Abstract
Background
The tripeptide glutathione (GSH) is the most abundant free radical scavenger synthesized endogenously in humans. Increasing mechanistic, clinical, and epidemiological evidence demonstrates that GSH status is significant in acute and chronic diseases. Despite ease of delivery, little controlled clinical research data exist evaluating the effects of oral GSH supplementation.
Objectives
The study objectives were to determine the effect of oral GSH supplementation on biomarkers of systemic oxidative stress in human volunteers.
Design
This was a randomized, double-blind, placebo-controlled clinical trial.
Setting/location
The study was conducted at Bastyr University Research Institute, Kenmore, WA and the Bastyr Center for Natural Health, Seattle, WA.
Subjects
Forty (40) adult volunteers without acute or chronic disease participated in this study.
Intervention
Oral GSH supplementation (500 mg twice daily) was given to the volunteers for 4 weeks.
Outcome measures
Primary outcome measures included change in creatinine-standardized, urinary F2-isoprostanes (F2-isoP) and urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG). Changes in erythrocyte GSH concentrations, including total reduced glutathione (GSH), oxidized glutathione (GSSG), and their ratio (GSH:GSSG) were also measured by tandem liquid chromatography/mass spectrometry. Analysis of variance was used to evaluate differences between groups.
Results
There were no differences in oxidative stress biomarkers between treatment groups at baseline. Thirty-nine (39) participants completed the study per protocol. Changes in creatinine standardized F2-isoP (ng/mg creatinine) (0.0±0.1 versus 0.0±0.1, p=0.38) and 8-OHdG (μg/g creatinine) (-0.2±3.3 versus 1.0±3.2, p=0.27) were nonsignificant between groups at week 4. Total reduced, oxidized, and ratio measures of GSH status were also unchanged.
Conclusions
No significant changes were observed in biomarkers of oxidative stress, including glutathione status, in this clinical trial of oral glutathione supplementation in healthy adults.
Introduction
Reduced glutathione (GSH) is a low-molecular-weight, water-soluble tripeptide, composed of the amino acids cysteine, glutamic acid, and glycine.1 GSH is an important antioxidant and plays a major role in the detoxification of endogenous metabolic products, including lipid peroxides, and xenobiotic compounds including pollutants, heavy metals, and drugs.2,3 Intracellular GSH exists in both the oxidized disulfide form (GSSG) or in reduced (GSH) state; the ratio between GSH and GSSG is held in dynamic balance depending on many factors including the tissue of interest, intracellular demand for conjugation reactions, intracellular demand for reducing power, extracellular demand for reducing potential, and the complex interplay between several regulatory enzymes including glutathione peroxidase (GPx), glutathione-S-transferase (GST) and γ-glutamyltransferase (GGT).1 In the absence of adequate GSH concentrations, numerous oxidative and nitrosative reactive intermediates persist, including superoxide, hydroxide, peroxide, and peroxynitrite radicals, which all can lead to modification of cellular macromolecules including lipid membranes (measured in vivo by F2-isoprostanes) and DNA adduct formation (measured in vivo by 8-hydroxy-2′-deoxyguanosine [8-OHdG]).1,4
As oxidative and nitrosative processes continue, and cellular modification increases, physiologic function becomes altered secondary to impaired cellular messaging.4 As such, there is considerable overlap in the pathogenesis of metabolic disease, environmental toxicity, and physiologic aging. Supporting this concept, total glutathione concentration appears to declines with aging, as demonstrated in both rat models of aging and in human aging (particularly after age 45 in humans), possibly due to a reduced ability to synthesize GSH.5–7 Suboptimal GSH concentration has been associated with aging-related induction of oxidized and glycated proteins, similar to metabolic disease.4 Furthermore, reduced glutathione concentration and/or a disproportionate ratio of GSH:GSSG has been associated with a number of diseases, including cancer, human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), hepatitis, type 2 diabetes, Parkinson's disease, and cystic fibrosis.2,8–14 Although no experimental research to date has demonstrated disease prevention from GSH supplementation or other strategies to specifically increase GSH, observational research suggests increased dietary glutathione intake has been associated with reduced risk for oral cancer.
