL-6 with psychological variables when these correlation coefficients were controlled for IL-6 morning scores. Three of the seven significant associations remained significant, implying that additional information was included in ΔIL-6 that was not provided by IL-6 morning levels. Two of the sevem significant associations were slightly reduced but remained in the range of 0.05 < p < .10. Only two of the seven significant associations between ΔIL-6 and psychological variables were substantially explained by morning levels of IL-6. Vice versa, if evening IL-6 scores instead of morning IL-6 scores were used as a control variable in partial correlation analyses, five of the seven associations between nocturnal IL-6 decrease and psychological variables remained significant or showed a trend to significance (at least p < .07). Again, this confirms that nocturnal IL-6 decrease scores include additional information compared with general concentration scores.
TABLE 3.
Partial Correlations After Controlling for Morning Scores and for Sleep Variables
| r Without Controlling for Other Influences | rp After Controlling for Morning Scores | rp After Controlling for Objective Sleep Variables | |
|---|---|---|---|
| 7Δ IL-6 with | |||
| CHUS Uplift Frequency | .22* | .21* | .22* |
| CHUS Uplift Severity | (.21*) | .15 | .19† |
| MFSIsf Fatigue Score | −.27** | −.26** | −.30** |
| POMS Tension | −.22* | −.19† | −.25* |
| POMS Anger | −.23* | −.15 | −.28** |
| POMS Fatigue | −.26** | −.25* | −.30** |
| POMS Total | (−.21*) | −.19† | −.26* |
| Δ CRP with | |||
| PSS10 Stress score | (.20*) | .15 | .19* |
| PSQI subj. sleep duration | .22* | .16 | .20* |
| ΔsTNF-R1 with | |||
| POMS Fatigue | (−.22*) | −.19† | −.22* |
| Δ Epinephrine with | |||
| PSS10 Stress score | −.24* | −.30* | −.31** |
| CES-D Depression Scores | −.29* | −.28* | −.31** |
| Δ Norepinephrine with | |||
| PSQI subj. sleep duration (variable included only for exploratory reasons) | −.25* | −.26* | −.25* |
Numbers in parentheses are correlation coefficients that should not be interpreted as significant after applying the modified Holm’s method for multiple testing.
p < .05;
p < .01;
.05 < p < .10.
IL = interleukin; CHUS = Combined Hassles and Uplift Scale; MFSIsf = Multidimensional Fatigue Symptom Inventory-Short Form; POMS = Profile of Mood States; CRP = C-reactive protein; PSS10 = Perceived Stress Scale; sTNF-RI = soluble TNF-receptor I; CES-D = Center for Epidemiological Studies Depression Scale; PSQI = Pittsburgh Sleep Quality Index.
Although objective sleep variables did not show significant associations with nocturnal IL-6 changes, it could still be possible that they contributed to these associations. Therefore, we reanalyzed the associations of ΔIL-6 with psychological variables controlling for the three objective sleep variables. The last column of Table 3 shows that nearly all associations between ΔIL-6 with psychological variables remained or even increased after controlling for objective sleep variables.
Because seven psychological variables correlated with ΔIL-6, we also computed linear stepwise regression analysis including ΔIL-6 as criterion, and all seven psychological variables showing significant associations with ΔIL-6 as potential predictor variables. Two psychological variables remained in the regression model, which explained a significant amount of variance (F = 7.8, df 2,95; p < .001). The POMS Fatigue Scale covered the more negative emotions (standardized beta = −0.32; p < .001), whereas the uplift frequency added additional information to the prediction of ΔIL-6 (standardized beta + 0.22; p < .05).
To visualize these associations with an example, we dichotomized the variable fatigue using split-half (POMS fatigue score of >3 equals “high fatigue”) and computed a repeated-measure analysis of variance with time point as repeated measure and fatigue as group factor. Neither fatigue nor time point revealed significant main effects on IL-6, but the interaction was significant (F = 7.5; df 1,110; p < .008). This interaction is shown in Figure 1, and it further underlines that nocturnal change scores can reveal information that is not included in the evening or morning level scores.
Figure 1.
