Abstract
Histidine-rich glycoprotein (HRG) is an abundant protein that binds fibrinogen and other plasma proteins in a Zn2+-dependent fashion but whose function is unclear. HRG has antimicrobial activity, and its incorporation into fibrin clots facilitates bacterial entrapment and killing and promotes inflammation. Although these findings suggest that HRG contributes to innate immunity and inflammation, little is known about the HRG-fibrin(ogen) interaction. By immunoassay, HRG-fibrinogen complexes were detected in Zn2+-supplemented human plasma, a finding consistent with a high affinity interaction. Surface plasmon resonance determinations support this concept and show that in the presence of Zn2+, HRG binds the predominant γA/γA-fibrinogen and the γ-chain elongated isoform, γA/γ′-fibrinogen, with Kd values of 9 nm. Likewise, 125I-labeled HRG binds γA/γA- or γA/γ′-fibrin clots with similar Kd values when Zn2+ is present. There are multiple HRG binding sites on fibrin(ogen) because HRG binds immobilized fibrinogen fragment D or E and γ′-peptide, an analog of the COOH terminus of the γ′-chain that mediates the high affinity interaction of thrombin with γA/γ′-fibrin. Thrombin competes with HRG for γ′-peptide binding and displaces 125I-HRG from γA/γ′-fibrin clots and vice versa. Taken together, these data suggest that (a) HRG circulates in complex with fibrinogen and that the complex persists upon fibrin formation, and (b) by competing with thrombin for γA/γ′-fibrin binding, HRG may modulate coagulation. Therefore, the HRG-fibrin interaction may provide a novel link between coagulation, innate immunity, and inflammation.
Keywords: Fibrin, Fibrinogen, Surface Plasmon Resonance (SPR), Thrombin, Zinc, Histidine-rich Glycoprotein, γ′ Fibrinogen
Introduction
Fibrinogen is the soluble precursor of fibrin, a critical component of blood clots that endows them with strength and elasticity. Fibrinogen is a glycoprotein composed of three pairs of polypeptide chains, termed Aα, Bβ, and γ, that are connected by disulfide bonds (1). Approximately 10–15% of circulating fibrinogen has a variant γ-chain termed the γ′-chain, which results from differential processing of the γA-chain mRNA transcript (2–4). The γ′-chain is distinguished from the γA-chain by the presence of an acidic 20-residue extension at its COOH terminus (2, 5).
Thrombin catalyzes the conversion of fibrinogen to insoluble fibrin, and during this process some thrombin remains bound to the fibrin network (6). Exosites 1 and 2 are two regulatory domains that flank the active site of thrombin and mediate its binding to fibrin (7, 8). Exosite 1 of thrombin interacts with the central E-domain of fibrin, whereas exosite 2 binds only to the COOH terminus of the γ′-chain. Consequently, thrombin binds γA/γA-fibrin in a univalent fashion with a Kd value of 2–4 μm. In contrast, both exosites are engaged when thrombin binds to γA/γ′-fibrin, resulting in a higher affinity interaction (Kd value of 0.08–0.18 μm) (5, 9). Fibrin-bound thrombin remains active, and the protease is protected from inhibition by fluid-phase inhibitors, such as antithrombin and heparin cofactor II (6). Because of its bivalent interaction with γA/γ′-fibrin, thrombin bound to γA/γ′-fibrin is more protected from inhibition by fluid-phase inhibitors than thrombin bound to γA/γA-fibrin (10).
Like thrombin, histidine-rich glycoprotein (HRG)3 binds to fibrinogen and is incorporated into fibrin clots (11). Although the plasma concentration of HRG ranges from 1.6 to 2 μm, the concentration in platelet-rich thrombi may be higher because HRG is stored in the alpha granules of platelets and is released when platelets are activated (12, 13). A 75-kDa glycoprotein, HRG, is composed of two NH2-terminal cystatin-like domains, a central histidine-rich region (HRR) flanked by two proline-rich regions, and a COOH-terminal domain (14). In addition to fibrinogen, HRG also binds plasminogen, heparan sulfate, and divalent cations, such as Zn2+ (12, 15, 16). Therefore, HRG is hypothesized to be an important accessory or adapter protein that brings different ligands together under specific conditions (14).
HRG-deficient mice exhibit a shorter prothrombin time and accelerated fibrinolysis compared with wild-type mice, raising the possibility that HRG modulates coagulation and fibrinolysis (17). In addition to its potential role in hemostasis, HRG also has been implicated in innate immunity and inflammation (18). HRG exhibits antifungal and antimicrobial activity in vitro, and these activities are enhanced at low pH or in the presence of Zn2+, conditions that promote ligand binding (19, 20). The antimicrobial activity of HRG has also been demonstrated in vivo and appears to be fibrin-dependent. Thus, compared with wild-type mice, HRG-deficient mice are more susceptible to the lethal effect of Streptococcus pyogenes infection and are rescued with HRG supplementation (21). This phenomenon is fibrin-dependent because fibrin is essential for HRG-mediated bacterial entrapment and killing, processes that prevent bacterial dissemination. In addition, the HRG-fibrin interaction modulates inflammation because HRG-deficient mice exhibit attenuated abscess formation in response to subcutaneous injection of bacteria. Based on these findings, it has been postulated that HRG plays a fibrin-dependent role in both inflammation and innate immunity (21).