Because of the theoretical benefits of maintaining antioxidant defenses to combat various acute and chronic diseases, reducing the consequences of aging and reducing tissue damage from exposure to environmental oxidants, therapeutic approaches to increase systemic and/or tissue-specific glutathione concentrations are of considerable investigational interest. Erythrocyte GSH (RBC GSH) serves as a convenient biological reserve in which to measure GSH status, and several valid measurement methods are available, with enzymatic recycling being the most sensitive.15–18 Importantly, the reactive nature of GSH causes challenges in sample stability and necessitates correct sample collection methods.19 The bioavailability of orally administered cysteine is believed to be the rate-limiting step for the synthesis of glutathione,10 although the amino acids glutamate/glutamine are also integral to GSH synthesis.11,20 Most studies aimed to increase GSH concentration have used glutathione precursors, such as N-acetylcysteine or sodium salts of GSH,2 based on the assumption that oral reduced GSH is poorly absorbed and/or oxidized in the gastrointestinal tract. However, to lesser degrees, intravenous, intramuscular, intrabronchial (i.e., nebulized), and intranasal glutathione administrations have all been used to increase circulating or tissue-specific GSH levels.12,13,21
Minimal experimental evidence has demonstrated significant increases in blood or intracellular levels of GSH through oral supplementation with reduced GSH. Animal in vivo data suggest that oral GSH is absorbed in rats.6,7 Specifically, Hagen et al. demonstrated a doubling of plasma GSH (15–30 μmol/L) within 120 minutes after oral administration in rats,7 and demonstrated dietary GSH is absorbed through a principal absorption site in the jejunum.7 However, the absorption of GSH in humans has not been adequately demonstrated, and may prove more challenging. The human gastrointestinal tract contains significant amounts of the enzyme GGT, which recycles GSH precursors and may prevent significant intact absorption of GSH per se from oral supplementation. Few human clinical trials have evaluated the effects of oral GSH supplementation. Witschi et al. administered a single oral dose of up to 3 g of GSH to seven healthy subjects and did not observe an increase in blood GSH levels, concluding “it is not feasible to increase circulating GSH to a clinically beneficial extent by the oral administration of 3 g of GSH.”22 Yet three studies have evaluated the impact of reduced GSH supplementation combined with oxidative chemotherapeutic agents in patients with various cancers23–25; all three trials demonstrated reduced adverse effects of the chemotherapy without negative effects on the effectiveness of the regimen, suggesting some physiologic effect from oral dosing. Unfortunately GSH status was not measured in any of these trials.
In order to evaluate the impact of longer term, oral GSH supplementation on GSH status and in vivo oxidation status, this study performed the first randomized, double-blinded, placebo-controlled trial of orally administered GSH in healthy, adult humans. Because GSH status is dynamic, the outcome measures were extended beyond GSH status alone to include 8-OHdG and F2-isoprostanes (F2-isoP), in case a change in oxidation status was exemplified by evidence of reduced oxidation products rather than a change in GSH or GSH:GSSG ratio (i.e., improvement in tissue-level oxidative stress not represented by RBC indices). It was hypothesized that there would be measurement of a direct increase in RBC GSH concentration, or an increase in GSH:GSSG ratio, or a reduction in at least one biomarker of oxidation products (i.e., 8-OHdG or F2-isoP).
Experimental Procedure
Study design
A randomized, double-blind, placebo-controlled trial in health human adults was conducted. Oral glutathione capsules (500 mg twice per day) or placebo capsules (dicalcium phosphate, 500 mg twice per day) were self-administered 15 minutes before breakfast and dinner for 4 weeks. Outcome measures included 8-OHdG, F2-isoP, and RBC GSH, GSSG, and GSH:GSSG and were determined at baseline and following 4 weeks of supplementation.
Participants
Forty (40) healthy, nonsmoking men (n=7) and women (n=33) aged 21–62 years (mean=40.7±11.8) with body–mass index ranging from 19 to 29 kg/m2 provided written informed consent participated in the study. Clinical procedures were performed at the Bastyr Center for Natural Health (Seattle, WA). Eligibility was established with a telephone questionnaire followed by a clinical examination to obtain height, weight, blood pressure, and screening hematology and blood chemistry. All enrolled participants were weight stable, free from acute or chronic diseases, and did not eat a diet or take medications that might interfere with outcome markers including antioxidant supplements, high intake of dietary polyphenols including coffee, or anti-inflammatory medications. This clinical trial was reviewed and approved by the Institutional Review Board of Bastyr University (Kenmore, WA).
