Abstract
Objective
The purpose of this study was to determine if: 1) insulin-like growth factor binding protein-1 (IGFBP-1) in amniotic fluid (AF) exhibited proteolytic cleavage in cases of intra-amniotic inflammation; and 2) if the matrix metalloproteinases (MMP-3, MMP-8, MMP-9) in AF are associated with the degradation of IGFBP-1 in AF.
Methods
AF samples (n=20) were obtained from preterm gestations with and without intra-amniotic inflammation. The form of IGFBP-1 in AF was assessed by Western blot analysis and AF MMP-8 concentration was measured by ELISA. Densitometric analysis of Western blot was performed and the fragmented/intact IGFBP-1 ratio was calculated. Proteolysis of AF IGFBP-1 by MMPs was evaluated by incubating AF with exogenous human MMP-3, MMP-8 or MMP-9, and by incubating recombinant human IGFBP-1 in AF with and without inflammation.
Results
1) IGFBP-1 was present in AF without inflammation as an intact form; however, the fragmented form was dominant in AF with inflammation; 2) the ratio of fragmented/intact IGFBP-1 was significantly higher in AF with inflammation than in AF without inflammation; 3) a higher ratio of fragmented/intact IGFBP-1 was associated with a higher concentration of MMP-8; 4) in-vitro proteolysis experiments showed that AF IGFBP-1 was degraded by exogenous human MMP-3, MMP-8 and MMP-9; 5) recombinant human IGFBP-1 was fragmented in AF with inflammation, but not in AF without inflammation.
Conclusion
The fragmented form of AF IGFBP-1 was significantly increased in AF with intra-amniotic inflammation, and MMPs produced in AF with intra-amniotic inflammation was associated with the proteolytic change of AF IGFBP-1.
Keywords: Intra-amniotic inflammation, matrix metalloproteinase (MMP), AF MMP-8
Introduction
Intra-amniotic inflammation/infection is a risk factor for impending preterm delivery and adverse pregnancy outcome [2, 11, 14, 28, 31]. Matrix metalloproteinases (MMPs), proteolytic enzymes derived at the site of inflammation, play a central role in the mechanisms responsible for preterm labor or premature rupture of membranes resulting in preterm birth. The amniotic fluid (AF) concentrations of some MMPs (MMP-3, MMP-8 and MMP-9) have been reported to be significantly elevated in the presence of intra-amniotic infection/inflammation [3, 5, 23, 26].
During pregnancy, the insulin-like growth factor binding protein-1 (IGFBP-1) is produced in decidua and exists in high concentration in AF and maternal serum [22, 29]. Insulin-like growth factor binding proteins (IGFBPs) have high affinity to insulin-like growth factors (IGFs) and antagonize the binding of IGFs to their receptors [12]. Several studies indicated that MMPs act as IGFBP protease and proteolytic cleavage of IGFBPs by specific MMPs has been reported [7,8,10,17,20].
The purpose of this study was to investigate if the cleavage of IGFBP-1 by MMPs (MMP-3, MMP-8 and MMP-9) would occur in intra-amniotic inflammation, and if the form of IGFBP-1 would change from intact to fragmented.
Materials and Methods
Amniotic Fluid
The form of IGFBP-1 was assessed by Western blot analysis in 20 AF samples obtained from preterm gestation. Characteristics of the study cases are described in Table 1. AF samples were collected by transabdominal amniocentesis or at the time of cesarean section and were centrifuged and stored in polypropylene tubes at −70°C until studied. AF was collected after written informed consent was obtained. The Institutional Review Board of our institution approved the collection of biological materials and data from these patients for research purposes. The Seoul National University has a Federal Wide Assurance with the Office for Human Research Protections of the Department of Health and Human Services of the United States.
Table 1.
Characteristics of the study populations
| AF without inflammation (n=9) | AF with inflammation (n=11) |
P value | |
|---|---|---|---|
| Maternal age (y) | 31 (23–34) | 31 (25–43) | NS |
| Nuliparity | 6 (67%) | 4 (36%) | NS |
| Gestational age at amniocentesis (wk) | 32.1 (26.1–36.7) | 31.1 (17.9–35.6) | NS |
| Gestational age at delivery (wk) | 39.4 (36.1–41.4) | 31.7 (18.1–35.7) | <.001 |
| Indication for amniocentesis | NS | ||
| Preterm labor | 6 (67%) | 6 (55%) | |
| Preterm PROM | 2 (22%) | 4 (36%) | |
| Others | 1 (11%) | 1 (9%) |
AF, amniotic fluid; NS, not significant; PROM, premature rupture of membranes.