Despite emerging evidence that the HRG-fibrin(ogen) interaction is physiologically important, little is known about the biochemical foundation of this interaction or its functional consequences. To address these gaps in knowledge, we set out to (a) quantify the binding of HRG to fibrin(ogen), (b) identify the HRG binding domains on fibrin(ogen), and (c) determine whether there is overlap between the HRG and thrombin binding domains on fibrin(ogen).
EXPERIMENTAL PROCEDURES
Materials
Reagents
Human thrombin, prothrombin, and plasminogen-free fibrinogen were from Enzyme Research Laboratories (South Bend, IN). Plasmin and factor XIII were from Haematologic Technologies Inc. (Essex Junction, VT). Prionex was from Pentapharm (Basel, Switzerland). A 20-amino acid analog of the COOH terminus of the γ′-chain of fibrinogen, γ′-peptide (VRPEHPAETEYDSLYPEDDL), was prepared by Bachem Bioscience, Inc. (King of Prussia, PA), and a sheep antibody against this peptide was from Affinity Biologicals (Ancaster, ON). The two Tyr residues within the γ′-peptide were modified with phosphate groups in place of sulfate to enhance stability (22). The γ′-peptide-directed IgG was subjected to affinity chromatography using immobilized γ′-peptide (SulfoLink Immobilization Kit for Peptides, Thermo Scientific, Rockford, IL). d-Phe-Pro-Arg chloromethyl ketone and d-Tyr-Pro-Arg chloromethyl ketone (FPRck and YPRck, respectively) were from EMD Chemicals (Gibbstown, NJ). Fibrinogen was rendered factor XIII-free, and γA/γA- and γA/γ′-fibrinogen were separated by fractionation on DEAE-Sepharose (GE Healthcare) and characterized as previously described (10, 23). HRG was purified by metal-chelate chromatography, and HRG-deficient plasma was prepared as described previously (24). Non-immune sheep IgG and a human HRG-directed IgG from sheep were prepared by Affinity Biologicals, and the HRG-specific IgG fraction was isolated by affinity chromatography using immobilized HRG (24). Unless otherwise specified, other reagents were from Sigma.
Preparation of Fibrinogen Fragments
Fragment X was prepared by limited plasmin digestion of fibrinogen (23). Fragments D and E were generated by plasmin digestion of γA/γA-fibrinogen (25). Digested material was applied to a 12-ml UNO Q-12 ion exchange column (Bio-Rad) using a Bio-Rad Biologic Duoflow system at a flow rate of 5 ml/min. To elute non-specifically bound proteins, the column was washed with 30 ml of 0.02 m sodium phosphate, 0.01 m citric acid, pH 7.6. Fragment D was eluted with 100 ml of 0.1 m sodium phosphate, 0.05 M citric acid, pH 5.0, whereas fragment E was subsequently eluted with 40 ml of the same buffer at pH 4.4 (26). Protein-containing fractions were identified by absorbance at 280 nm and pooled. Purified fragments were dialyzed into 10 mm Hepes-NaOH, 150 mm NaCl, pH 7.4 (HBS) and concentrated. Final concentrations were determined at 280 nm (27). The integrity of the fragments was assessed by SDS-PAGE analysis on 4–15% polyacrylamide gels (Ready-Gel, Bio-Rad) under reducing and non-reducing conditions. Samples were stored in aliquots at −80 °C.
Preparation of γ′-directed IgG Fab Fragments
For some experiments, fragment antibody binding (Fab) regions from the affinity-purified γ′-peptide-directed IgG were generated by papain digestion (28), isolated with a Fab preparation kit (Pierce), assessed for purity by SDS-PAGE analysis, and then concentrated and dialyzed against HBS.
Labeling of Proteins
γ′-Peptide was labeled with fluorescein isothiocyanate as previously described (9). α–Thrombin was radiolabeled by reaction with 125I-labeled YPRck, and HRG was radiolabeled with Na125I (McMaster University Nuclear Reactor, Hamilton, ON) using Iodo-beads (Pierce) as described (29). 125I-YPRck-thrombin and 125I-HRG concentrations were 5–6 μm, as determined by absorbance at 280 nm, with radioactivity of 500,000–800,000 cpm/μg of protein. FPRck-thrombin was prepared as described (30).