Study drug
l-Glutathione was provided by Kohjin Co. Ltd., Japan. Kohjin's l-glutathione has qualified for GRAS (Generally Recognized As Safe) status. Kosher and Halal certified, Kohjin's GSH is manufactured through a fermentation process using Food and Drug Administration–approved nongenetically modified torula yeast in a current Good Manufacturing Practices–certified laboratory facility. GSH and placebo pills were encapsulated by Natural Factors, Coquitlam, British Columbia, Canada. The batch of GSH used in this study was analyzed to verify physical, chemical, and microbiological parameters. The GSH study drug contained no less than 98% reduced glutathione, <1.4% oxidized glutathione, and was free of microbial contamination and heavy metals.
Blood and urine sample collection
Blood samples were collected by venipuncture after an overnight fast according to standard laboratory procedures. Red blood cells were washed, lysed, and mixed (<10 minutes) with a precipitating acid solution to minimize spontaneous oxidation of GSH.19 Samples were placed on ice and stored at −70°C for batch analysis; all samples were measured within 90 days of collection. First morning urine samples were collected by the participants at home, brought to the clinic and frozen (-70°C). All urines samples were assayed in batches for 8-OHdG and F2-isoP. Urinary creatinine was measured from the same urine samples in order to standardize measurements.
Urinary 8-hydroxydeoxyguanosine and F2-isoprostane measurements
Urine samples were analyzed by Genox Corp. (Baltimore, MD) using a competitive enzyme-linked immunosorbent assay (ELISA) for quantification of both 8-OHdG and F2-isoP. Briefly, after thawing, 50-μL samples and standards were added to 8-OHdG conjugate plates followed by the primary antibody solution. Samples were incubated for 1 hour at 37°C. Plates were washed and a secondary antibody solution was applied for 1 hour at 37°C. After washing, 100 μL of a chromagenic substrate (3,3′5,5′-tetramethylbenzidene) was added and allowed to react for 15 minutes. Optical density of each sample was measured at 490 nm and compared to a standard curve to determine final concentration.19
Red blood cell glutathione measurement
Glutathione was measured using enzymatic recycling methodology.18 Samples were analyzed in triplicate by Kronos Laboratory (Phoenix, AZ) for total GSH, GSSG, reduced GSH, and the ratio (GSH:GSSG).
Dietary intake
A food frequency questionnaire (FFQ) was completed at the beginning of the study. Dietary intake of cysteine, glutamate, and methionine was quantified based on FFQ at the Fred Hutchinson Cancer Research Center, Seattle, WA. Dietary intake was measured for possible adjustment purposes in our final analysis.
Adverse events
Possible adverse events were recorded using the Monitoring of Side Effects Scale questionnaire (DSHS Form 10-334) completed at baseline, week 2, and week 4.
Adherence
Adherence was assessed by interview and pill count at week 2 and 4 clinical visits.
Data analysis
Data were analyzed using SPPS version 15.0 (SPSS Inc., Chicago, IL). The distributions of all outcome variables were confirmed to be normally distributed, allowing analysis of variance and paired t tests to evaluate the primary endpoint(s) of differences in the mean change in urinary 8-OHdG, urinary F2-isoP, and RBC GSH:GSSG from baseline to week 4 compared between the active and placebo groups. Secondary endpoints included changes in RBC GSH and GSSG concentrations and adverse events. Changes in routine blood chemistries including electrolytes, hepatic, and renal functioning were also measured and compared between groups to confirm safety.
Results
Thirty-nine (39) participants completed the study; 1 participant was lost to follow-up after her baseline visit. Because our primary analyses related to mechanistic efficacy rather than effectiveness, baseline data from the 1 withdrawal was not included and final data were not estimated, rather these data were omitted from the final analysis. Subjects were generally similar between the two groups (Table 1).
Table 1.
