Abstract
The radical S-adenosylmethionine (AdoMet) superfamily currently comprises thousands of proteins that participate in numerous biochemical processes across all kingdoms of life. These proteins share a common mechanism to generate a powerful 5′-deoxyadenosyl radical, which initiates a highly diverse array of biotransformations. Recent studies are beginning to reveal the role of radical AdoMet proteins in the catalysis of highly complex and chemically unusual transformations, e.g. the ThiC-catalyzed complex rearrangement reaction. The unique features and intriguing chemistries of these proteins thus demonstrate the remarkable versatility and sophistication of radical enzymology.
Keywords: Biosynthesis, Enzyme Mechanisms, Enzymes, Radicals, S-Adenosylmethionine (SAM)
Introduction
These past 2 decades have witnessed an explosive growth of knowledge concerning enzymes that involve free radical-mediated catalysis (1). Just like the enormous number of methodologies involving radicals created by chemists for organic synthesis over recent years (2), nature has evolved diverse strategies for utilization of radical chemistry to benefit life processes. The high reactivity of radical species not only promotes the chemically difficult reactions by overcoming the high kinetic and/or thermodynamic barriers but also leads to some exquisite transformations that are unlikely to occur via polar mechanisms. One prominent example in radical biochemistry is the large class of radical S-adenosylmethionine (AdoMet)3 enzymes, which perform catalysis with AdoMet and specialized [4Fe-4S] clusters through a radical-associated mechanism (3–15).
Since the discovery of the first member, lysine 2,3-aminomutase (LAM), in 1970 (16), the radical AdoMet superfamily is now believed to comprise thousands of members that participate in numerous biochemical processes across all kingdoms of life. These proteins contain a motif of three cysteine residues (typically as CXXXCXXC) to nucleate a [4Fe-4S] cluster, which binds AdoMet in a bidentate fashion and serves as an electron donor to cleave AdoMet reductively (Fig. 1A). A powerful oxidant 5′-deoxyadenosyl (Ado) radical is thus generated, which abstracts a hydrogen atom from the substrate and initiates a highly diverse array of reactions relevant to modifications not only of nucleic acid and protein biosynthesis but also of vitamin, coenzyme, and antibiotic biosynthesis. Bioinformatic studies guided by known crystal structures have revealed that, despite low shared homology, radical AdoMet superfamily proteins all possess a conserved TIM barrel core fold to accommodate AdoMet and different substrates ranging from small molecules to proteins, DNA, and RNA (17). The Ado radical chemistry is also found in adenosylcobalamin (AdoCbl)-dependent family enzymes. In this case, the Ado radical is produced via the homolytic cleavage of the cobalt–carbon bond of the corrin cofactor (10, 18, 19). However, the number and diversity of the AdoCbl-dependent enzymes are in no way comparable with those of the radical AdoMet enzymes. It has been proposed that radical AdoMet proteins are of ancient origin and among the earliest biological catalysts (5). This is perhaps not surprising in view of the huge number, wide distributions, and catalytic diversity of radical AdoMet enzymes.
FIGURE 1.
Reactions of the radical AdoMet enzymes. A, binding of AdoMet to the [4Fe-4S] cluster and the reversible carbon–sulfur bond cleavage to produce the Ado radical. The unique iron for binding of AdoMet is highlighted in yellow. B, ThiC-catalyzed formation of HMP-P from AIR. The unexpected deuterium labeling patterns are highlighted in the yellow. C, NosL/NocL-catalyzed MIA biosynthesis.
The reactions catalyzed by the radical AdoMet superfamily include mainly glycyl radical generation, sulfur insertion, methylation, methylthiolation, oxidation, isomerization, elimination (fragmentation), etc. Table 1 is a representative but not comprehensive collection of the typical reactions of radical AdoMet proteins. A more fascinating aspect of radical AdoMet proteins is their potential to catalyze highly complex structural rearrangements. The complexity of some of these reactions is astonishing. For example, in the conversion of aminoimidazole ribonucleotide (AIR) to 4-amino-5-hydroxymethyl-2-methylpyrimidine phosphate (HMP-P) catalyzed by ThiC (20, 21), 10 of 15 connections between each atom (not including hydrogen atoms) are broken, leading to the product HMP-P with a completely reprogrammed atom connection pattern (Fig. 1B). The fact that such a chemically complicated conversion can be actually catalyzed by such a single monofunctional enzyme is amazing, extending far beyond our initial understanding of enzymatic reactions.
TABLE 1.
Reactions of radical AdoMet enzymes and their representative examples
ARR, anaerobic ribonucleotide reductase; BSS, benzylsuccinate synthase; HPD, 4-hydroxyphenylacetate decarboxylase.