Methods
Surface Plasmon Resonance (SPR)
The interaction of HRG with immobilized γA/γA- or γA/γ′-fibrin(ogen), biotinylated-γ′-peptide, or fragments X, D, or E was assessed by SPR using a BIAcore 1000 (GE Healthcare) as previously described (24, 30) but with some modifications. Briefly, proteins were covalently linked to separate flow cells of a carboxymethylated dextran (CM4) biosensor chip at a flow rate of 5 μl/min using an amine coupling kit (GE Healthcare). Proteins were immobilized using 10 mm acetate buffer at varying pH values to maximize adsorption. γA/γA- or γA/γ′-fibrinogen or fragments X and D were immobilized at pH 5.5 to ∼3000–7000 response units (RU). Fragment E was immobilized at pH 4.5 to ∼2000–3000 RU. For fibrin binding studies, immobilized γA/γA- or γA/γ′-fibrinogen was converted to fibrin by three successive 60-min injections of 100 nm thrombin at 5 μl/min (30). To prepare streptavidin-conjugated CM4 flow cells, 0.4 mg/ml streptavidin (Sigma) at pH 4.5 was injected. Biotinylated γ′-peptide (30) was adsorbed to the immobilized streptavidin to 200–300 RU. Remaining reactive groups were neutralized with 1 m ethylenediamine, and non-specifically adsorbed proteins were removed by treatment with 0.5 m NaCl. An unmodified flow cell served as the control. All SPR procedures were done in HBS containing 0.005% Tween 20 and 2 mm CaCl2, and flow cells were regenerated with 250 mm imidazole and 2 mm EDTA between runs.
To measure the affinity of HRG for immobilized fibrinogen, fibrin, or fibrinogen fragments, aliquots of HRG (0–1 μm) in buffer containing 20 μm ZnCl2 were injected at a flow rate of 30 μl/min. To quantify Zn2+ dependence, binding of 200 nm HRG to immobilized γA/γA- or γA/γ′-fibrinogen was monitored in the presence of varying concentrations of ZnCl2 (0–60 μm) using dual injection mode. The binding of thrombin to immobilized fragment D or E was monitored by injection of FPRck-thrombin (0–15 μm) into flow cells.
The γ′-peptide-directed IgG was used to assess the contribution of the γ′-chain to the interaction of HRG with immobilized γ′-peptide or fibrinogen. A saturating amount of γ′-peptide-directed IgG or a non-immune IgG (2 μm) was first injected into flow cells containing immobilized γ′-peptide or γA/γ′- or γA/γA-fibrinogen. The binding of HRG to fibrinogen or fibrinogen fragments was then measured as described above, except that flow cells were re-saturated with 0.5 μm γ′-peptide-directed IgG or control IgG before each HRG injection.
Binding of HRG to immobilized γ′-peptide in the absence or presence of FPRck-thrombin was monitored using a BIAcore T200. Biotinylated γ′-peptide was adsorbed to streptavidin-conjugated CM4 flow cells to 100 RU in the presence of 20 μm ZnCl2 at a flow rate of 5 μl/min. An unmodified flow cell served as a control. Using dual injection mode, 1 μm HRG was injected at 10 μl/min followed by a second injection of FPRck-thrombin or prothrombin at concentrations ranging from 0 to 8 μm. Flow cells were regenerated with 250 mm imidazole, 2 mm EDTA, and 1 m NaCl. All experiments were performed at least twice.
SPR Data Analysis
Kd values were determined by kinetic analysis of on- and off-rates of HRG binding to immobilized ligands using Scrubber2 version 2.0a (Bio-Logic Software Co., Campbell, Australia) as described previously (24, 30). For further assessment of binding, the amount of HRG bound at the equilibrium position (Req) was determined using the Langmuir 1:1 binding model (BIAEvaluation software Version 3.2) and was plotted against the titrant concentration. Molar stoichiometries were determined as described in the BIAtechnology handbook (BIAcore 1000). The correction factor to account for the orientation of the immobilized fibrinogen and fibrin was 0.25, which corresponds to 25% of the amount of immobilized fibrin accessible to the γ′-peptide-directed IgG as determined in a separate study (10). The correction factor for immobilized γ′-peptide was 0.7, which corresponds to 70% correct orientation of peptide accessible to an analyte (BIAcore).
Interaction of 125I-HRG with Fibrin Clots
In a series of microcentrifuge tubes, γA/γA- or γA/γ′-fibrinogen, in concentrations ranging from 0 to 1.25 μm, was clotted with 10 nm thrombin in the presence of 40 nm 125I-HRG as previously described (30). Clots were formed in 20 mm Tris-HCl and 150 mm NaCl, pH 7.4 (TBS), containing 0.005% Tween (TBS-Tween) and 2 mm CaCl2 and 20 μm ZnCl2 or 10 μm sodium diethyldithiocarbamate trihydrate. The concentration of 125I-HRG bound to fibrin was calculated by subtracting the concentration in the clot supernatant from the value obtained in controls prepared in the absence of fibrinogen. Plots of bound 125I-HRG versus fibrin concentration were analyzed by nonlinear regression of a rectangular hyperbola to determine Kd. Experiments were performed twice in duplicate.