Cases complicated with preeclampsia, intrauterine fetal growth retardation, major congenital anomalies or multiple pregnancy were not included.
Values expressed as median (range) or number (percentage).
AF inflammation was defined as an elevated AF MMP-8 concentration (>23 ng/ml), as previously reported [27]. MMP-8 concentration was measured with a commercially available enzyme-linked immunosorbent assay (Amersham Pharmacia Biotech, Inc, Bucks, UK). The sensitivity of the test is 0.3 ng/mL. Intra- and inter-assay coefficients of variation were 3.1% and 9.5%, respectively.
Western blot analysis
1–2 uL of human AF was diluted with sodium dodecyl sulfate (SDS)-loading buffer and boiled for 10 minutes and subjected to SDS–PAGE using 13.5% gels. Proteins were then transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA) at 30 V for 1 hour. Nonspecific binding was blocked overnight in a 5% milk protein in 0.1% TBS-T (0.1% [vol/vol] Tween 20 in Tris-buffer saline) at 4°C. After washing, the membrane was incubated with 1:1000 dilution of rabbit anti-human IGFBP-1 polyclonal antibody (1:1000; Upstate, Lake Placid, NY) diluted in the blocker at room temperature for 1½ hours. The membrane was washed and incubated with 1:5000 dilution of alkaline phosphatase-labeled goat anti-rabbit antibody (Zymed Laboratories, San Francisco, CA) dilutedin the 0.1% TBS-T at room temperature for 1 hour. A BCIP/NBT tablet (Sigma, St Louis, MO) diluted in distilled water was used for development.
AF IGFBP-1 proteolysis by MMPs
The ability of human MMPs to degrade AF IGFBP-1 was evaluated by incubating 2 uL of AF with exogenous human MMP-3, MMP-8 or MMP-9 (6pmol for each MMP; Calbiochem, Darmstadt, Germany) in a solution of 50 mM Tris (pH 7.5) containing 150 mM NaCl, 10 mM CaCl2, and 0.05% Triton (final volume 20 uL). To activate proenzyme, 1 mM p-aminophenylmercuric acetate (APMA) solution (Calbiochem, Darmstadt, Germany) was used. Incubation was performed at 37°C for 12–24 hours, and the reaction was terminated by adding SDS-PAGE sample buffer and boiling the mixture.
Recombinant human IGFBP-1 (rhIGFBP-1) proteolysis in amniotic fluid
rhIGFBP-1 (100 ng; R&D systems, Minneapolis, MN) was incubated in 10 uL of AF with or without inflammation. The solution was incubated at 37°C for 6–12 hours, and the reaction was stopped by adding reducing buffer and boiling the mixture. A time course of IGFBP-1 degradation in AF was evaluated by analyzing the aliquots with Western blot.
Densitometric analysis
Densitometric analysis of the results of Western blot (scanned images of membranes) was performed with the TINA 2.0 software program (Raytest, Staubenhardt). Ratio of proteolytic fragment to intact IGFBP-1 was calculated. The fragmented/intact IGFBP-1 ratio in AF between groups with and without inflammation were compared with the Mann-Whitney U test. A P-value of <.05 was considered significant.