Baseline Characteristics of Trial Participants
| Characteristic | l-Glutathione (N=20) | Placebo (N=20) | p-Value |
|---|---|---|---|
| Demographics | |||
| Age (yr) | 42.8 (11.9) | 38.7 (11.5) | 0.28 |
| Female gender (%) | 20 | 15 | — |
| Anthropometrics | |||
| BMI | 23.7 (3.0) | 23.6 (1.9) | 0.85 |
| Systolic BP | 122.4 (13.1) | 119.5 (10.4) | 0.46 |
| Diastolic BP | 79.3 (9.9) | 76.8 (7.3) | 0.38 |
| Serum biochemical levels | |||
| Glucose, fasting (mg/dL) | 87.9 (12.6) | 92.6 (12.3) | 0.24 |
| Dietary intake | |||
| Cysteine (g/day) | 1.2 (0.7) | 0.8 (0.2) | 0.04 |
| Glutamic acid (g/day) | 15.4 (8.4) | 11.0 (2.9) | 0.04 |
| Methionine (g/day) | 1.7 (1.1) | 1.2 (0.4) | 0.06 |
| Total protein (g/day) | 80.5 (46.5) | 55.9 (15.3) | 0.04 |
| Oxidative stress indices | |||
| 8-OHdG (μg) | 26.5 (19.0) | 20.6 (9.8) | 0.23 |
| 8-OHdG/creatinine (μg/mg) | 0.2 (0.1) | 0.2 (0.1) | 0.25 |
| F2-isoP (ng) | 847.5 (674.3) | 730.2 (616.8) | 0.57 |
| F2-isoP/creatinine (ng/mg) | 6.0 (2.8) | 5.3 (2.9) | 0.46 |
| GSH, reduced (μmol/L) | 832.7 (215.1) | 751.0 (326.3) | 0.36 |
| GSSG, oxidized (μmol/L) | 25.8 (9.2) | 26.2 (16.2) | 0.92 |
| GSH:GSSG, ratio | 34.6 (11.3) | 34.6 (17.3) | 0.99 |
| Total GSH (μmol/L) | 858.5 (220.8) | 777.2 (337.9) | 0.38 |
BMI, body–mass index; BP, blood pressure; 8-OHdG, 8-hydroxy-2′-deoxyguanosine; F2-isoP, F2-isoprostanes; GSH, glutathione; GSSG, oxidized glutathione.
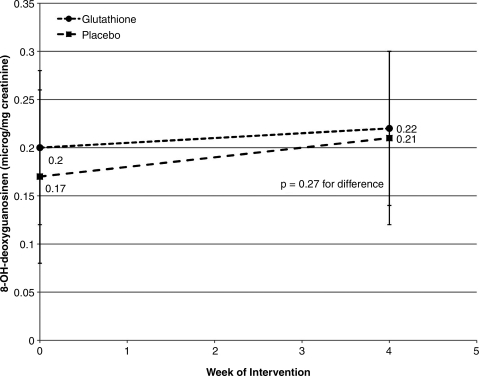
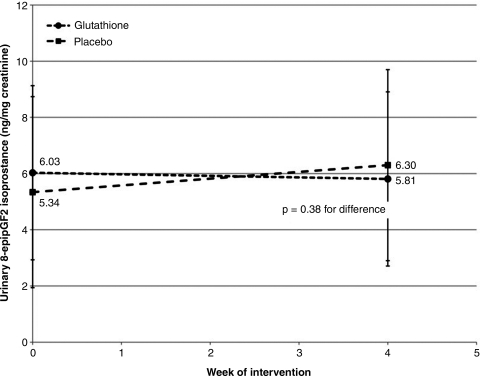
Differences between groups for observed changes in primary or secondary endpoints were without notable trend and did not reach statistical significance (Figs. 1–3). Differences in creatinine-standardized urinary F2-isoP between baseline and week 4 were unchanged following supplementation (0.0±0.1 ng/mg creatinine versus 0.0±0.1 ng/mg creatinine, p=0.38). Similarly, differences in 8-OHdG were not statistically significant between treatment groups (-0.2±3.3 μg/mg creatinine versus 1.0±3.2 μg/mg creatinine, p=0.27).
FIG. 1.
Effect of oral glutathione (GSH) on urinary 8-hydroxy-2′-deoxyguanosine. This figure demonstrates no effect of oral GSH supplementation at 500 mg twice daily for 4 weeks on creatinine-standardized, urinary GSH concentration in healthy, human volunteers; p=0.27 for between-group difference in mean change.