Effect of Competitors on the Binding of 125I-HRG to Fibrin Clots
Clots were formed in TBS-Tween containing 2 mm CaCl2 and 20 μm ZnCl2. Fab fragments derived from γ′-peptide-directed IgG were used to assess the contribution of the γ′-chain to 125I-HRG binding to clots. After preincubation of 0.25 μm γA/γA- or γA/γ′-fibrinogen with γ′-peptide-directed Fab fragments or control sheep IgG (0–4 μm) for 1 h at 23 °C, 60 nm 125I-HRG was added, and clots were generated with 20 nm thrombin. To determine whether HRG and thrombin share fibrin binding sites, the effect of FPRck-thrombin on the binding of 125I-HRG was assessed. Samples containing 2 μm γA/γA- or γA/γ′- fibrinogen, 20 nm 125I-HRG, and FPRck-thrombin (0–8 μm) in 0.25% Prionex were clotted with 20 nm thrombin. The effect of varying concentrations of HRG on the binding of 125I-YPRck-thrombin was assessed in a reciprocal experiment. Samples containing 2 μm γA/γA- or 0.25 μm γA/γ′-fibrinogen, 20 nm 125I-YPRck-thrombin, and HRG (0–2 μm) were clotted with 10 nm thrombin. The fraction of 125I-HRG or 125I-YPRck-thrombin bound was determined as described above. All experiments were performed twice in duplicate.
HRG Diffusion from Preformed Fibrin Clots
The rate of 125I-HRG dissociation from γA/γA- or γA/γ′-fibrin clots was determined as previously described (10, 30). Briefly, fibrin clots were formed around plastic inoculation loops (Bac-Loop, Thermo-Fisher Scientific, Waltham, MA) by adding 10 nm thrombin to solutions containing 5 μm γA/γA- or γA/γ′-fibrinogen, 20 nm factor XIII, and 50 nm 125I-HRG in TBS-Tween containing 2 mm CaCl2 and 20 μm ZnCl2. After incubation for 45 min at 23 °C, clots were removed and immersed in buffer containing 2 mm CaCl2 plus 20 μm ZnCl2, 2 mm CaCl2 plus 10 μm diethyldithiocarbamate trihydrate, or 2 m NaCl plus 2 mm EDTA. The fraction of clot-associated 125I-HRG remaining at various times was determined, and time courses were fit to a two-phase exponential decay curve (Table Curve, Jandel Scientific, San Rafael, CA) (10). Experiments were repeated three times.
Detection of HRG-Fibrinogen Complexes in Plasma
HRG-fibrinogen complexes in plasma were detected using a previously described sandwich ELISA (24) with some modifications. Briefly, 100 μl of fibrinogen-directed capture antibody (Affinity Biologicals) diluted to 20 μg/ml in 50 mm NaHCO3, pH 9.6, was added to wells of a 96-well Immunlon 4 HBX plate (Thermo Scientific) and incubated overnight at 4 °C. To block nonspecific binding, 200 μl of 10 mg/ml bovine serum albumin (Sigma) was added to each well and incubated for 1 h at 23 °C. Wells were washed 3 times with 150 μl of phosphate-buffered saline (PBS) containing 10 μm ZnCl2 and 0.1% Tween 20. Normal and HRG-deficient plasma samples were dialyzed against TBS to remove citrate, reconstituted with 18 μm ZnCl2, and then serially diluted up to 1600-fold with HBS containing 1% ovalbumin, 0.1% Tween 20, 2 mm CaCl2, 100 nm hirudin (Behring), and 18 μm ZnCl2. To prepare a reference curve, HRG-fibrinogen complexes were generated by incubating varying concentrations of HRG (0–1.6 μg/ml) with fibrinogen (0–0.8 μg/ml) in a 2:1 molar ratio. 100 μl of each of these mixtures or plasma was added to wells and incubated for 2 h at 23 °C. After three sequential washes with PBS containing ZnCl2, HRG-fibrinogen complexes were detected in purified and plasma systems using a HRG-directed IgG-horseradish peroxidase (HRP) conjugate, prepared as specified by the manufacturer using a Lightning-link HRP conjugation kit (Cedarlane, Burlington, ON). After incubation for 1 h at 23 °C, bound HRP conjugates were detected as specified by the supplier. Experiments were repeated three times.
Interaction of HRG with Fluorescein-labeled γ′-Peptide in the Absence or Presence of ZnCl2
The binding of 1.1 μm HRG to 0.05 μm fluorescein-γ′-peptide was monitored by fluorescence in the absence or presence of Zn2+ using a PerkinElmer Life Sciences LS 50B luminescence spectrometer (9). Briefly, the base-line fluorescence (Io) was determined at excitation and emission wavelengths (slit widths) of 492 (5 nm) and 532 nm (2.5 nm), respectively, and an emission filter at 515 nm. The mixture was then titrated with aliquots of ZnCl2 up to 20 μm, and fluorescence intensity (I) was monitored after each addition. I/Iovalues were plotted against the concentration of ZnCl2, and the data were subjected to nonlinear regression analysis as previously described (9).
Statistical Analyses
Results are presented as the mean ± S.D., and the significance of differences in the means was determined using t tests. For these analyses, p < 0.05 was considered statistically significant.