Results
Difference in the form of IGFBP-1 in AF with and without inflammation
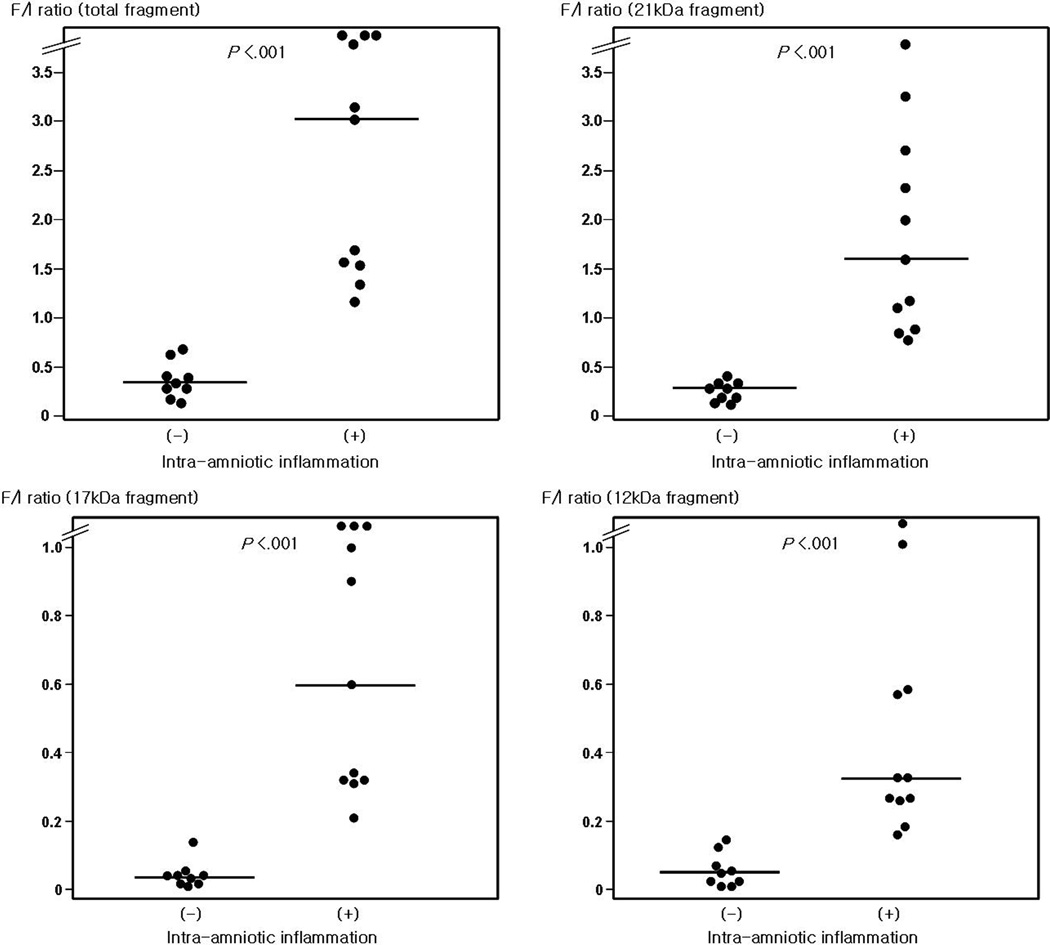
Results of Western blot analysis for IGFBP-1 in AF showed that IGFBP-1 was present in AF without inflammation as an intact form of 30 kDa (Figure 1). However, the intact form of IGFBP-1 decreased and fragments were seen at 21, 17 and 12 kDa on the result of immunoblot for AF with inflammation. A total of 20 cases were analyzed, and the ratio of fragmented/intact IGFBP-1 was significantly higher in AF with inflammation than in AF without inflammation (Figure 2).
Figure 1.
Western blot analysis of amniotic fluid (AF) IGFBP-1 protein; IGFBP-1 was present in AF without inflammation as an intact form of 30 kDa (lane 1–4), however intact form of IGFBP-1 decreased and fragments were seen at 21, 17 and 12 kDa in AF with inflammation (lane 5–8). Representative cases are shown.
Figure 2.
Densitometric analysis of amniotic fluid (AF) IGFBP-1 protein; The ratio of fragmented/intact IGFBP-1 was significantly higher in AF with inflammation than that in AF without inflammation (for total fragment: median 0.41 [range, 0.13–0.69] vs median 3.0 [range, 1.17–8.77]; for 21 kDa fragment: median 0.33 [range, 0.13–0.49] vs median 1.57, [range, 0.73–3.75]; for 17 kDa fragment: median 0.05 [range, 0.00–0.15] vs median 0.60, [range, 0.21–3.35]; for 12 kDa fragment: median 0.06 [range, 0.00–0.15] vs median 0.34, [range, 0.17–1.67]. P<0.001, respectively).