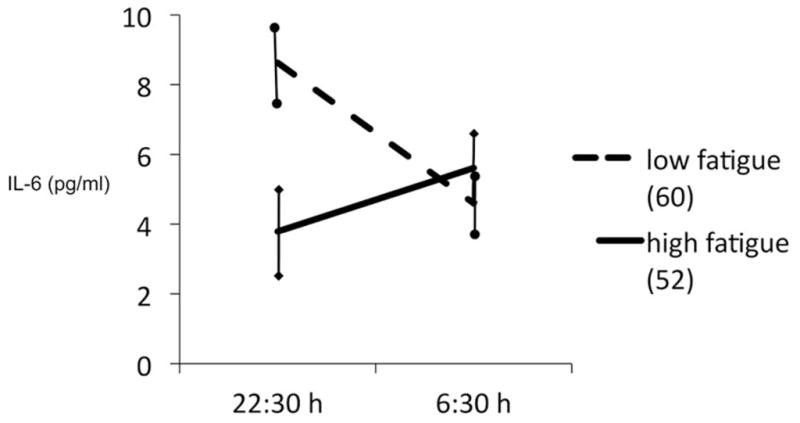
Bedtime and awake concentrations of interleukin (IL)-6 in people with high and low fatigue scores. Observed means; bars indicate standard error of the mean values; groups according to split-half procedure of Profile of Mood States fatigue score. Analysis of variance interaction term F = 7.5; df 1,110; p < .008.
Psychological Correlates of Nocturnal Decrease of Other Biological Variables
The nocturnal CRP changes were associated with only one of the psychological variables, namely, the total stress score PSS10 (r = .20; p < .05), and this association was questioned by adjustment for multiple testing. If this association was controlled for morning scores of CRP, it declined to r = .15 (NS). The nocturnal changes of sTNF-R1 were associated with mood variables, namely, POMS fatigue (−0.22; p < .05), whereas the associations with POMS anger (−0.19) and the MFSI Fatigue Scale (−0.16) failed to reach significance. Moreover, the significant association of sTNF-R1 with POMS fatigue is questioned after adjustment for multiple testing.
The nocturnal epinephrine decrease was significantly associated with depression and stress (Center for Epidemiological Studies Depression Scale, r = −.29; PSS10, −.24; p < .05). It also showed relevant associations with other mood scales (POMS tension, −0.20; POMS vigor, + 0.21; total fatigue score, −0.23; all NS), but due to the smaller sample size of the catecholamines, these associations failed to reach significance levels. Interestingly, these associations remained completely unchanged after controlling for morning scores of epinephrine and for objective sleep variables (Table 3). Therefore, the nocturnal decrease of epinephrine and its association with depression and subjective stress were not explained by the general level of catecholamines. This result again underlines the relevance of analyzing nocturnal change scores in addition to general level scores. The nocturnal changes of norepinephrine were not associated with any of the psychological variables included in this study. Only the subjective sleep duration, which was included in these analyses due to exploratory reasons (compared with objective sleep duration), showed some association with this variable.
DISCUSSION
Our results showed that the nocturnal change scores for immune variables and catecholamines provide additional information that is not provided by analyzing morning levels of these biological variables. The nocturnal decrease of IL-6 showed several associations with psychological variables, such as mood, fatigue, and the frequency of uplifting events. However, the direction of causality remains open, as all data were based on correlations. That is, it is possible that an insufficient nocturnal readjustment of biological systems induces or facilitates the development of negative mood and feelings of fatigue. Alternatively, uplifting events and low scores of negative mood could facilitate nocturnal IL-6 decreases, whereas feelings of fatigue, being exhausted, or other mood variables could hinder the nocturnal IL-6 decrease. The negative associations between stress level and depressive symptoms with nocturnal decrease of epinephrine point to the same direction as the IL-6 results. Depressive symptoms and the total stress level seem to hinder a nocturnal decrease of epinephrine or are amplified by low overnight recovery of the adrenergic systems.