| Reaction/enzyme | Function | Ref. |
|---|---|---|
| Protein radical generation | ||
| PFL activase | PFL glycyl radicalization | 5, 10 |
| ARR activase | ARR glycyl radicalization | 5, 10 |
| BSS activase | BSS glycyl radicalization | 5, 10 |
| GDH activase | GDH glycyl radicalization | 5, 10 |
| HPD activase | HPD glycyl radicalization | 5, 10 |
| Sulfur insertion | ||
| BioB | Biotin synthesis | 5, 10 |
| LipA | Lipoyl synthesis | 5, 10 |
| Oxidation | ||
| Formylglycine synthase | Anaerobic sulfatase maturation | 5, 10 |
| BtrN | Butirosin biosynthesis | 10 |
| Isomerization | ||
| LAM | LAM | 5, 10, 16 |
| BlsG | Arginine 2,3-aminomutase | 5, 10 |
| Eam | Glutamate 2,3-aminomutase | 5, 10 |
| Littorine mutase | Alkaloid biosynthesis | 5 |
| Methylation of aromatic heterocycle | ||
| RlmN, Cfr | rRNA modification | 67–69, 73 |
| TsrT (TsrM) | Thiostrepton biosynthesis | 74, 75 |
| NosN/NocN | Nosiheptide/nocathiacin biosynthesis | 37, 38 |
| TpdI, TpdL, TpdU | GE2270 and thiomuracin biosynthesis | 79 |
| CloN6 | Clorobiocin biosynthesis | 5 |
| Methylation of sp3 carbon | ||
| Fom3 | Fosfomycin biosynthesis | 5, 10 |
| Fms7 | Fortimicin biosynthesis | 5 |
| CndI | Chondrochloren biosynthesis | 80 |
| Elimination (fragmentation) | ||
| SplB | Spore photoproduct lyase | 5, 10 |
| DesII | Desosamine biosynthesis | 64, 65 |
| HemN | Coproporphyrinogen III oxidase | 5, 10 |
| ThiH | Thiamine biosynthesis | 26, 39 |
| HydG | Metal cofactor biosynthesis | 40, 41 |
| Methylthiolation | ||
| MiaB | Methylthiolation of tRNA | 5, 10 |
| RimO | Methylthiolation of protein | 5, 10 |
| Complex structural rearrangement | ||
| ThiC | Thiamine biosynthesis | 20, 21, 30 |
| NosL/NocL | Nosiheptide/nocathiacin biosynthesis | 37, 42, 43 |
| MoaA | Moco biosynthesis | 51, 52 |
| QueE | Deazapurine biosynthesis | 63 |
| Others | ||
| CofG, CofH | Coenzyme F420 biosynthesis | 5 |
| TWY1 | Wybutosine biosynthesis | 5, 10 |
| PqqE | Pyrroloquinoline quinone biosynthesis | 5 |
| AviX12 | Epimerization in avilamycin A | 5 |
Two aspects should be underscored for appreciation of these amazing catalysts. First, to achieve a complex transformation, the enzyme uses multiple steps specifically designed to sequentially tailor the substrate and radical intermediates in a strictly controlled pathway. This is an ingenious but very complicated process, the elucidation of which will surely provide valuable insights into our understanding of radical enzymology. Second, it is noteworthy that distinct from many radical enzymes, where the radicals are usually associated with metal centers or reside in conjugated systems, such as flavin, thiamine, pterin, and quinone, etc., many of the radicals produced by radical AdoMet enzymes are free alkyl radicals. Because these radicals are usually extremely reactive and unstable, how the enzymes accurately control the reactivity of these radicals in a regio- and stereospecific fashion in the multistep conversions currently seems a biochemical puzzle. Although little is known about the detail, accumulating evidence indicates that both the amino acid residues in the active site and the overall protein scaffold may play an important role in controlling the radical chemistry (22). Engineering the key protein residues to accommodate different substrate analogs or to reprogram the catalytic pathway to afford novel outcomes of catalysis is thus promising, as the latter was actually achieved in the study of biotin synthase (23).
This minireview focuses primarily on recent studies regarding some complex and chemically unusual transformations catalyzed by radical AdoMet enzymes. “Complex” refers mainly to the multistep process, whereas “unusual” means the unprecedented nature and/or the unique mechanism of the reactions. We attempt to demonstrate here that the unique features and intriguing chemistries of these enzymes not only constitute a fascinating puzzle for aficionados of radical mechanism but also facilitate the understanding of the versatility of radical enzymology.
HMP-P Synthase ThiC in Thiamine Biosynthesis
Thiamine (vitamin B1) is an essential compound in all living organisms and participates in several key cellular processes, such as carbohydrate and amino acid metabolism (24). Thiamine consists of a thiazole and a pyrimidine heterocycle, which are synthesized separately and assembled together by thiamine phosphate synthase (25). The thiazole and pyrimidine of thiamine are biosynthesized through different pathways in different organisms. In prokaryotes, thiazole formation is well understood and involves at least five enzymes that transform 1-deoxy-d-xylulose 5-phosphate, cysteine, and either glycine (aerobic pathway) or tyrosine (anaerobic pathway) in a sequential condensation (25, 26). In contrast, pyrimidine biosynthesis in prokaryotes remained enigmatic for some time. Although extensive genetic and biochemical studies had been carried out (27, 28), the mechanism of pyrimidine formation came to light only recently with two independent reports on the functional characterization of the radical AdoMet enzyme ThiC (20, 21). ThiC is the only enzyme involved in catalyzing the complex transformation of AIR to HMP-P.
ThiC does not contain the canonical CXXXCXXC motif in the N-terminal domain, as do most of the radical AdoMet enzymes. Accordingly, it was not regarded as belonging to the radical AdoMet family in early studies. Instead, ThiC contains a CXXCXXXXC motif. The indispensability of this motif was confirmed by a mutational study showing that replacement of any cysteine residue with alanine abolished thiamine biosynthesis (29). Modeling a [4Fe-4S] cluster in the apo-ThiC crystal structure guided by a biotin synthase backbone supported the function of the CXXCXXXXC motif for binding a [4Fe-4S] cluster (16), which has been shown to exist in the reconstituted protein by EPR, UV-visible, and Mössbauer spectrometries (20, 21). The in vitro conversion of AIR to HMP-P is AdoMet-dependent, and 5′-deoxyadenosine (AdoH) is the coproduct in the reaction. Together, these results indicate that ThiC is a new member of the radical AdoMet enzyme superfamily, catalyzing the most complex rearrangement reaction known in primary metabolism.