F/I ratio, IGFBP-1 protein fragmented/intact form ratio by densitometric analysis
The relationship between the change of IGFBP-1 and the degree of AF inflammation measured by AF MMP-8 concentration
Figure 3 describes that a higher ratio of fragmented/intact IGFBP-1 was associated with a higher concentration of AF MMP-8. Fragment detection decreased in AF without inflammation (Figure 3A, lanes 1 and 2). As AF MMP-8 concentration increased, fragments of IGFBP-1 increased and intact IGFBP-1 decreased. At higher MMP-8 concentrations, intact IGFBP-1 almost disappeared (Figure 3A, lanes 5–7).
Figure 3.
Degradation of IGFBP-1 in amniotic fluid (AF) according to the AF MMP-8 concentration; (A) Representative cases are shown: (1)–(2) AF without inflammation and (3)–(7) AF with inflammation. AF with a higher degree of inflammation measured by AF MMP-8 concentration had a higher ratio of fragmented/intact IGFBP-1. (B) Strong correlation is shown between AF MMP-8 concentrations and the ratio of fragmented/intact IGFBP-1 in AF (r=0.86; p<0.001; Spearman rank correlation test). F/I ratio, IGFBP-1 protein fragmented/intact form ratio by densitometric analysis.
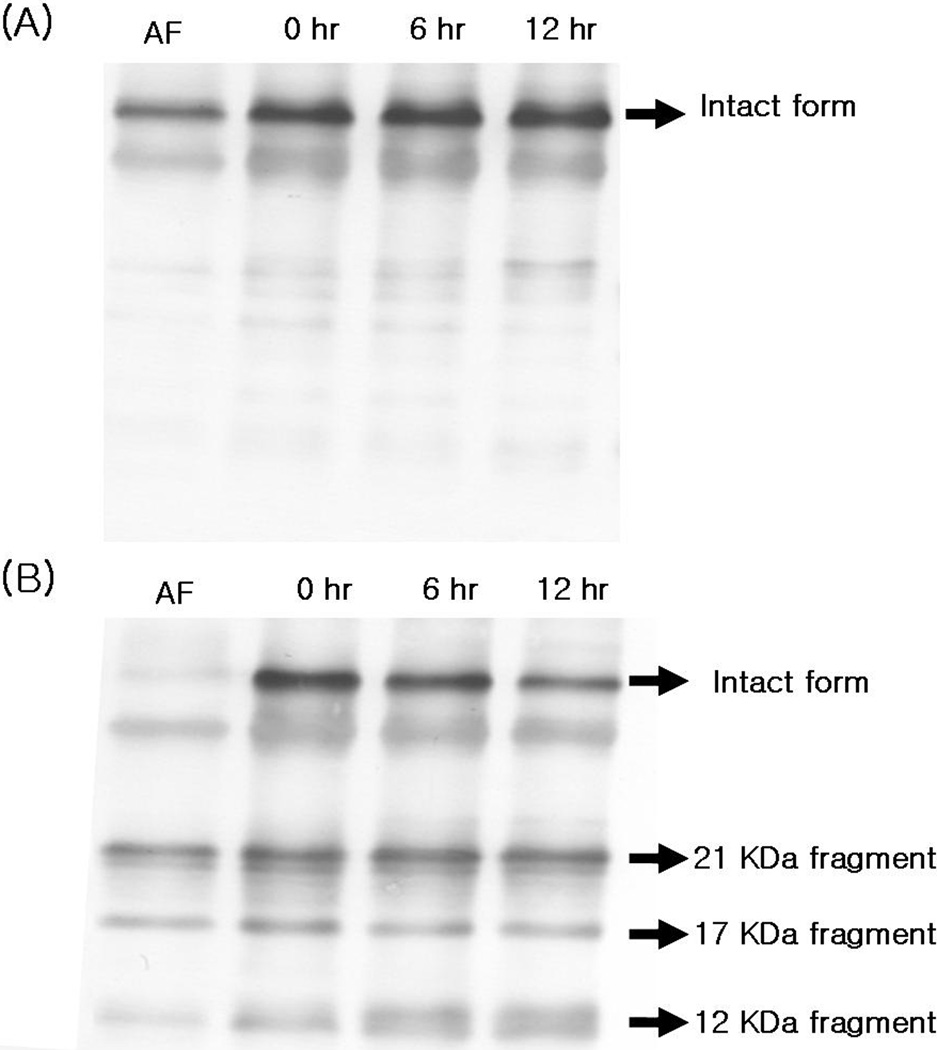
rhIGFBP-1 degradation in AF with inflammation
Figure 4 shows the change of exogenous rhIGFBP-1 in AF with and without inflammation. Experiments were done on 4 cases of AF. MMP-8 concentrations of AF with inflammation were 415.2 and 2199.9 ng/mL, and those of control AF were < 0.3 ng/mL. After 12 hours of incubation, intact rhIGFBP-1 was gradually degraded in AF with inflammation and 12 kDa fragment increased. However, there was no change in the form of rhIGFBP-1 in AF without inflammation.