Nocturnal variations of human biological systems play a major role for readjustment and for the preparation of daily adaptation processes. Typically, most (but not all) biological systems reduce their activity during the night hours. Heart rate and blood pressure decrease, usually in association with the reduction of secretion of major stress hormones, and it has been shown that nocturnal change scores of cardiovascular activity (“nocturnal dipping”) (2,3) add information to the general levels of heart rates and blood pressure. The secretion of catecholamines decreases overnight (36). Cortisol as a prominent representative of the hypothalamic-pituitary-adrenal axis shows strong diurnal and nocturnal variations, with a reincrease of cortisol secretion in the late night/early morning hours (20). Generally, high amplitude rhythms are regarded as “healthy,” whereas flattened circadian neuroendocrine rhythms have been reported in the elderly and in people with depression (37). Poor sleep is also considered to cause a disruption of circadian rhythms (11).
Despite earlier studies and overviews, information on diurnal and nocturnal changes for cytokines and cytokine-specific receptors is still limited (38–42). Although the hypothalamic-pituitary-adrenal axis obviously influences cytokines (43), there is also direct evidence for nocturnal variations of immune parameters. The concentration of CD4-helper cells and CD8-surpressor cells is lowest in the early morning hours (44). Sleep seems to increase the number of IL-12-producing monocytes but decreases the number of IL-10-producing monocytes (45), hereby modulating the Type 1/Type 2 helper T cells (Th1/Th2) relation of the immune response.
Impaired night time sleep has been found to be associated with elevated plasma IL-6 levels (21,46). Hong et al. (18) showed that morning IL-6 levels were positively correlated with WASO and negatively correlated with sleep efficiency, a result we did not replicate for nocturnal change scores. Nocturnal IL-6 excretion was increased in patients with primary insomnia (19), which could be also related to insomnia-associated states of negative mood. This effect seems to be most pronounced around midnight (22). Therefore, it has been postulated that the circadian secretion of IL-6 correlates with sleepiness (46,47). This assumption was confirmed by our results, showing that the nocturnal IL-6 decrease was negatively associated with feelings of fatigue, a result confirmed by both scales assessing fatigue. On the other hand, positive daytime experiences (uplift frequency) were associated with a more pronounced decrease of nocturnal IL-6 concentrations. However, the cited studies assessing diurnal and nocturnal variations of IL-6, as well as our IL-6 results, should be interpreted with caution. Diurnal variations are confounded by blood drawing procedures (48,49), and differences in blood drawing techniques between studies could have led to inconclusive results for IL-6. The nocturnal changes of CRP were only weakly related to stress and subjective sleep duration, and after adjustment for multiple testing, the correlation with stress dropped under the significance level. It may be that the amplitude change in CRP over 24 hours was too small to be detected in this sample, or the timing of such changed levels departed from our sampling at 10:30 PM and 6:30 AM. Similarly, for sTNF-R1, diurnal and nocturnal variations were unclear. In our sample, the mean scores even increased from bed time to awake. Comparable results indicating a slight increase of soluble TNF receptors have been reported elsewhere (42). Therefore, the reported association of nocturnal sTNF-R1 changes with fatigue should not be overinterpreted, especially because it was questioned after α adjustment.
Catecholamines are not only relevant for their role in the sympathetic nervous system, but are prominently influenced by emotional factors. Brummett et al. (50) were able to show that positive affect was inversely related to the average level of norepinephrine. In our data, the nocturnal decrease of epinephrine was associated with low levels of daily stress and low depression scores. Interestingly, this association was even stronger after controlling for morning levels of epinephrine and after controlling for objective sleep variables. Again, this underlines that the nocturnal change scores included information that went beyond the mean level scores, and that these nocturnal changes were linked to psychological well-being. Moreover, in light of the association of IL-6 concentrations with blood pressure (51), complex interactions of catecholaminergic systems and immunity should be considered.
Our results also provide some information on the concept of fatigue. The similarity of results for different fatigue scales confirms the homogeneity of the construct. On the other hand, fatigue and sleepiness seem to relate to different underlying concepts, with fatigue being less associated with nonrestorative sleep (52). This is confirmed by lacking associations of fatigue with objective sleep parameters in our study, whereas fatigue was closer related with variables of emotional well-being.