RESULTS
Interactions of HRG with γA/A- or γA/γ′-Fibrinogen
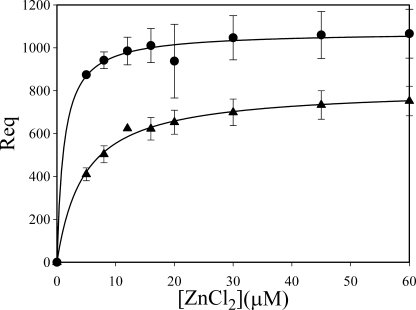
Although HRG has previously been shown to bind fibrinogen (11), the distinction between γA/γA- and γA/γ′-fibrinogen binding has not been investigated. SPR was used to characterize the interaction between HRG and the two isoforms of fibrinogen. γA/γA- or γA/γ′-fibrinogen was immobilized on separate flow cells of a CM4 sensor chip, and an unmodified flow cell served as the control. HRG did not bind either form of fibrinogen in the presence of Ca2+ alone (data not shown). Because Zn2+ facilitates the binding of ligands to HRG (14), we examined the effect of Zn2+ on the HRG-fibrinogen interaction. The Req increased as a function of the Zn2+ concentration and saturated at ∼30 μm ZnCl2 (Fig. 1). At each Zn2+ concentration, more HRG bound to γA/γ′-fibrinogen than to γA/γA-fibrinogen. The apparent Kd values of ZnCl2 necessary to promote HRG binding to γA/γA- or γA/γ′-fibrinogen were 4.9 ± 0.2 and 1.2 ± 0.8 μm, respectively. The Zn2+ dependence of the interaction of HRG with fibrin(ogen) is in apparent contradiction to previous work demonstrating HRG binding to fibrin without Zn2+ addition (11). Because no binding was detected in the absence of Zn2+ in our study, it is likely that the HRG preparation used in the previous report contained sufficient amounts of Zn2+ to enable the interaction. For the remainder of the study, ZnCl2 was used at a concentration of 20 μm. This concentration was chosen because the physiological concentration of Zn2+ in plasma ranges from 10 to 20 μm (15, 31). As evidenced from the similarity in saturation profiles illustrated in Fig. 1, more than 80% of HRG is bound to both forms of fibrinogen at 20 μm ZnCl2.
FIGURE 1.
Effect of ZnCl2 on the binding of HRG to γA/γA- or γA/γ′-fibrinogen. γA/γA-Fibrinogen (triangles) or γA/γ′-fibrinogen (circles) was immobilized to 6000–7000 RU on separate flow cells of a CM4 BIAcore chip. An unmodified flow cell served as control. HRG (0.2 μm) was injected into flow cells in the absence or presence of ZnCl2 at the concentrations indicated. The RU at equilibrium (Req) was calculated and, after background correction, is plotted against the input ZnCl2 concentrations. Data represent the mean ± S.D. of two experiments, and lines represent nonlinear regression analyses of the data.
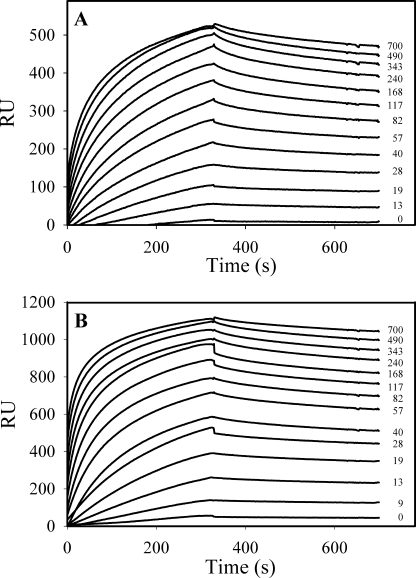
To determine the affinity of HRG for fibrinogen, increasing concentrations of HRG were sequentially injected into flow cells containing immobilized fibrinogen in the presence of 20 μm ZnCl2. The sensograms reveal slow association and dissociation phases for HRG binding to both isoforms of fibrinogen (Fig. 2, A and B). Kd values were obtained by kinetic analysis of the on- and off-rates by globally fitting the binding data. In the presence of Zn2+, HRG binds γA/γA- and γA/γ′-fibrinogen with similar affinity, Kd values of 8.8 ± 0.9 and 8.9 ± 3.9 nm, respectively (Table 1). These values agree with the previously reported Kd of 6.7 nm determined by immunoassay (11). Plots of calculated Req values for each HRG concentration revealed saturable binding and demonstrated that binding of HRG to γA/γ′-fibrinogen was significantly (p < 0.005) higher than that for γA/γA-fibrinogen (Fig. 3), suggesting that 2-fold more HRG is bound to the γA/γ′-fibrinogen isoform. The molar stoichiometries for the interaction of HRG with γA/γA-fibrinogen and γA/γ′-fibrinogen are 1.7 ± 0.3 and 3.2 ± 0.5, respectively (Table 1). Because the extended COOH terminus of the γ′-chain is the feature that distinguishes γA/γ′-fibrinogen from γA/γA-fibrinogen, the increased binding of HRG to γA/γ′-fibrinogen suggests that the γ′-chain provides the additional HRG binding site.