Figure 4.
rhIGFBP-1 proteolysis in amniotic fluid (AF) without (A) or with (B) inflammation; 100 ng of rhIGFBP-1 was incubated in 10 uL of AFs at 37°C. There was no change in the form of rhIGFBP-1 in AF without inflammation (A). However, intact rhlGFBP-1 (30 kDa) was gradually degraded in AF with inflammation and 12 kDa fragment increased (B). Independent experiments were done on 4 cases of AFs and representative cases were shown.
Time-course of IGFBP-1 proteolysis by exogenous MMP-3, MMP-8 and MMP-9
Figure 5 demonstrated that AF IGFBP-1 was degraded by exogenous human MMP-3, MMP-8 and MMP-9 in a time-dependent manner. MMP-3 cleaved intact IGFBP-1 in AF into 3 fragments of 23, 21 and 12 kDa. MMP-9 degraded into 21, 17 and 12 kDa fragments. MMP-8 proteolyzed AF IGFBP-1 into 4 fragments of 21, 17, 14 and 12 kDa. However, there was no change in the form of AF IGFBP-1 in the control AF.
Figure 5.
Proteolysis of amniotic fluid (AF) IGFBP-1 in the presence MMP-3 (B), MMP-9 (C) and MMP-8 (D); 2 uL of AFs were incubated with exogenous human MMP-3, MMP-8 or MMP-9 (enzyme/substrate molar ratio =1:1) in a solution of 50 mM Tris (pH 7.5) containing 150 mM NaCl, 10 mM CaCl2, and 0.05% Triton (final volume 20 uL) at 37°C for 12–24 hours. AF IGFBP-1 was degraded by exogenous human MMP-3, MMP-8 and MMP-9 in a time-dependent manner. However, there was no change in the form of AF IGFBP-1 in the control AF (A).
Comment
Principal findings of this study
1) IGFBP-1 was present as an intact form in AF without inflammation. However, a fragmented form of IGFBP-1 was dominant in AF with inflammation; 2) the ratio of fragmented/intact IGFBP-1 was significantly higher in AF with inflammation than in AF without inflammation; 3) a higher ratio of fragmented/intact IGFBP-1 was associated with a higher degree of inflammation; 4) rhIGFBP-1 was fragmented in AF with inflammation, but not in AF without inflammation; and 5) in vitro experiments showed that an intact form of IGFBP-1 in AF was degraded by exogenous human MMP-3, MMP-8 and MMP-9 in a time-dependent manner.
Degradation of IGFBP-1 in AF with inflammation
IGFBP-1 originates from decidua and exists in a high concentration during pregnancy [8, 10, 12, 19]. Substantial evidence indicates that IGFBP-1 is likely to play an important role in human pregnancy. In particular, it is thought to be associated with placental development [10, 17, 19, 22]. Elevated decidual concentration of IGFBP-1 is known to inhibit invasion of trophoblast [19. 29]. A recent study indicated that cleavage of IGFBP-1 by MMP-3 and MMP-9 might increase IGF bioavailability and control placental development in early pregnancy [10]. Women with preeclampsia have the higher serum IGFBP-1 concentration compared with those with normal pregnancies [4, 16, 18]. The IGFBP system is also thought to be involved in fetal growth [6, 9, 30]. However, the role of IGFBP system in an intra-uterine inflammation during pregnancy has not been discussed. This study demonstrated that IGFBP-1 in AF was cleaved by MMPs produced in an inflammatory condition. Our data suggest a possible important role of AF IGFBP-1 in the pathologic process of AF inflammation leading to preterm birth.