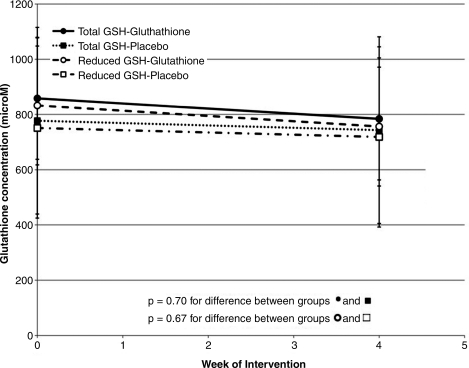
FIG. 3.
Effects of oral glutathione (GSH) on in vivo erythrocyte glutathione indices. This figure demonstrates no effect of oral GSH supplementation at 500 mg twice daily compared to placebo for 4 weeks on glutathione indices, including reduced GSH and total GSH concentration, in healthy, human volunteers; p=0.70 and 0.67 for between-group difference in mean change for total GSH and reduced GSH, respectively.
FIG. 2.
Effect of oral glutathione (GSH) on urinary isoprostanes. This figure demonstrates no effect of oral GSH supplementation at 500 mg twice daily compared to placebo for 4 weeks on creatinine-standardized, urinary F2-isoprostane concentration in healthy, human volunteers; p=0.38 for between-group difference in mean change.
Concentrations of reduced, oxidized, total (GSH+GSSG) and ratio measures of GSH status were unchanged by oral supplementation; p>0.05 for each between-group comparison. Differences in total reduced GSH were: −74.5±294.8 μmol/L versus −34.5±334.7 μmol/L for the GSH group and the placebo group, respectively (p=0.67 between groups). Similarly, the ratios of reduced GSH to GSSG were not significantly different between treatment groups; final ratios were −3.6±2.6 versus −3.6±3.5 for GSH and placebo, respectively (p=0.99 between groups). Trends were apparent for differences in baseline intake of cysteine, methionine, and total protein intake; however, baseline GSH status showed no association with dietary intake of precursor amino acids or total dietary protein, suggesting our results were not confounded by these differences (p>0.05 for all associations between GSH indices and dietary amino acid intake for all participants, data not shown).
No serious or systematic adverse effects were reported. There were no isolated or combined alterations in clinical laboratory measures, including hematology, hepatic, and renal function tests during GSH supplementation. Side-effects were generally mild, and included the following: 5 participants reported increased flatulence and loose stools; 2 participants reported flushing; and 1 participant reported weight gain. Adherence was calculated as >90% for all participants in each group.
In summary, despite high adherence to oral supplementation and a low frequency of side-effects, oral glutathione supplementation did not result in significant changes in biomarkers of lipid peroxidation, DNA adduct formation or glutathione status in healthy adults.
Discussion
To the authors' knowledge, this double-blind, randomized, placebo-controlled clinical trial is the first well-controlled and longest clinical trial of oral GSH supplementation performed in adult humans. However, despite the biochemical plausibility, optimal dose, recommended timing of administration, and appropriate choice of outcome measures, no significant changes were observed in oxidative stress biomarkers or erythrocyte glutathione concentrations following 4 weeks of oral GSH supplementation in healthy adults.