Finally, we summarize some weaknesses and strengths of this study. Due to multiple testing, some of the reported associations could be expected just by chance. Although we used an adjustment procedure for multiple testing, some of the reported correlations could still be artifacts. We favor the view that the correlation coefficients should only be interpreted if they are either confirmed by other studies, or are part of a group of correlation coefficients indicating a comparable direction (as for example for IL-6). Another limitation was the assessment of nocturnal changes with only one evening assessment (10:30 PM), and one assessment after awakening (6:30 AM), but without continuous assessments over 24 hours. Comparable to cortisol, some of the immune variables and catecholamines might show a rise in the early morning hours which reduces or reverses the nocturnal decrease of the corresponding system (53). Some researchers used more assessment points and computed cosinor modulation. However, these models are frequently based on small sample sizes, whereas our study was based on a large sample size with standardized assessment procedures, including objective sleep measures.
Further limitations resulted from the study design. We tried to disentangle the effects of blood sampling and polysomnographic sleep assessments, which resulted in a two-night design, but the design cannot exclude the possibility of a “first-night effect” influencing the blood sampling. To fully disentangle this would have required a lengthier hospitalization which, in turn, would have been more burdensome. The associations between immune parameters and sleep variables could be weakened by the fact that these measures were separated by one day, but the fact remains that associations were found despite the time lag between assessments. Finally, it might have been advantageous to have had the self-rating scales completed at one consistent time of day, but most of these scales ask the respondent to report on activities over the course of several days (e.g., PSQI sleep quality asks for the last 7 days). Thus, we doubt that time of day of scale administration was a major confound.
To our knowledge, this is one of the first studies demonstrating that nocturnal change scores of immune parameters and catecholamines contain additional information that is not covered by the general morning baseline level of these variables. Therefore, continuing the research of nocturnal change scores as a potential index of healthy adaptation processes and their association with psychosocial factors is warranted.
Acknowledgments
This work was supported, in part, by Grants HL36005 and HL44915 from the National Institutes of Health (J.E.D.).
Glossary
- Δ
delta, difference score
- AHI
Apnea-Hypopnea Index
- CRP
C-reactive protein
- IL
interleukin
- MFSI
Multidimensional Fatigue Symptom Inventory
- POMS
Profile of Mood States
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- PSS-10
Perceived Stress Scale
- sTNF-RI
soluble tumor necrosis factor-receptor I
- TNF
tumor necrosis factor
- TST
total sleep time
- WASO
wake after sleep onset
References
- 1.Reddy AB, O’Neill JS. Healthy clocks, healthy body, healthy mind. Trends Cell Biol. 2009;20:36–44. doi: 10.1016/j.tcb.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Routledge F, McFetridge-Durdle J. Nondipping blood pressure patterns among individuals with essential hypertension: a review of the literature. Eur J Cardiovasc Nurs. 2007;6:9–26. doi: 10.1016/j.ejcnurse.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 3.Tomfohr L, Cooper DC, Mills PJ, Nelesen RA, Dimsdale JE. Everyday discrimination and nocturnal blood pressure dipping in black and white Americans. Psychosom Med. 2010;72:266–72. doi: 10.1097/PSY.0b013e3181d0d8b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eguchi K, Hoshide S, Ishikawa J, Pickering TG, Schwartz JE, Shimada K, Kario K. Nocturnal nondipping of heart rate predicts cardiovascular events in hypertensive patients. J Hypertens. 2009 Oct 14; doi: 10.1097/HJH.0b013e328330a938. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scuteri A, Spalletta G, Cangelosi M, Gianni W, Assisi A, Brancati AM, Modestino A, Caltagirone C, Volpe M. Decreased nocturnal systolic blood pressure fall in older subjects with depression. Aging Clin Exp Res. 2009;21:292–7. doi: 10.1007/BF03324918. [DOI] [PubMed] [Google Scholar]