IGFBP-1 as a substrate of MMP-8
This study reveals that MMP-3, MMP-8 and MMP-9 are responsible for the proteolysis of IGFBP-1 in AF. Several studies have reported that IGFBPs play a role of a substrate for MMPs [7, 10, 13, 17, 21, 22, 24, 25]. Proteolysis of IGFBPs is the mechanism by which MMPs control the bioactivity of the IGF system. IGFBP-1 has been reported as a substrate for MMP-2, MMP-3, MMP-7, MMP-9, and MMP-11 [7, 10, 21, 25]. The proteolysis of IGFBP-1 by MMP-3 and MMP-9 was also demonstrated in the present study. However, to our best knowledge, this is the first study showing that MMP-8 is responsible for the proteolysis of IGFBP-1. This novel finding suggests that MMP-8 should regulate bioavailability of the IGF system in an inflammatory condition by means of proteolysis of IGFBP-1.
AF IGFBP-1 fragment as a possible biomarker of preterm birth
Our data showed that a specific proteolytic fragment of IGFBP-1 might be a novel biomarker of intra-amniotic inflammation leading to preterm birth. Indeed, a proteomics-based study has found 11 kDa proteolytic fragment of IGFBP-1 in AF and pooled maternal serum of patients with intra-amniotic infection [15]. Our study proved the mechanism for the production of fragments of IGFBP-1, which was MMPs-related proteolysis in AF with inflammation. Moreover, our data showed that not only the 11 kDa fragment but other fragments (21, 17 and 14 kDa) should be considered.
Intra-uterine inflammation/infection, as well as other pathologic mechanisms (e.g. activation of the maternal or fetal HPA axis, systemic inflammation, decidual hemorrhage, and pathologic distension of uterus) can result in preterm birth [1]. Present data only suggests a proteolytic fragment of IGFBP-1 as a marker of AF inflammation/infection. However, proteolysis of IGFBP-1 may occur to regulate IGF system of the fetal/maternal surface in the absence of intra-amniotic inflammation. Coppock et al.[10] demonstrated that decidual cells produced protease as well as IGFBP-1 in the first trimester, and cleavage of IGFBP-1 by MMP-3 and MMP-9 occurred to control placental development. Moreover, “decidual activation” is considered as a common biochemical pathway involving initiating parturition. Therefore, future research is needed to evaluate if a specific proteolytic fragment of IGFBP-1 could be a biomarker of preterm birth resulting from other mechanisms of disease.
Acknowledgement
This work was supported in part by Korea Science and Engineering Foundation (KOSEF) grant No. R01-2006-000-10607-0, funded by the Ministry of Science and Technology of Korea (MOST) and in part by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
References
- 1.Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. J Matern Fetal Neonatal Med. 2006;19(12):773–782. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- 2.Andrews WW, Hauth JC, Goldenberg RL. Infection and preterm birth. Am J Perinatol. 2000;17:357–365. doi: 10.1055/s-2000-13448. [DOI] [PubMed] [Google Scholar]
- 3.Angus SR, Segel SY, Hsu CD, Locksmith GJ, Clark P, Sammel MD, et al. Amniotic fluid matrix metalloproteinase-8 indicates intra-amniotic infection. Am J Obstet Gynecol. 2001;185:1232–1238. doi: 10.1067/mob.2001.118654. [DOI] [PubMed] [Google Scholar]
- 4.Anim-Nyame N, Hills FA, Sooranna SR, Steer PJ, Johnson MR. A longitudinal study of maternal plasma insulin-like growth factor binding protein-1 concentrations during normal pregnancy and pregnancies complicated by pre-eclampsia. Hum Reprod. 2000;15(10):2215–2219. doi: 10.1093/humrep/15.10.2215. [DOI] [PubMed] [Google Scholar]