The mechanism of ThiC catalysis has been probed by early isotopic labeling studies in vivo (27) and in vitro using cell free extracts (28), establishing the origin of all atoms in the HMP-P scaffold. Recent labeling studies in a defined ThiC reconstitution system revealed that the missing C1′ and C3′ of AIR are converted to formate and carbon monoxide, respectively (30). More insights have been gained by using deuterated AIR at various positions and by characterization of the resultant product AdoH by LC-MS and 1H NMR spectrometry. The optimal assay condition was set both by using flavodoxin, flavodoxin reductase, and NADPH to reduce the [4Fe-4S] cluster of ThiC and by obviation of the prolonged reaction time to minimize the uncoupled AdoH production. The assay showed that no deuterium was incorporated into AdoH with 1′-, 2′-, or 3′-labeled AIR, indicating that hydrogen abstraction does not occur on these sites. Surprisingly, deuterium incorporation was observed when both 4′-deuterated AIR and 5′-dideuterated AIR were used as substrates. When perdeuterated AIR was used as a substrate, monodeuterated AdoH and dideuterated AdoH were both observed in the reaction mixture. These results indicate that the Ado radical abstracts two hydrogen atoms at C4′ and C5′ during a single reaction cycle. The iterative hydrogen abstraction is unprecedented in radical AdoMet enzymology. In this case, the Ado radical serves not only as the initiator of the reaction but also as a “relay station” for radical transfer, exhibiting the ingenuity and sophistication of radical AdoMet family enzymes in catalyzing complex reactions. The proposed mechanism of this daunting catalysis is described in supplemental Fig. 1. It should be noted that the involvement of the protein-based radical(s), which was detected when ThiC was treated with AdoMet and sodium dithionite, cannot be excluded (31).
3-Methyl-2-indolic Acid (MIA) Synthase NosL/NocL in Thiopeptide Biosynthesis
Thiopeptides are a large group of naturally occurring, thiazole-containing, and highly modified macrocyclic peptides (32) that are biosynthesized through post-translational modifications on ribosomally synthesized precursor peptides (33, 34). According to the structural characteristics, thiopeptides are divided into five subgroups. The e-series subgroup members, such as nosiheptide and nocathiacin, possess a unique indolic acid moiety that is independent of the precursor peptide (32–34). Early studies in nosiheptide biosynthesis indicated that the indolic acid moiety was derived from l-Trp (35). The C1 and indole C2′ linkage of l-Trp in the conversion shown in Fig. 1C is quite reminiscent of Friedel-Crafts chemistry (36). However, Friedel-Crafts acylation generally requires a very strong Lewis acid (such as anhydrous aluminum chloride, etc.) as the catalyst, which cannot be provided under physiological conditions. Although extensive isotopic labeling studies had been performed (36), the mechanism of this conversion remained merely speculative.
Characterization of the biosynthetic gene clusters of nosiheptide (37) and nocathiacin (38) provided the basis for probing the mechanism of indolic acid moiety formation at the genetic and biochemical levels. The sequences allowed identification of the radical AdoMet enzymes NosL and NocL in nosiheptide and nocathiacin biosynthesis, respectively. NosL and NocL share high sequence similarity and are also homologous to ThiH, which is involved in anaerobic thiamine biosynthesis (26, 39), and HydG, which is required for [Fe-Fe] hydrogenase maturation (40, 41). Both ThiH and HydG are radical AdoMet enzymes that cleave the Cα–Cβ bond of l-Tyr. As indolic acid moiety synthesis also involves the Cα–Cβ (C2–C3) bond cleavage of aromatic amino acids, this indicates that NosL and NocL might be suitable candidates to catalyze this conversion. Indeed, nosiheptide production is completely abolished in the nosL deletion mutant strain and can be readily restored either by simple addition of synthetic MIA to the fermentation culture (37) or by heterologous expression of nocL in the mutant chromosome (38). The conversion of l-Trp to MIA has been reconstituted subsequently in vitro by using NosL (42) or NocL (43) as the only catalyst (Fig. 1C), demonstrating that NosL and NocL are radical AdoMet MIA synthases.
Two strategies have been employed in studying NosL/NocL, and they have contributed substantially to characterization of the enzyme mechanism. One is heterologous expression of the proteins in Escherichia coli. This obviates the inefficient in vitro conversion and produces a high yield of MIA, even without coexpression of the cysteine desulfurase gene iscS for [Fe-S] cluster assembly (44). A series of isotopic labeling experiments have thus been performed in this efficient heterologous expression system, establishing the origin of almost every atom of MIA (42). Moreover, the utility of this system promotes the exploration of the enzyme specificity, allowing for the production of a regiospecifically halogenated MIA that could be further incorporated into the thiopeptide framework to afford a novel halogenated thiopeptide member (42). The second strategy is trapping and/or directly examining the catalytic intermediates. A unique feature of the NosL/NocL in vitro assay is that a substantial amount of the shunt product (3-methylindole) is produced in addition to the authentic product (MIA). Interestingly, the production of 3-methylindole, presumably by inadvertent trapping of the indolyl intermediate resulting from Cα–Cβ cleavage, is actually adjustable by changing the reductant sodium dithionite concentration in the assay (42, 43). Combined with the detection of another shunt product (glyoxylate) in the reaction, these observations firmly established a fragmentation-recombination mechanism that is involved in MIA synthesis.