This study has important limitations. Despite 80% power to detect a twofold reduction in 8-OHdG status compared to placebo, no significant differences were detected between groups or within group between baseline and postintervention. The lack of a suggestion of trend and the lack of a point estimate suggesting an increase in GSH status or reduction in oxidative stress suggest type 2 error is not responsible for our findings. Yet it cannot be ruled out that a response may be possible in a larger sample, a longer duration of supplementation, and/or a shorter assessment period (hours–days versus weeks). Also, these findings in healthy adults are unable to be generalized to individuals with acute or chronic health conditions associated with reduced glutathione status, including metabolic syndrome, diabetes, neurodegenerative disease, cancer, HIV/AIDS, pulmonary disease, and/or acute infection.14
Additional limitations may be present in the measurement methods in this study. The authors are confident in their results for measures of glutathione, as “gold-standard” methodology was employed in this trial. However, immunoassays for measurement of 8-OHdG and F2-isoP have been criticized, although results employing this technology being widely published.26–28 Recent publications critiquing ELISA-based 8-OHdG methods suggest that ELISA-based values tended to be higher values than “gold-standard” LC/MS-based measurement.29 However, several steps were taken in this trial protocol to reduce sources of variability that should have improved the ability to detect a trend for change, if one was present. Because change in status was compared from baseline to week 4 between groups, the exact concentration of each biomarker is less important than the net change; thus, these differences in measurement methods may not be significant to this study. Also, the samples were stored properly at −70°C from collection until measurement and all measurements were batched and performed by the same technician in order to reduce measurement variability. Also, GSH status was measured in the fasting state only, defined by the protocol as at least a 12-hour, overnight fast, further reducing possible sources of variability. Finally, Garratt et al. reported that ELISA and LC/MS methods were moderately highly correlated (r=0.63, p<0.0001), suggesting that any overall trend for change in status should have been evident, despite this study's choice of ELISA-based measurement methods.
Although the choice in this study to measure oxidation status in the fasting state likely reduced variability in the final measures, this choice was also a limitation. Given that postprandial oxidative stress occurs, and impacts short-term vascular function, it is possible that GSH supplementation has transient positive effects on postprandial oxidative stress and/or endothelial function, which was not evaluated in this trial.30
Although the clinical use of GSH and GSH precursors is rather limited to the use of N-acetylcysteine in acetaminophen toxicity, as a mucolytic in respiratory disease, and experimentally in the prevention of contrast-induced nephropathy, this study has important clinical implications for consumers and for providers in nonallopathic disciplines, such as complementary and alternative medicine, in which oral nutritional supplementation is commonplace.31–35 The results of this study determined that short-term, oral intake of GSH does not improve glutathione status, nor reduce markers of oxidative stress in healthy adults, and thus routine supplementation may not offer health benefits in the absence of disease or oxidative challenge.
Conclusions
Research should continue to investigate potential therapeutics to modify glutathione balance because of the importance of glutathione in health and disease. Future studies of oral GSH administration should consider reduced GSH status as an inclusion criteria, demonstrate increased levels of oxidative stress in study candidates prior to inclusion, and/or be performed in populations with increased oxidative burden (e.g., type 2 diabetes). Given the apparent safety of reduced GSH supplementation, safety is not an apparent barrier to continued clinical trials of GSH supplementation in relevant disease states.
Acknowledgments