- 6.Holt-Lunstad J, Steffen PR. Diurnal cortisol variation is associated with nocturnal blood pressure dipping. Psychosom Med. 2007;69:339–43. doi: 10.1097/PSY.0b013e318050d6cc. [DOI] [PubMed] [Google Scholar]
- 7.Mikolajczak M, Quoidbach J, Vanootighem V, Lambert F, Lahaye M, Fillée C, de Timary P. Cortisol awakening response (CAR)’s flexibility leads to larger and more consistent associations with psychological factors than CAR magnitude. Psychoneuroendocrinology. 2010;35:752–7. doi: 10.1016/j.psyneuen.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Dahlgren A, Kecklund G, Theorell T, Åkerstedt T. Day-to-day variation in saliva cortisol—relation with sleep, stress and self-rated health. Biol Psychol. 2009;82:149–55. doi: 10.1016/j.biopsycho.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Vreeburg SA, Hoogendijk WJG, van Pelt J, DeRijk RH, Verhagen JCM, van Dyck R, Smit JH, Zitman FG, Penninx BWJH. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66:617–26. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 10.Gustafsson PE, Janlert U, Theorell T, Hammarström A. Life-course socioeconomic trajectories and diurnal cortisol regulation in adulthood. Psychoneuroendocrinology. 2010;35:613–23. doi: 10.1016/j.psyneuen.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Kumari M, Badrick E, Ferrie J, Perski A, Marmot M, Chandola T. Self-reported sleep duration and sleep disturbance are independently associated with cortisol secretion in the Whitehall II study. J Clin Endocrinol Metab. 2009;94:4801–9. doi: 10.1210/jc.2009-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinan TG. Inflammatory markers in depression. Curr Opin Psychiatry. 2009;22:32–6. doi: 10.1097/YCO.0b013e328315a561. [DOI] [PubMed] [Google Scholar]
- 13.Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–69. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 14.Rief W, Pilger F, Ihle D, Bosmans E, Egyed B, Maes M. Immunological differences between patients with major depression and somatization syndrome. Psychiatry Res. 2001;105:165–74. doi: 10.1016/s0165-1781(01)00338-9. [DOI] [PubMed] [Google Scholar]
- 15.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;64:446–57. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 16.Bryant Howren M, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med. 2009;71:171–86. doi: 10.1097/PSY.0b013e3181907c1b. [DOI] [PubMed] [Google Scholar]
- 17.Pervanidou P, Kolaitis G, Charitaki S, Margeli A, Ferentinos S, Bakoula C, Lazaropoulou C, Papassotiriou I, Tsiantis J, Chrousos GP. Elevated morning serum interleukin (IL)-6 or evening salivary cortisol concentrations predict posttraumatic stress disorder in children and adolescents six months after a motor vehicle accident. Psychoneuroendocrinology. 2007;32:991–9. doi: 10.1016/j.psyneuen.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Hong S, Mills PJ, Loredo JS, Adler KA, Dimsdale JE. The association between interleukin-6, sleep, and demographic characteristics. Brain Behav Immun. 2005;19:165–72. doi: 10.1016/j.bbi.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 19.Burgos I, Richter L, Klein T, Fiebich B, Feige B, Lieb K, Voderholzer U, Riemann D. Increased nocturnal interleukin-6 excretion in patients with primary insomnia: a pilot study. Brain Behav Immun. 2006;20:246–53. doi: 10.1016/j.bbi.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Perry MG, Kirwan JR, Jessop DS, Hunt LP. Overnight variations in cortisol, interleukin 6, tumour necrosis factor alpha and other cytokines in people with rheumatoid arthritis. Ann Rheum Dis. 2009;68:63–8. doi: 10.1136/ard.2007.086561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vgontzas AN, Zoumakis M, Bixler EO, Lin H-M, Prolo P, Vela-Bueno A, Kales A, Chrousos GP. Impaired nighttime sleep in healthy old versus young adults is associated with elevated plasma interleukin-6 and cortisol levels: physiologic and therapeutic implications. J Clin Endocrinol Metab. 2003;88:2087–95. doi: 10.1210/jc.2002-021176. [DOI] [PubMed] [Google Scholar]