The proposed mechanism of MIA synthesis is briefly illustrated in Fig. 1C. In analogy to ThiH and HydG catalysis, the fragmentation may occur on a tryptophan radical, which is produced by hydrogen abstraction by the Ado radical from the N1 of l-Trp. The homolytic cleavage of the Cα–Cβ bond of the tryptophan radical, resulting in 3-methyleneindole and a glycyl radical, is supported by computational studies and has been confirmed by trapping the glycyl radical as a glycine-dansyl derivative by a rapid-quench method (42). The putative glycyl radical has been observed by EPR spectroscopy during the steady-state phase of NocL catalysis (43). In contrast to the relatively well understood fragmentation mechanism, little is known about the subsequent recombination process by which the two 3-methyleneindole and glycyl radical fragments are tailored to afford MIA and the coproducts formaldehyde and ammonia. As the Cα of the glycyl radical carries the largest spin density, it should be more reactive compared with the carboxyl group. Why the reaction does not proceed with the attack of the Cα of the glycyl radical on the π system, as found in glutamate mutase (10, 18), is unclear. It is possibly because the glycyl radical is tightly sequestered in the enzyme's active site, leaving the Cα too far to react with the indole ring. Two possible mechanisms are envisioned as shown in supplemental Fig. 2. One is the direct addition of the glycyl radical to 3-methyleneindole via a polar mechanism, during which an enzyme-mediated proton transfer might promote this process. Alternatively, the glycyl radical may undergo fragmentation after protonation of the Cα to produce a carboxyl radical (45), which recombines with the indole ring and reinstalls the carboxyl group. Given the considerable production of 3-methylindole in the assay, the recombination may be inefficient and serve as one of the limiting steps in catalysis. However, future studies are needed to address the detailed mechanism of MIA biosynthesis, particularly the intriguing recombination process.
MoaA in Molybdenum Cofactor (Moco) Biosynthesis
Moco, the biological active form of molybdenum, is an essential component of a diverse group of redox enzymes (46). Moco consists of a mononuclear molybdenum atom coordinated by the dithiolene moiety of a tricyclic pyranopterin (Fig. 2A). The biosynthesis of the cofactor is an ancient, ubiquitous, and highly conserved pathway that includes three major steps (47). The first step is the conversion of GTP to an oxygen-sensitive 6-alkyl pterin with a cyclic phosphate, which is commonly referred to as precursor Z (48). A similar reaction is also found in folate and pterin biosynthesis, such as the formation of dihydroneopterin triphosphate from GTP catalyzed by GTP cyclohydrolase I (GCH I) (49). However, precursor Z biosynthesis is distinct, as the C8 of the purine is inserted between the 2′- and 3′-ribose carbons via an as yet unknown rearrangement, rather than being released as formate in the GCH I-catalyzed reaction.
FIGURE 2.
GTP-derived biosynthesis of Moco and deazapurine. A, role of MoaA and MoaC in Moco synthesis. B, key steps in queuosine biosynthesis. The reaction catalyzed by the radical AdoMet protein QueE is highlighted in yellow. CPH4, 6-carboxy-5,6,7,8-tetrahydropterin; CDG, 7-carboxy-7-deazaguanine.
The remarkable rearrangement of precursor Z biosynthesis is catalyzed by MoaA and the accessory protein MoaC (which correspond to MOCS1A and MOCS1B (encoded by a bicistronic mRNA), respectively, in humans (50)). MoaA is a radical AdoMet protein containing a typical (αβ)6 TIM barrel fold in the N terminus, with a [4Fe-4S] cluster ligated by the characteristic CXXXCXXC motif. However, the C terminus of MoaA contains an additional [4Fe-4S] cluster, which is similar to the N-terminal cluster featuring a vacant ligation site of the fourth iron atom (51). The function of the C-terminal cluster for binding of the substrate GTP was supported by the crystal structure of MoaA in complex with GTP (52). Additional function(s) of the C-terminal [4Fe-4S] cluster may also be involved, as the simple anchoring of the GTP could be easily accomplished by amino acid residues, and the cluster has also been shown to be redox-active (53). The closest distance between the two [Fe-S] clusters is 17 Å, defining a large active site pocket interrupted by the hydrophilic channel (51). The active site is constructed from predominantly basic residues, which are ideally positioned to compensate the negative charges of the GTP phosphate groups. Most of these residues are crucial for catalysis, as mutations of them resulted in either completely abolished or dramatically decreased MoaA activity (52).
The involvement of the accessory protein MoaC during catalysis makes the mechanism of the conversion complicated. Although the crystal structure of the MoaC protein has been resolved (54, 55), the catalytic role of MoaC and its relationship with MoaA are still unknown. One proposal is that MoaA generates a stable glycyl radical on the MoaC scaffold to perform catalysis, in analogy to the binary enzyme system of pyruvate formate-lyase (PFL) and PFL activase. However, this seems to be unlikely, as substitution of the two strict conserved glycine residues of MoaC with alanine leads to only a minor reduction of activity (54). An alternative scenario could be that MoaA first synthesizes an unknown intermediate from GTP, which is sequestered by MoaC and converted to precursor Z. The fact that MoaA is able to convert GTP to a diaminopyrimidine-type product in the absence of MoaC is consistent with this notion (52). Characterization of this diaminopyrimidine product will certainly shed light on the elusive mechanism of MoaA and MoaC catalysis.