FIGURE 2.
Determination of the affinity of HRG for γA/γA- or γA/γ′-fibrinogen in the presence of ZnCl2. γA/γA-Fibrinogen (A) or γA/γ′-fibrinogen (B) was adsorbed to separate flow cells on a CM4 chip. HRG (0–700 nm) was injected into flow cells for 300 s in the presence of 20 μm ZnCl2, and the cells were then washed with HBS buffer containing 2 mm CaCl2 and 20 μm ZnCl2 for 500 s to monitor dissociation. HRG concentrations in nm are indicated adjacent to each sensogram tracing. These are data from a single experiment, which was performed three times.
TABLE 1.
Dissociation constants and stoichiometries for the binding of HRG to γA /γA-fibrin(ogen), γA /γ′-fibrin(ogen), or γ′-peptide
The binding of HRG to immobilized fibrin(ogen) isoforms or γ′-peptide in the presence of 20 μm Zn2+ was quantified using SPR. Kd values were determined by kinetic analysis of the data, and stoichiometries were calculated according to the BIAtechnology handbook.
| Kd | Stoichiometry | |
|---|---|---|
| nm | HRG/molecule | |
| γA/γA-Fibrinogen | 8.8 ± 0.9 | 1.7 ± 0.3 |
| γA/γ′-Fibrinogen | 8.9 ± 3.9 | 3.2 ± 0.5 |
| γA/γA-Fibrin | 19.3 ± 2.6 | 1.8 ± 0.3 |
| γA/γ′-Fibrin | 10.9 ± 0.9 | 2.9 ± 0.6 |
| γ′-Peptide | 0.8 ± 0.01 | 0.7 ± 0.02 |
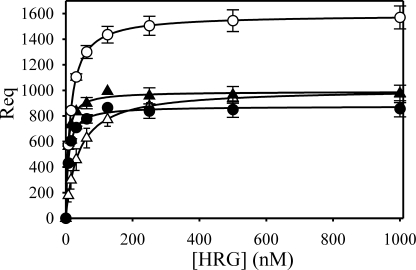
FIGURE 3.
Effect of the γ′-peptide-directed IgG on HRG binding to γA/γA- or γA/γ′-fibrinogen. Flow cells containing immobilized γA/γA-fibrinogen (triangles) or γA/γ′-fibrinogen (circles) were pretreated with (closed) or without (open) 0.5 μm γ′-peptide-directed IgG before injection of HRG (0–1.0 μm). Symbols represent the mean ± S.D. of two experiments, and lines represent nonlinear regression analyses of the data.
Effect of the γ′-Peptide-directed IgG on the Binding of HRG to γA/γA- or γA/γ′-Fibrinogen
To confirm that HRG binds specifically to the γ′-chain of γA/γ′-fibrin(ogen), we examined the effect of an affinity-purified IgG directed against this region. As an initial control, we demonstrated specific binding of the antibody to immobilized γA/γ′-fibrinogen but not to γA/γA-fibrinogen (data not shown). We next examined the effect of the antibody on HRG binding to γA/γ′- or γA/γA-fibrinogen. The γ′-chain-directed antibody reduced HRG binding to γA/γ′-fibrinogen to that observed with γA/γA-fibrinogen (Fig. 3). As a control, a sheep non-immune IgG was used; the control IgG had no effect on the binding of HRG to fibrinogen (data not shown). Because the interaction of HRG with γA/γA-fibrinogen is already of high affinity, the addition of the γ′-peptide-directed IgG did not alter the affinity of HRG for γA/γ′-fibrinogen. However, in the presence of the antibody, the molar stoichiometry of HRG for γA/γ′-fibrinogen was similar to that for γA/γA-fibrinogen (1.8 ± 0.5 and 1.8 ± 0.6, respectively), providing further support for the concept that the γ′-chain on γA/γ′-fibrinogen represents a unique HRG binding site. These data suggest that HRG has multiple high-affinity binding sites on fibrinogen and that blocking one binding site has minimal effects on the others.
HRG Binding to the γ′-Peptide
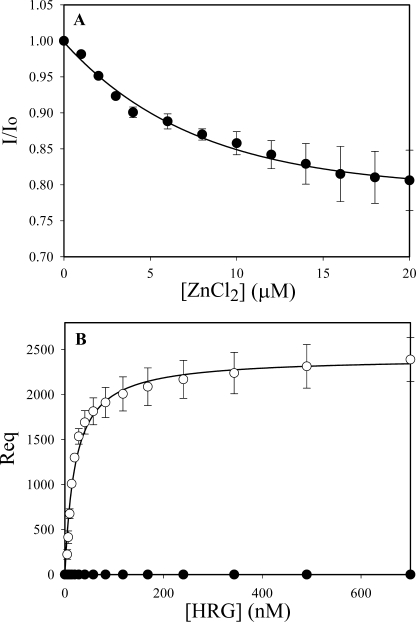
To confirm that the γ′-chain affords HRG an additional binding site, binding of HRG to synthetic γ′-peptide was examined. First, the interaction of HRG with fluorescein-γ′-peptide was examined by fluorescence. Neither HRG nor Zn2+ alone altered the fluorescence intensity of the f-γ′-peptide, suggesting that in the absence of Zn2+, there is no interaction. However, when Zn2+ was titrated in the presence of HRG, the fluorescence intensity of fluorescein-γ′-peptide decreased in a dose-dependent and saturable manner (Fig. 4A), suggesting that Zn2+ facilitates the binding of HRG to the peptide. Nonlinear regression analysis of the data revealed that the apparent Kd for ZnCl2 required to promote the HRG-fluorescein-γ′-peptide interaction was 9.1 ± 4.5 μm. This value is similar to the apparent Kd of 1.2 μm for Zn2+-mediated promotion of HRG binding to γA/γ′-fibrinogen (Fig. 1).