- 22.Vgontzas AN, Zoumakis M, Papanicolaou DA, Bixler EO, Prolo P, Lin HM, Vela-Bueno A, Kales A, Chrousos GP. Chronic insomnia is associated with a shift of interleukin-6 and tumor necrosis factor secretion from nighttime to daytime. Metabolism. 2002;51:887–92. doi: 10.1053/meta.2002.33357. [DOI] [PubMed] [Google Scholar]
- 23.Vgontzas AN, Pejovic S, Zoumakis E, Lin HM, Bixler EO, Basta M, Fang J, Sarrigiannidis A, Chrousos GP. Daytime napping after a night of sleep loss decreases sleepiness, improves performance, and causes beneficial changes in cortisol and interleukin-6 secretion. Am J Physiol Endocrinol Metab. 2007;292:E253–R261. doi: 10.1152/ajpendo.00651.2005. [DOI] [PubMed] [Google Scholar]
- 24.Burioka N, Miyata M, Fukuoka Y, Endo M, Shimizu E. Day-night variations of serum Interleukin-6 in patients with severe obstructive sleep apnea syndrome before and after continuous positive airway pressure (CPAP) Chronobiol Int. 2008;25:827–34. doi: 10.1080/07420520802384101. [DOI] [PubMed] [Google Scholar]
- 25.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring for Sleep Stages of Human Subjects DCUS. Bethesda: Government Printing Office; 1968. [Google Scholar]
- 26.Curran SL, Andrykowski MA, Studts JL. Short form of the Profile of Mood States (POMS-SF): psychometric information. Psychol Assess. 1995;7:80–3. [Google Scholar]
- 27.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. J Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- 28.DeLongis A, Folkman S, Lazarus RS. The impact of daily Stress on health and mood: psychological and social resources as mediators. J Pers Soc Psychol. 1988;54:486–95. doi: 10.1037//0022-3514.54.3.486. [DOI] [PubMed] [Google Scholar]
- 29.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 30.Stein KD, Jacobsen PB, Blanchard CM, Thors C. Further validation of the multidimensional fatigue symptom inventory-short form. J Pain Symptom Manage. 2004;27:14–23. doi: 10.1016/j.jpainsymman.2003.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buysse DJ, Reynolds Iii CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 32.Roberts WL, Moulton L, Law TC, Farrow G, Cooper-Anderson M, Savory J, Rifai N. Evaluation of nine automated high-sensitivity C-reactive protein methods: implications for clinical and epidemiological applications. Part 2 Clin Chem. 2001;47:418. [PubMed] [Google Scholar]
- 33.Kennedy B, Ziegler MG. A more sensitive and specific radioenzymatic assay for catecholamines. Life Sci. 1990;47:2143–53. doi: 10.1016/0024-3205(90)90314-h. [DOI] [PubMed] [Google Scholar]
- 34.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86:726–8. doi: 10.2105/ajph.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holm SA. A simple sequentially rejective multiple test procedure. Scand J Stat. 1979;6:65–70. [Google Scholar]
- 36.Rasch B, Dodt C, Mölle M, Born J. Sleep-stage-specific regulation of plasma catecholamine concentration. Psychoneuroendocrinology. 2007;32:884–91. doi: 10.1016/j.psyneuen.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 37.Pandi-Perumal SR, Moscovitch A, Srinivasan V, Spence DW, Cardinali DP, Brown GM. Bidirectional communication between sleep and circadian rhythms and its implications for depression: lessons from agomelatine. Prog Neurobiol. 2009;88:264–71. doi: 10.1016/j.pneurobio.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Lorton D, Lubahn CL, Estus C, Millar BA, Carter JL, Wood CA, Bellinger DL. Bidirectional communication between the brain and the immune system: Implications for physiological sleep and disorders with disrupted sleep. Neuroimmunomodulation. 2006;13:357–74. doi: 10.1159/000104864. [DOI] [PubMed] [Google Scholar]