Distinct from GCH I, which breaks the N7–C8 and N9–C8 bonds of GTP to produce a diaminopyrimidine intermediate via a zinc-dependent hydrolytic process, the mechanism of MoaA involves breakage of the GTP N7–C8 and/or N9–C8 bond by radical chemistry. Based on the crystal structure of MoaA in complex with GTP and AdoMet, it was proposed that the possible sites in the GTP for hydrogen abstraction by the Ado radical might be the purine C8 atom and either the C2′ or C3′ atom of the ribose (52). However, other possibilities cannot be excluded. A mechanism including the hydrogen abstraction at the C4′ atom is proposed, as shown in supplemental Fig. 3. Future studies using isotopically labeled GTP will provide more information on the mechanism of MoaA and MoaC catalysis.
QueE in Deazapurine Biosynthesis
Compounds containing pyrrolopyrimidine nucleoside (collectively termed deazapurines) are an important class of structurally diverse compounds that are widespread in biological systems and include various antibiotics (56) and the hypermodified tRNA bases queuosine (57) and archaeosine (58). Isotopic labeling studies indicated that deazapurine base was derived from a purine precursor with the loss of a C8 atom (59). Biosynthetic studies of queuosine, toyocamycin, and sangivamycin revealed that four conserved enzymes might be involved to afford the deazapurine ring (60–62), including GCH I, a 6-pyruvoyltetrahydropterin synthase-like protein (QueD homolog), a radical AdoMet protein (QueE homolog), and an ATPase (QueC homolog). By sequentially using these four enzymes, the conversion of GTP to preQ0, a precursor of queuosine, has been reconstituted recently in vitro (63), revealing a substantial structural rearrangement in deazapurine biosynthesis (Fig. 2B).
Of particular interest is the conversion of 6-carboxy-5,6,7,8-tetrahydropterin to 7-carboxy-7-deazaguanine catalyzed by the radical AdoMet enzyme QueE, which serves as the key enzyme of the deazapurine framework synthesis. This reaction involves the extrusion of an ammonium from the 6-membered tetrahydropyrazine ring to afford the 5-membered imidazole ring. Currently, there is no clue to the mechanism of the enzyme, but this ammonium elimination bears some analogy to the AdoCbl-dependent elimination reaction catalyzed by diol dehydratase, glycerol dehydratase (GDH), and ethanolamine ammonia-lyase (15) and to the deamination reaction catalyzed by the radical AdoMet protein DesII (64, 65). These enzymes all use an Ado radical to abstract a hydrogen atom from the C2 of the substrates to produce an intermediate radical. The cleavage of the C1–OH or C1–NH2 bond is then promoted by the hydroxyl group on the C2 atom, which stabilizes the resultant radical cation (an illustrative mechanism is shown in supplemental Fig. 4). A mechanism of QueE catalysis has been proposed based on this point, as shown in supplemental Fig. 5. Further evidence is needed to address the mechanism of this key reaction in deazapurine biosynthesis.
RlmN and Cfr in Ribosome Methylation
Methylation is an essential reaction involved in a wide range of life processes, such as gene expression, protein modification, RNA metabolism, lipid and secondary metabolite biosynthesis, etc. The majority of methylations are AdoMet-dependent, proceeding with the nucleophilic attack of the substrates on the methyl group of the AdoMet sulfonium via an Sn2 displacement (66). However, this requires the nucleophilicity of the substrates, and methylation of the unreactive sp3 carbons or electrophilic substrates should invoke different pathways.
Recent studies on the ribosome methyltransferases RlmN and Cfr revealed a novel radical-associated mechanism for methylation (67–69). Cfr methylates the C8 atom of adenosine 2503 of 23 S rRNA located in the peptidyl transferase center of the bacterial large ribosomal subunit. Such a modification confers resistance on bacteria to five classes of antibiotics that act upon the peptidyl transferase center (70). On the other hand, RlmN methylates the C2 position of the same nucleotide (adenosine 2503), which is a housekeeping modification that is important in translational fidelity and the nascent peptide response (71, 72). The electrophilic imine carbon of the C2 and C8 of adenine seems to preclude the Sn2-type methylation mechanism. Indeed, both RlmN and Cfr contain the characteristic CXXXCXXC motifs, and their radical AdoMet chemistry has been demonstrated by in vitro analyses using complete 23 S rRNA, RNA fragments, and even small nucleotide oligomers as substrates (67–69).
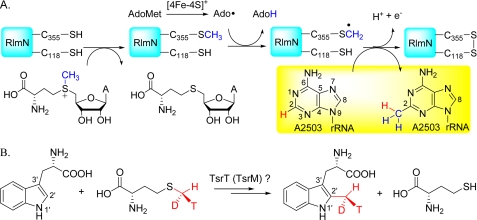
Elegant labeling studies have been performed independently by two laboratories. Yan and Fujimori (68) used deuterated AdoMet ([methyl-2H3]AdoMet) and adenine C2-deuterated rRNA fragments for assay, whereas Grove et al. (69) used [methyl-2H3]AdoMet and deuterated protein that was produced in and isolated from an E. coli methionine auxotroph cultured in the presence of [methyl-2H3]methionine. Both sets of data are consistent with an unprecedented protein-based radical mechanism for methylation (Fig. 3A). During catalysis, the protein initially undergoes methylation by the first AdoMet molecule, possibly via a typical Sn2 mechanism at a conserved cysteine residue (Cys-355 in RlmN), to result in a methylthio group. The Ado radical produced by reductive cleavage of the second AdoMet molecule then abstracts a hydrogen atom from the methylthio group, generating a protein-based methylene radical that initiates the radical-based methyl transfer. This mechanism was strongly supported by the observation of the methylated Cys-355 residue in the RlmN crystal structure (73). Further studies are expected to reveal how the protein is methylated by the first AdoMet molecule and how the catalytic turnover proceeds. It should also be kept in mind that this strategy for radical methylation of aromatic heterocycles may not be the only one. The C2′ methylation of tryptophan in thiostrepton biosynthesis, which may also employ radical chemistry (74, 75), does not involve a methylene intermediate, as the reactions proceed with net retention of the methyl group configuration of AdoMet (Fig. 3B) (76–78). In addition, the methyl group of l-[methyl-13C,2H3]methionine is incorporated with complete retention of all three deuterium atoms (77). Of course, the mechanism of this methylation is another fascinating enigma that remains to be solved.