FIGURE 4.
Binding of HRG to γ′-peptide. A, the binding of 1.1 μm HRG to fluorescein-labeled γ′-peptide (50 nm) was monitored in the presence of ZnCl2 (0–20 μm) at λex = 492 nm and λem = 532 nm. Initial fluorescence was determined in the presence of HRG but in the absence of ZnCl2 (Io). Aliquots of ZnCl2 were then added, and the fluorescence intensity (I) was measured after each addition. I/Io values are plotted versus ZnCl2 concentrations. B, binding of HRG to immobilized γ′-peptide was measured by SPR. Biotinylated γ′-peptide was adsorbed to a streptavidin-immobilized CM4 chip. Binding of HRG (0–700 nm) to γ′-peptide in the presence of 20 μm ZnCl2 was then determined in the absence (open symbols) or presence (closed symbols) of the γ′-peptide-directed IgG. Corrected Req values are plotted against the input HRG concentrations. Symbols represent the mean ± S.D. of two experiments, and lines represent nonlinear regression analyses of the data.
To confirm these results, the interaction of HRG with γ′-peptide was examined by SPR. Biotinylated γ′-peptide was adsorbed to a streptavidin-modified flow cell, and HRG binding was monitored in the presence of 20 μm ZnCl2. HRG bound immobilized γ′-peptide in a concentration-dependent and saturable manner, and binding was blocked by the γ′-peptide-directed IgG (Fig. 4B). Based on kinetic analysis, HRG binds the γ′-peptide with a Kd value of 0.79 ± 0.01 nm in the presence of Zn2+; there is no detectable binding in the absence of Zn2+ (data not shown). These data offer independent confirmation that HRG binds to the COOH terminus of the γ′-chain of γA/γ′-fibrinogen in a Zn2+-dependent fashion.
Effect of the γ′-Peptide-directed IgG on the Binding of HRG to γA/γA- or γA/γ′-Fibrin
Having shown that HRG binds fibrinogen with high affinity in a Zn2+-dependent fashion, we next used SPR to determine the affinity of HRG for fibrin. To convert immobilized fibrinogen to fibrin, flow cells were treated with thrombin (30). HRG bound to both isoforms of fibrin with affinities (Table 1) and Req values similar to those for fibrinogen, suggesting that HRG binding is unaltered when fibrinogen is converted to fibrin. To complement the SPR studies, we also assessed the binding of 125I-HRG to fibrin clots. Clots containing varying concentrations of fibrinogen were prepared, and the amount of 125I-HRG in the supernatants of compacted clots was determined. In keeping with our SPR data, 125I-HRG did not bind to fibrin clots in the absence of Zn2+ (data not shown). With 20 μm Zn2+, 125I-HRG bound to γA/γA- and γA/γ′-fibrin clots with Kd values of 105.9 ± 21.0 and 33.3 ± 6.2 nm, respectively (Fig. 5A). Similar results were obtained in the reciprocal experiment using varying concentrations of 125I-HRG and a fixed concentration of fibrinogen (data not shown). The binding constants obtained here are comparable with the previously reported Kd value of 250 nm for the interaction of 125I-HRG with fibrin formed from unfractionated fibrinogen, which consists of both isoforms of fibrinogen (11). Taken together, these results suggest that HRG binds fibrinogen and remains bound when fibrinogen is converted to fibrin.
FIGURE 5.