This clinical trial was funded by Kohjin Co., Ltd., Japan, and the authors were supported in part by the National Center for Complementary and Alternative Medicine (NIH/NCCAM), (1 K99 AT004711-01A1) and the National Center for Research Resources (1KL2RR025015-01). We would like to thank Mr. Phillip Palmer for his assistance in study coordination, Dr. Jamey Wallace for use of the Bastyr Center for Natural Health for clinical procedures, and Vanessa Bolejack for her statistical support.
Disclosure Statement
No competing financial interests exist.
References
- 1.Dickinson DA. Forman HJ. Glutathione in defense and signaling: Lessons from a small thiol. Ann N Y Acad Sci. 2002;973:488–504. doi: 10.1111/j.1749-6632.2002.tb04690.x. [DOI] [PubMed] [Google Scholar]
- 2.Exner R. Wessner B. Manhart N. Roth E. Therapeutic potential of glutathione. Wien Klin Wochenschr. 2000;112:610–616. [PubMed] [Google Scholar]
- 3.Lee DH. Steffes MW. Jacobs DR., Jr Can persistent organic pollutants explain the association between serum gamma-glutamyltransferase and type 2 diabetes? Diabetologia. 2008;51:402–407. doi: 10.1007/s00125-007-0896-5. [DOI] [PubMed] [Google Scholar]
- 4.Evans JL. Goldfine ID. Maddux BA. Grodsky GM. Oxidative stress and stress-activated signaling pathways: A unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- 5.Liu RM. Dickinson DA. Decreased synthetic capacity underlies the age-associated decline in glutathione content in Fisher 344 rats. Antioxid Redox Signal. 2003;5:529–536. doi: 10.1089/152308603770310176. [DOI] [PubMed] [Google Scholar]
- 6.Favilli F. Marraccini P. Iantomasi T. Vincenzini MT. Effect of orally administered glutathione on glutathione levels in some organs of rats: Role of specific transporters. Br J Nutr. 1997;78:293–300. doi: 10.1079/bjn19970147. [DOI] [PubMed] [Google Scholar]
- 7.Hagen TM. Wierzbicka GT. Bowman BB, et al. Fate of dietary glutathione: Disposition in the gastrointestinal tract. Am J Physiol. 1990;259:G530–G535. doi: 10.1152/ajpgi.1990.259.4.G530. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia S. Shukla R. Venkata Madhu S, et al. Antioxidant status, lipid peroxidation and nitric oxide end products in patients of type 2 diabetes mellitus with nephropathy. Clin Biochem. 2003;36:557–562. doi: 10.1016/s0009-9120(03)00094-8. [DOI] [PubMed] [Google Scholar]
- 9.Jariwalla RJ. Lalezari J. Cenko D, et al. Restoration of blood total glutathione status and lymphocyte function following alpha-lipoic acid supplementation in patients with HIV infection. J Altern Complement Med. 2008;14:139–146. doi: 10.1089/acm.2006.6397. [DOI] [PubMed] [Google Scholar]
- 10.Wu G. Fang YZ. Yang S, et al. Glutathione metabolism and its implications for health. J Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 11.Klimberg VS. McClellan JL. Organ CH., Jr Honorary lectureship: Glutamine, cancer, and its therapy. Am J Surg. 1996;172:418–424. doi: 10.1016/s0002-9610(96)00217-6. [DOI] [PubMed] [Google Scholar]
- 12.Prousky J. The treatment of pulmonary diseases and respiratory-related conditions with inhaled (nebulized or aerosolized) glutathione. Evid Based Complement Altern Med. 2008;5:27–35. doi: 10.1093/ecam/nem040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauser RA. Lyons KE. McClain T, et al. Randomized, double-blind, pilot evaluation of intravenous glutathione in Parkinson's disease. Mov Disord. 2009;24:979–983. doi: 10.1002/mds.22401. [DOI] [PubMed] [Google Scholar]
- 14.Franco RSO. Pappa A. Panayiotidis MI. The central role of glutathione in the pathophysiology of human disease. Arch Physiol Biochem. 2007;113:234–258. doi: 10.1080/13813450701661198. [DOI] [PubMed] [Google Scholar]
- 15.Araujo AR. Saraiva ML. Lima JL. Determination of total and oxidized glutathione in human whole blood with a sequential injection analysis system. Talanta. 2008;74:1511–1519. doi: 10.1016/j.talanta.2007.09.028. [DOI] [PubMed] [Google Scholar]
- 16.Redegeld FA. van Opstal MA. Houdkamp E. van Bennekom WP. Determination of glutathione in biological material by flow-injection analysis using an enzymatic recycling reaction. Anal Biochem. 1988;174:489–495. doi: 10.1016/0003-2697(88)90048-6. [DOI] [PubMed] [Google Scholar]
- 17.Tietze F. Enzymic method for quantitative determination of nanogram amounts of total and oxidized glutathione: Applications to mammalian blood and other tissues. Anal Biochem. 1969;27:502–522. doi: 10.1016/0003-2697(69)90064-5. [DOI] [PubMed] [Google Scholar]