FIGURE 3.
Radical-mediated methylation of aromatic heterocycles. A, RNA adenine methylation catalyzed by RlmN. B, C2′ methylation of l-Trp, possibly involving catalysis by the radical AdoMet methyltransferase TsrT (TsrM), proceeds with the net retention of the methyl group configuration.
Conclusion and Outlook
The radical AdoMet superfamily is well known for its remarkable catalytic diversity. This was reinforced by recent studies on the characterization of several complex and chemically unusual transformations. Understanding the mechanism of these intriguing transformations will certainly enrich our knowledge about enzymology and radical chemistry. As discussed above, various strategies can be employed to dissect the enzymatic process. Besides isotopic labeling, which is invariably one of the most powerful tools for mechanistic study, other methods, such as trapping and/or detection of intermediates during catalysis, can also provide important clues. Because of the complexity of the catalytic pathway and the extremely short lifetime of radical intermediates, the comprehensive elucidation of the detailed catalytic process seems to be a considerable challenge. Computational studies and comparative analysis may thus be helpful to address the mechanistic proposal in some cases. Although many mechanistic aspects still await elucidation, characterization of these biotransformations provides us with excellent platforms to understand the elegance and sophistication of radical biochemistry. Given the enormous number of functionally uncharacterized radical AdoMet enzymes identified in myriad pathways, one may expect that more and more novel examples of radical AdoMet chemistry will be revealed and appreciated in the future.
Supplementary Material
This work was supported in part by National Natural Science Foundation Grants 20832009, 30525001, and 20921091; Ministry of Science and Technology Grant 2009ZX09501-008; National Basic Research Program (973 Program) Grant 2010CB833200; Chinese Academy of Sciences Grant KJCX2-YW-H08; and Science and Technology Commission of Shanghai Municipality Grant 09QH1402700. This minireview will be reprinted in the 2011 Minireview Compendium, which will be available in January, 2012.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
- AdoMet
- S-adenosylmethionine
- LAM
- lysine 2,3-aminomutase
- Ado
- 5′-deoxyadenosyl
- AdoCbl
- adenosylcobalamin
- AIR
- aminoimidazole ribonucleotide
- HMP-P
- 4-amino-5-hydroxymethyl-2-methylpyrimidine phosphate
- AdoH
- 5′-deoxyadenosine
- MIA
- 3-methyl-2-indolic acid
- Moco
- molybdenum cofactor
- GCH I
- GTP cyclohydrolase I
- PFL
- pyruvate formate-lyase
- GDH
- glycerol dehydratase.
REFERENCES
- 1. Frey P. A., Hegeman A. D., Reed G. H. (2006) Chem. Rev. 106, 3302–3316 [DOI] [PubMed] [Google Scholar]
- 2. Renaud P., Sibi M. P. (eds) (2001) Radicals in Organic Synthesis, Wiley-VCH, Weinheim, Germany [Google Scholar]
- 3. Sofia H. J., Chen G., Hetzler B. G., Reyes-Spindola J. F., Miller N. E. (2001) Nucleic Acids Res. 29, 1097–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marsh E. N., Patwardhan A., Huhta M. S. (2004) Bioorg. Chem. 32, 326–340 [DOI] [PubMed] [Google Scholar]
- 5. Frey P. A., Hegeman A. D., Ruzicka F. J. (2008) Crit. Rev. Biochem. Mol. Biol. 43, 63–88 [DOI] [PubMed] [Google Scholar]
- 6. Cheek J., Broderick J. B. (2001) J. Biol. Inorg. Chem. 6, 209–226 [DOI] [PubMed] [Google Scholar]
- 7. Roach P. L. (2011) Curr. Opin. Chem. Biol. 15, 267–275 [DOI] [PubMed] [Google Scholar]
- 8. Frey P. A., Magnusson O. T. (2003) Chem. Rev. 103, 2129–2148 [DOI] [PubMed] [Google Scholar]
- 9. Jarrett J. T. (2003) Curr. Opin. Chem. Biol. 7, 174–182 [DOI] [PubMed] [Google Scholar]
- 10. Marsh E. N., Patterson D. P., Li L. (2010) ChemBioChem 11, 604–621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wang S. C., Frey P. A. (2007) Trends Biochem. Sci. 32, 101–110 [DOI] [PubMed] [Google Scholar]
- 12. Vey J. L., Drennan C. L. (2011) Chem. Rev. 111, 2487–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Atta M., Mulliez E., Arragain S., Forouhar F., Hunt J. F., Fontecave M. (2010) Curr. Opin. Struct. Biol. 20, 684–692 [DOI] [PubMed] [Google Scholar]
- 14. Booker S. J., Cicchillo R. M., Grove T. L. (2007) Curr. Opin. Chem. Biol. 11, 543–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Walsby C. J., Ortillo D., Yang J., Nnyepi M. R., Broderick W. E., Hoffman B. M., Broderick J. B. (2005) Inorg. Chem. 44, 727–741 [DOI] [PubMed] [Google Scholar]