- 39.Bryant PA, Trinder J, Curtis N. Sick and tired: does sleep have a vital role in the immune system? Nat Rev Immunol. 2004;4:457–67. doi: 10.1038/nri1369. [DOI] [PubMed] [Google Scholar]
- 40.Pedrovsky N. Towards a unified model of neuroendocrine-immune interaction. Immunol Cell Biol. 2001;79:350–7. doi: 10.1046/j.1440-1711.2001.01029.x. [DOI] [PubMed] [Google Scholar]
- 41.Haus E, Smolensky MH. Biologic rhythms in the immune system. Chronobiol Int. 1999;16:581–622. doi: 10.3109/07420529908998730. [DOI] [PubMed] [Google Scholar]
- 42.Haack M, Pollmächer T, Mullington JM. Diurnal and sleep-wake dependent variations of soluble TNF- and IL-2 receptors in healthy volunteers. Brain Behav Immun. 2004;18:361–7. doi: 10.1016/j.bbi.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 43.Zunszain PA, Anacker C, Cattaneo A, Carvalho LA, Pariante CM. Glucocorticoids, cytokines and brain abnormalities in depression. Progr Neuropsychopharmacol Biol Psychiatry. 2010 Apr 18; doi: 10.1016/j.pnpbp.2010.04.011. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dimitrov S, Benedict C, Heutling D, Westermann J, Born J, Lange T. Cortisol and epinephrine control opposing circadian rhythms in T cell subsets. Blood. 2009;113:5134–43. doi: 10.1182/blood-2008-11-190769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lange T, Dimitrov S, Fehm H-L, Westermann J, Born J. Shift of monocyte function toward cellular immunity during sleep. Arch Intern Med. 2006;166:1695–1700. doi: 10.1001/archinte.166.16.1695. [DOI] [PubMed] [Google Scholar]
- 46.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Trakada G, Chrousos GP. IL-6 and its circadian secretion in humans. Neuroimmunomodulation. 2005;12:131–40. doi: 10.1159/000084844. [DOI] [PubMed] [Google Scholar]
- 47.Mullington JM, Haack M, Toth M, Serrador JM, Meier-Ewert HK. Cardiovascular, inflammatory, and metabolic consequences of sleep deprivation. Prog Cardiovasc Dis. 2009;51:294–302. doi: 10.1016/j.pcad.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Haack M, Kraus T, Schuld A, Dalal M, Koethe D, Pollmächer T. Diurnal variations of interleukin-6 plasma levels are confounded by blood drawing procedures. Psychoneuroendocrinology. 2002;27:921–31. doi: 10.1016/s0306-4530(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 49.Dimitrov S, Lange T, Benedict C, Nowell MA, Jones SA, Scheller J, Rose-John S, Born J. Sleep enhances IL-6 trans-signaling in humans. FASEB J. 2006;20:2174–6. doi: 10.1096/fj.06-5754fje. [DOI] [PubMed] [Google Scholar]
- 50.Brummett BH, Boyle SH, Kuhn CM, Siegler IC, Williams RB. Positive affect is associated with cardiovascular reactivity, norepinephrine level, and morning rise in salivary cortisol. Psychophysiology. 2009;46:862–9. doi: 10.1111/j.1469-8986.2009.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim K-I, Lee J-H, Chang H-J, Cho Y-S, Youn T-J, Chung W-Y, Chae I-H, Choi D-J, Park KU, Kim C-H. Association between blood pressure variability and inflammatory marker in hypertensive patients. Circulation. 2008;72:293–8. doi: 10.1253/circj.72.293. [DOI] [PubMed] [Google Scholar]
- 52.Neu D, Mairesse O, Hoffmann G, Valsamis JB, Verbanck P, Linkowski P, Le Bon O. Do ‘sleepy’ and ‘tired’ go together? Rasch analysis of the relationships between sleepiness, fatigue and nonrestorative sleep complaints in a nonclinical population sample. Neuroepidemiology. 2010;35:1–11. doi: 10.1159/000301714. [DOI] [PubMed] [Google Scholar]
- 53.Cutolo M, Straub RH. Circadian rhythms in arthritis: hormonal effects on the immune/inflammatory reaction. Autoimmun Rev. 2008;7:223–8. doi: 10.1016/j.autrev.2007.11.019. [DOI] [PubMed] [Google Scholar]