- 18.Rahman I. Kode A. Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 19.Roberts JC. Francetic DJ. The importance of sample preparation and storage in glutathione analysis. Anal Biochem. 1993;211:183–187. doi: 10.1006/abio.1993.1254. [DOI] [PubMed] [Google Scholar]
- 20.Amores-Sanchez MI. Medina MA. Glutamine, as a precursor of glutathione, and oxidative stress. Mol Genet Metab. 1999;67:100–105. doi: 10.1006/mgme.1999.2857. [DOI] [PubMed] [Google Scholar]
- 21.Allen J. Inhaled glutathione for the prevention of air pollution-related health effects: A brief review. Altern Ther Health Med. 2008;14:42–44. [PubMed] [Google Scholar]
- 22.Witschi A. Reddy S. Stofer B. Lauterburg BH. The systemic availability of oral glutathione. Eur J Clin Pharmacol. 1992;43:667–669. doi: 10.1007/BF02284971. [DOI] [PubMed] [Google Scholar]
- 23.Milla P. Airoldi M. Weber G, et al. Administration of reduced glutathione in FOLFOX4 adjuvant treatment for colorectal cancer: Effect on oxaliplatin pharmacokinetics, Pt-DNA adduct formation, and neurotoxicity. Anticancer Drugs. 2009;20:396–402. doi: 10.1097/CAD.0b013e32832a2dc1. [DOI] [PubMed] [Google Scholar]
- 24.Smyth JF. Bowman A. Perren T, et al. Glutathione reduces the toxicity and improves quality of life of women diagnosed with ovarian cancer treated with cisplatin: Results of a double-blind, randomised trial. Ann Oncol. 1997;8:569–673. doi: 10.1023/a:1008211226339. [DOI] [PubMed] [Google Scholar]
- 25.Cascinu S. Cordella L. Del Ferro E, et al. Neuroprotective effect of reduced glutathione on cisplatin-based chemotherapy in advanced gastric cancer: A randomized double-blind placebo-controlled trial. J Clin Oncol. 1995;13:26–32. doi: 10.1200/JCO.1995.13.1.26. [DOI] [PubMed] [Google Scholar]
- 26.Gmitterova K. Heinemann U. Gawinecka J, et al. 8-OHdG in cerebrospinal fluid as a marker of oxidative stress in various neurodegenerative diseases. Neurodegener Dis. 2009;6:263–269. doi: 10.1159/000237221. [DOI] [PubMed] [Google Scholar]
- 27.Himmetoglu S. Dincer Y. Bozcali E, et al. Oxidative DNA damage and antioxidant defense after reperfusion in acute myocardial infarction. J Investig Med. 2009;57:595–599. doi: 10.2310/JIM.0b013e31819d87c1. [DOI] [PubMed] [Google Scholar]
- 28.Yano T. Shoji F. Baba H, et al. Significance of the urinary 8-OHdG level as an oxidative stress marker in lung cancer patients. Lung Cancer. 2009;63:111–114. doi: 10.1016/j.lungcan.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Garratt LW. Mistry V. Singh R, et al. Interpretation of urinary 8-oxo-7,8-dihydro-2′-deoxyguanosine is adversely affected by methodological inaccuracies when using a commercial ELISA. Free Radic Biol Med. 2010;48:1460–1464. doi: 10.1016/j.freeradbiomed.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 30.Ceriello A. Taboga C. Tonutti L, et al. Evidence for an independent and cumulative effect of postprandial hypertriglyceridemia and hyperglycemia on endothelial dysfunction and oxidative stress generation: Effects of short- and long-term simvastatin treatment. Circulation. 2002;106:1211–1218. doi: 10.1161/01.cir.0000027569.76671.a8. [DOI] [PubMed] [Google Scholar]
- 31.Bradley R. Oberg EB. Calabrese C. Standish LJ. Algorithm for complementary and alternative medicine practice and research in type 2 diabetes. J Altern Complement Med. 2007;13:159–175. doi: 10.1089/acm.2006.6207. [DOI] [PubMed] [Google Scholar]
- 32.Bradley R. Kozura E. Buckle H, et al. Description of clinical risk factor changes during naturopathic care for type 2 diabetes. J Altern Complement Med. 2009;15:633–638. doi: 10.1089/acm.2008.0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bradley R. Kozura E. Kaltunas J, et al. Observed changes in risk during naturopathic treatment of hypertension. Evid Based Complement Alternat Med. 2011;2011:826751. doi: 10.1093/ecam/nep219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shavit L. Korenfeld R. Lifschitz M, et al. Sodium bicarbonate versus sodium chloride and oral N-acetylcysteine for the prevention of contrast-induced nephropathy in advanced chronic kidney disease. J Interv Cardiol. 2009;22:556–563. doi: 10.1111/j.1540-8183.2009.00500.x. [DOI] [PubMed] [Google Scholar]
- 35.Sadowska AM. Verbraecken J. Darquennes K. De Backer WA. Role of N-acetylcysteine in the management of COPD. Int J Chron Obstruct Pulmon Dis. 2006;1:425–434. doi: 10.2147/copd.2006.1.4.425. [DOI] [PMC free article] [PubMed] [Google Scholar]