- 16. Chirpich T. P., Zappia V., Costilow R. N., Barker H. A. (1970) J. Biol. Chem. 245, 1778–1789 [PubMed] [Google Scholar]
- 17. Nicolet Y., Drennan C. L. (2004) Nucleic Acids Res. 32, 4015–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Banerjee R. (2003) Chem. Rev. 103, 2083–2094 [DOI] [PubMed] [Google Scholar]
- 19. Toraya T. (2003) Chem. Rev. 103, 2095–2127 [DOI] [PubMed] [Google Scholar]
- 20. Chatterjee A., Li Y., Zhang Y., Grove T. L., Lee M., Krebs C., Booker S. J., Begley T. P., Ealick S. E. (2008) Nat. Chem. Biol. 4, 758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Martinez-Gomez N. C., Downs D. M. (2008) Biochemistry 47, 9054–9056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Duschene K. S., Veneziano S. E., Silver S. C., Broderick J. B. (2009) Curr. Opin. Chem. Biol. 13, 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Farrar C. E., Jarrett J. T. (2009) Biochemistry 48, 2448–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pohl M., Sprenger G. A., Müller M. (2004) Curr. Opin. Biotechnol. 15, 335–342 [DOI] [PubMed] [Google Scholar]
- 25. Settembre E., Begley T. P., Ealick S. E. (2003) Curr. Opin. Struct. Biol. 13, 739–747 [DOI] [PubMed] [Google Scholar]
- 26. Kriek M., Martins F., Leonardi R., Fairhurst S. A., Lowe D. J., Roach P. L. (2007) J. Biol. Chem. 282, 17413–17423 [DOI] [PubMed] [Google Scholar]
- 27. Newell P. C., Tucker R. G. (1968) Biochem. J. 106, 279–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lawhorn B. G., Mehl R. A., Begley T. P. (2004) Org. Biomol. Chem. 2, 2538–2546 [DOI] [PubMed] [Google Scholar]
- 29. Dougherty M. J., Downs D. M. (2006) Microbiology 152, 2345–2353 [DOI] [PubMed] [Google Scholar]
- 30. Chatterjee A., Hazra A. B., Abdelwahed S., Hilmey D. G., Begley T. P. (2010) Angew. Chem. Int. Ed. Engl. 49, 8653–8656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Martinez-Gomez N. C., Poyner R. R., Mansoorabadi S. O., Reed G. H., Downs D. M. (2009) Biochemistry 48, 217–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bagley M. C., Dale J. W., Merritt E. A., Xiong X. (2005) Chem. Rev. 105, 685–714 [DOI] [PubMed] [Google Scholar]
- 33. Walsh C. T., Acker M. G., Bowers A. A. (2010) J. Biol. Chem. 285, 27525–27531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li C., Kelly W. L. (2010) Nat. Prod. Rep. 27, 153–164 [DOI] [PubMed] [Google Scholar]
- 35. Houck D. R., Chen L. C., Keller P. J., Beale J. M., Floss H. G. (1988) J. Am. Chem. Soc. 110, 5800–5806 [Google Scholar]
- 36. Mocek U., Knaggs A. R., Tsuchiya R., Nguyen T., Beale J. M., Floss H. G. (1993) J. Am. Chem. Soc. 115, 7557–7568 [Google Scholar]
- 37. Yu Y., Duan L., Zhang Q., Liao R., Ding Y., Pan H., Wendt-Pienkowski E., Tang G., Shen B., Liu W. (2009) ACS Chem. Biol. 4, 855–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ding Y., Yu Y., Pan H., Guo H., Li Y., Liu W. (2010) Mol. BioSystems 6, 1180–1185 [DOI] [PubMed] [Google Scholar]
- 39. Kriek M., Martins F., Challand M. R., Croft A., Roach P. L. (2007) Angew. Chem. Int. Ed. Engl. 46, 9223–9226 [DOI] [PubMed] [Google Scholar]
- 40. Driesener R. C., Challand M. R., McGlynn S. E., Shepard E. M., Boyd E. S., Broderick J. B., Peters J. W., Roach P. L. (2010) Angew. Chem. Int. Ed. Engl. 49, 1687–1690 [DOI] [PubMed] [Google Scholar]
- 41. Shepard E. M., Duffus B. R., George S. J., McGlynn S. E., Challand M. R., Swanson K. D., Roach P. L., Cramer S. P., Peters J. W., Broderick J. B. (2010) J. Am. Chem. Soc. 132, 9247–9249 [DOI] [PubMed] [Google Scholar]
- 42. Zhang Q., Li Y., Chen D., Yu Y., Duan L., Shen B., Liu W. (2011) Nat. Chem. Biol. 7, 154–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang Q., Chen D., Lin J., Liao R., Tong W., Xu Z., Liu W. (2011) J. Biol. Chem. 286, 21287–21294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Johnson D. C., Dean D. R., Smith A. D., Johnson M. K. (2005) Annu. Rev. Biochem. 74, 247–281 [DOI] [PubMed] [Google Scholar]
- 45. Bonifacic M., Stefanic I., Hug G. L., Armstrong D. A., Asmus K. D. (1998) J. Am. Chem. Soc. 120, 9930–9940 [Google Scholar]
- 46. Hille R. (1996) Chem. Rev. 96, 2757–2816 [DOI] [PubMed] [Google Scholar]
- 47. Rizzi M., Schindelin H. (2002) Curr. Opin. Struct. Biol. 12, 709–720 [DOI] [PubMed] [Google Scholar]
- 48. Wuebbens M. M., Rajagopalan K. V. (1995) J. Biol. Chem. 270, 1082–1087 [DOI] [PubMed] [Google Scholar]
- 49. Burg A. W., Brown G. M. (1968) J. Biol. Chem. 243, 2349–2358 [PubMed] [Google Scholar]