- 5.Athayde N, Romero R, Gomez R, Maymon E, Pacora P, Mazor M, et al. Matrix metalloproteinases-9 in preterm and term human parturition. J Matern Fetal Med. 1999;8(5):213–219. doi: 10.1002/(SICI)1520-6661(199909/10)8:5<213::AID-MFM3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 6.Ben Lagha N, Seurin D, Le Bouc Y, Binoux M, Berdal A, Menuelle P, et al. Insulin-like growth factor binding protein (IGFBP-1) involvement in intrauterine growth retardation: study on IGFBP-1 overexpressing transgenic mice. Endocrinology. 2006;147(10):4730–4737. doi: 10.1210/en.2006-0171. [DOI] [PubMed] [Google Scholar]
- 7.Bischof P, Meisser A, Campana A, Tseng L. Effects of decidua-conditioned medium and insulin-like growth factor binding protein-1 on trophoblastic matrix metalloproteinases and their inhibitors. Placenta. 1998;19(7):457–464. doi: 10.1016/s0143-4004(98)91038-4. [DOI] [PubMed] [Google Scholar]
- 8.Bunn RC, Fowlkes JL. Insulin-like growth factor binding protein proteolysis. Trends Endocrinol Metab. 2003;14(4):176–181. doi: 10.1016/s1043-2760(03)00049-3. [DOI] [PubMed] [Google Scholar]
- 9.Chevallier B, Lagarde A, Degrelle H, Belaisch-Allart J, Giraudet P, Gallet JP. Insulin-like growth factor binding protein 1 level in amniotic fluid: correlation with birth weight. Biol Neonate. 1998;73(6):404–406. doi: 10.1159/000014003. [DOI] [PubMed] [Google Scholar]
- 10.Coppock HA, White A, Aplin JD, Westwood M. Matrix metalloprotease-3 and -9 proteolyze insulin-like growth factor-binding protein-1. Biol Reprod. 2004;71(2):438–443. doi: 10.1095/biolreprod.103.023101. [DOI] [PubMed] [Google Scholar]
- 11.Eschenbach DA. Intrauterine infection and premature membrane rupture. Curr Opin Obstet Gynecol. 1989;1:23–26. [PubMed] [Google Scholar]
- 12.Fowler DJ, Nicolaides KH, Miell JP. Insulin-like growth factor protein-1 (IGFBP-1): a multifunctional role in the human female reproductive tract. Hum Reprod Updat. 2000;6(5):495–503. doi: 10.1093/humupd/6.5.495. [DOI] [PubMed] [Google Scholar]
- 13.Fowlkes JL, Enghild JJ, Suzuki K, Nagase H. Matrix metalloproteinases degrade insulin-like growth factor-binding protein-3 in dermal fibroblast cultures. J Biol Chem. 1994;269:25742–25746. [PubMed] [Google Scholar]
- 14.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol. 1992;166:1515–1528. doi: 10.1016/0002-9378(92)91628-n. [DOI] [PubMed] [Google Scholar]
- 15.Gravett MG, Novy MJ, Rosenfeld RG, Reddy AP, Jacob T, Turner M, et al. Diagnosis of intra-amniotic infection by proteomic profiling and identification of novel biomarkers. JAMA. 2004 Jul 28;292(4):462–469. doi: 10.1001/jama.292.4.462. [DOI] [PubMed] [Google Scholar]
- 16.Grobman WA, Kazer RR. Serum insulin, insulin-like growth factor-I, and insulin-like growth factor binding protein-1 in women who develop preeclampsia. Obstet Gynecol. 2001;97(4):521–526. doi: 10.1016/s0029-7844(00)01193-5. [DOI] [PubMed] [Google Scholar]
- 17.Hills FA, Elder MG, Chard T, Sullivan MH. Regulation of human villous trophoblast by insulin-like growth factors and insulin-like growth factor-binding protein-1. J Endocrinol. 2004;183(3):487–496. doi: 10.1677/joe.1.05867. [DOI] [PubMed] [Google Scholar]
- 18.Ingec M, Gursoy HG, Yildiz L, Kumtepe Y, Kadanali S. Serum levels of insulin, IGF-1, and IGFBP-1 in pre-eclampsia and eclampsia. Int J Gynaecol Obstet. 2004;84(3):214–219. doi: 10.1016/S0020-7292(03)00342-4. [DOI] [PubMed] [Google Scholar]