- 50. Hänzelmann P., Schwarz G., Mendel R. R. (2002) J. Biol. Chem. 277, 18303–18312 [DOI] [PubMed] [Google Scholar]
- 51. Hänzelmann P., Schindelin H. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 12870–12875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hänzelmann P., Schindelin H. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 6829–6834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hänzelmann P., Hernández H. L., Menzel C., García-Serres R., Huynh B. H., Johnson M. K., Mendel R. R., Schindelin H. (2004) J. Biol. Chem. 279, 34721–34732 [DOI] [PubMed] [Google Scholar]
- 54. Wuebbens M. M., Liu M. T., Rajagopalan K., Schindelin H. (2000) Structure Fold. Des. 8, 709–718 [DOI] [PubMed] [Google Scholar]
- 55. Kanaujia S. P., Jeyakanthan J., Nakagawa N., Balasubramaniam S., Shinkai A., Kuramitsu S., Yokoyama S., Sekar K. (2010) Acta Crystallogr. D 66, 821–833 [DOI] [PubMed] [Google Scholar]
- 56. Suhadolnik R. J. (1970) Nucleoside Antibiotics, pp. 298–353, Wiley InterScience, New York [Google Scholar]
- 57. Kasai H., Oashi Z., Harada F., Nishimura S., Oppenheimer N. J., Crain P. F., Liehr J. G., von Minden D. L., McCloskey J. A. (1975) Biochemistry 14, 4198–4208 [DOI] [PubMed] [Google Scholar]
- 58. Bai Y., Fox D. T., Lacy J. A., Van Lanen S. G., Iwata-Reuyl D. (2000) J. Biol. Chem. 275, 28731–28738 [DOI] [PubMed] [Google Scholar]
- 59. Suhadolnik R. J., Uematsu T. (1970) J. Biol. Chem. 245, 4365–4371 [PubMed] [Google Scholar]
- 60. Reader J. S., Metzgar D., Schimmel P., de Crécy-Lagard V. (2004) J. Biol. Chem. 279, 6280–6285 [DOI] [PubMed] [Google Scholar]
- 61. McCarty R. M., Bandarian V. (2008) Chem. Biol. 15, 790–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. de Crécy-Lagard V. (2007) Methods Enzymol. 425, 153–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. McCarty R. M., Somogyi A., Lin G., Jacobsen N. E., Bandarian V. (2009) Biochemistry 48, 3847–3852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Szu P. H., He X., Zhao L., Liu H. W. (2005) Angew. Chem. Int. Ed. Engl. 44, 6742–6746 [DOI] [PubMed] [Google Scholar]
- 65. Szu P. H., Ruszczycky M. W., Choi S. H., Yan F., Liu H. W. (2009) J. Am. Chem. Soc. 131, 14030–14042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Woodard R. W., Tsai M. D., Floss H. G., Crooks P. A., Coward J. K. (1980) J. Biol. Chem. 255, 9124–9127 [PubMed] [Google Scholar]
- 67. Yan F., LaMarre J. M., Röhrich R., Wiesner J., Jomaa H., Mankin A. S., Fujimori D. G. (2010) J. Am. Chem. Soc. 132, 3953–3964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yan F., Fujimori D. G. (2011) Proc. Natl. Acad. Sci. U.S.A. 108, 3930–3934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Grove T. L., Benner J. S., Radle M. I., Ahlum J. H., Landgraf B. J., Krebs C., Booker S. J. (2011) Science 332, 604–607 [DOI] [PubMed] [Google Scholar]
- 70. Long K. S., Poehlsgaard J., Kehrenberg C., Schwarz S., Vester B. (2006) Antimicrob. Agents Chemother. 50, 2500–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kowalak J. A., Bruenger E., McCloskey J. A. (1995) J. Biol. Chem. 270, 17758–17764 [DOI] [PubMed] [Google Scholar]
- 72. Toh S. M., Xiong L., Bae T., Mankin A. S. (2008) RNA 14, 98–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Boal A. K., Grove T. L., McLaughlin M. I., Yennawar N. H., Booker S. J., Rosenzweig A. C. (2011) Science 332, 1089–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Liao R., Duan L., Lei C., Pan H., Ding Y., Zhang Q., Chen D., Shen B., Yu Y., Liu W. (2009) Chem. Biol. 16, 141–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kelly W. L., Pan L., Li C. (2009) J. Am. Chem. Soc. 131, 4327–4334 [DOI] [PubMed] [Google Scholar]
- 76. Floss H. G., Lee S. (1993) Acc. Chem. Res. 26, 116–122 [Google Scholar]
- 77. Zhou P., O'Hagan D., Mocek U., Zeng Z., Yuen L. D., Frenzel T., Unkefer C. J., Beale J. M., Floss H. G. (1989) J. Am. Chem. Soc. 11, 7274–7276 [Google Scholar]
- 78. Frenzel T., Zhou P., Floss H. G. (1990) Arch. Biochem. Biophys. 278, 35–40 [DOI] [PubMed] [Google Scholar]
- 79. Morris R. P., Leeds J. A., Naegeli H. U., Oberer L., Memmert K., Weber E., LaMarche M. J., Parker C. N., Burrer N., Esterow S., Hein A. E., Schmitt E. K., Krastel P. (2009) J. Am. Chem. Soc. 131, 5946–5955 [DOI] [PubMed] [Google Scholar]
- 80. Rachid S., Scharfe M., Blöcker H., Weissman K. J., Müller R. (2009) Chem. Biol. 16, 70–81 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.