- 19.Irwin JC, Suen LF, Faessen GH, Popovici RM, Giudice LC. Insulin-like growth factor (IGF)-II inhibition of endometrial stromal cell tissue inhibitor of metalloproteinase-3 and IGF-binding protein-1 suggests paracrine interactions at the decidua:trophoblast interface during human implantation. J Clin Endocrinol Metab. 2001;86(5):2060–2064. doi: 10.1210/jcem.86.5.7451. [DOI] [PubMed] [Google Scholar]
- 20.Kabir-Salmani M, Shimizu Y, Sakai K, Iwashita M. Posttranslational modifications of decidual IGFBP-1 by steroid hormones in vitro. Mol Hum Reprod. 2005;11(9):667–671. doi: 10.1093/molehr/gah222. [DOI] [PubMed] [Google Scholar]
- 21.Manes S, Mira E, Barbacid MM, Cipres A, Fernandez-Resa P, Buesa JM, et al. Identification of insulin-like growth factor-binding protein-1 as a potential physiological substrate for human stromelysin-3. J Biol Chem. 1997;272:25706–25712. doi: 10.1074/jbc.272.41.25706. [DOI] [PubMed] [Google Scholar]
- 22.Martina NA, Kim E, Chitkara U, Wathen NC, Chard T, Giudice LC. Gestational age-dependent expression of insulin-like growth factor-binding protein-1 (IGFBP-1) phosphoisoforms in human extraembryonic cavities, maternal serum, and decidua suggests decidua as the primary source of IGFBP-1 in these fluids during early pregnancy. J Clin Endocrinol Metab. 1997;82(6):1894–1898. doi: 10.1210/jcem.82.6.3974. [DOI] [PubMed] [Google Scholar]
- 23.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–99. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 24.Miyamoto S, Yano K, Sugimoto S, Ishii G, Hasebe T, Endoh Y, et al. Matrix metalloproteinase-7 facilitates insulin-like growth factor bioavailability through its proteinase activity on insulin-like growth factor binding protein 3. Cancer Res. 2004;64:665–671. doi: 10.1158/0008-5472.can-03-1916. [DOI] [PubMed] [Google Scholar]
- 25.Nakamura M, Miyamoto S, Maeda H, Ishii G, Hasebe T, Chiba T, et al. Matrix metalloproteinase-7 degrade all insulin-like growth factor binding proteins and facilitates indulin0like growth factor bioavailability. Biochem Biophy Res Com. 2005;333:1011–1016. doi: 10.1016/j.bbrc.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 26.Park KH, Chaiworapongsa T, Kim YM, Espinoza J, Yoshimatsu J, Edwin S, et al. Matrix metalloproteinase-3 in parturition, premature rupture of the membranes, and microbial invasion of the amniotic cavity. J Perinat Med. 2003;31(1):12–22. doi: 10.1515/JPM.2003.002. [DOI] [PubMed] [Google Scholar]
- 27.Park JS, Romero R, Yoon BH, Moon JB, Oh SY, Han SY, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001;185:1156–1161. doi: 10.1067/mob.2001.117679. [DOI] [PubMed] [Google Scholar]
- 28.Ramsey PS, Lieman JM, Brumfield CG, Carlo W. Chorioamnionitis increases neonatal morbidity in pregnancies complicated by preterm premature rupture of membranes. Am J Obstet Gynecol. 2005;192:1162–1166. doi: 10.1016/j.ajog.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 29.Rutanen EM, Bohn H, Seppala M. Radioimmunoassay of placental protein 12: levels in amniotic fluid, cord blood, and serum of healthy adults, pregnant women, and patients with trophoblastic disease. Am J Obstet Gynecol. 1982;144(4):460–463. doi: 10.1016/0002-9378(82)90254-x. [DOI] [PubMed] [Google Scholar]
- 30.Watson CS, Bialek P, Anzo M, Khosravi J, Yee SP, Han VK. Elevated circulating insulin-like growth factor binding protein-1 is sufficient to cause fetal growth restriction. Endocrinology. 2006;147(3):1175–1186. doi: 10.1210/en.2005-0606. [DOI] [PubMed] [Google Scholar]
- 31.Yoon BH, Romero R, Moon JB, Shim SS, Kim M, Kim G, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2011;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]