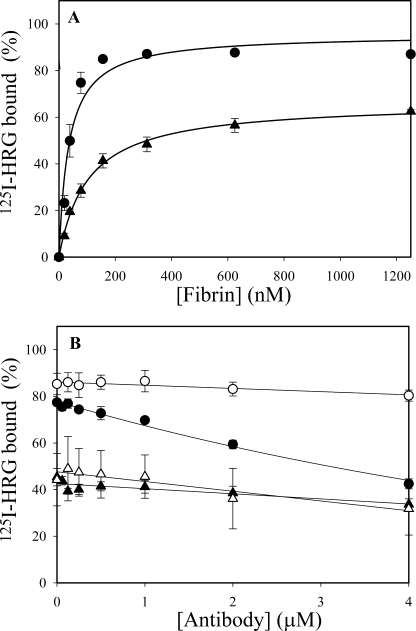
Binding of 125I-HRG to γA/γA- or γA/γ′-fibrin clots. A, 0–1.25 μm γA/γA- (triangles) or γA/γ′-fibrinogen (circles) was added to microcentrifuge tubes, and the binding of 125I-HRG (40 nm) to clots was assessed after thrombin addition. B, the binding of 125I-HRG (20 nm) to 0.25 μm γA/γA- (triangles) or γA/γ′-fibrin clots (circles) was assessed in the presence of 0–4 μm γ′-peptide-directed Fab fragments (closed symbols) or a control sheep IgG (open symbols). Experiments were performed in TBS-Tween containing 20 μm ZnCl2 and 2 mm CaCl2 and clots were generated with 10 nm thrombin. After incubation at 23 °C for 45 min, fibrin clots were pelleted by centrifugation, and the amount of free 125I-HRG in the supernatant was used to calculate the fraction bound. The percent of HRG bound to the clots is plotted versus fibrin or antibody concentrations. Symbols represent the mean ± S.D. of two experiments, each performed in duplicate, whereas the lines represent nonlinear regression analyses of the data.
Next, we examined the effect of varying concentrations of Fab fragments derived from the γ′-peptide-directed IgG on 125I-HRG binding to γA/γA- or γA/γ′-fibrin clots. Fab fragments had minimal effects on HRG binding to γA/γA-fibrin clots (Fig. 5B). In contrast, at 4 μm, the Fab fragments reduced HRG binding to γA/γ′-fibrin clots by 50%, providing further evidence that HRG binds to the γ′-chain of γA/γ′-fibrin. A non-immune sheep IgG was used as a control and demonstrated no effect.
HRG Binding to Fibrinogen Fragments
To localize the HRG binding domains on fibrinogen, binding of HRG to immobilized fibrinogen fragments was examined by SPR (data not shown). To avoid potential contribution of the γ′-chain to HRG binding, fragments X, D, and E were prepared from γA/γA-fibrinogen. In the presence of Zn2+, HRG bound fragment X with a Kd value of 63.5 ± 11.8 nm, suggesting that the αC-domain of fibrinogen does not represent the primary HRG binding site. HRG also bound fragments D and E with high affinity in a Zn2+-dependent manner, with Kd values of 8.0 ± 1.3 and 23.3 ± 2.2 nm, respectively. As a negative control, we demonstrated that HRG did not bind immobilized FPRck-thrombin in the absence or presence of Zn2+. As a positive control, we showed that FPRck-thrombin bound fragment E, with a Kd value of 5.0 ± 0.4 μm, but did not bind fragment D, findings in agreement with previously published results (25). Therefore, these data suggest that, in addition to its interaction with the γ′-chain, HRG binds to other unique sites on fibrinogen.
Diffusion of HRG from Fibrin Clots
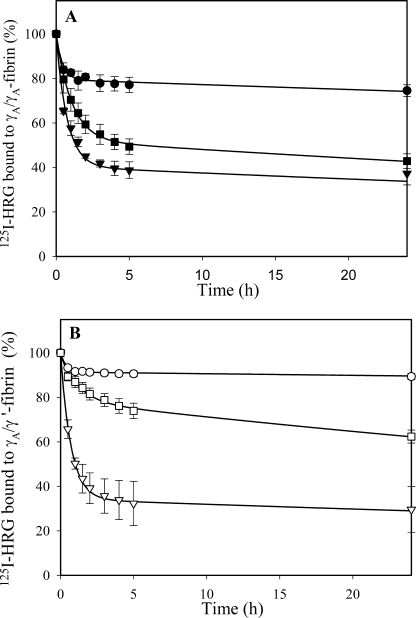
To identify differences in the binding of HRG to γA/γA- and γA/γ′-fibrin clots, we monitored the dissociation of 125I-HRG from preformed fibrin clots (Fig. 6). Diffusion in the presence of 2 m NaCl and 2 mm EDTA served as the base-line control because ionic and divalent cation-dependent interactions are abrogated (10, 30). In the presence of Zn2+, the rates of diffusion of 125I-HRG from γA/γA- and γA/γ′-fibrin clots were significantly (p < 0.05) slowed by 3- and 11-fold, respectively, compared with those determined in the presence of diethyldithiocarbamate trihydrate, a specific Zn2+ chelator (32). Consistent with the concept that γA/γ′-fibrin affords HRG an additional binding site, the rate of 125I-HRG diffusion from γA/γ′-fibrin was 3-fold slower than that from γA/γA-fibrin (p < 0.05). Collectively, our results offer independent confirmation that the HRG-fibrinogen interaction is Zn2+-dependent and that there is an additional HRG binding site on γA/γ′-fibrin.
FIGURE 6.
Dissociation of 125I-HRG from preformed γA/γA- or γA/γ′-fibrin clots. Aliquots of 5 μm γA/γA- (A) or γA/γ′-fibrinogen (B) containing 2 mm CaCl2, 20 μm ZnCl2, 20 nm factor XIII, and 50 nm 125I-HRG were clotted with 10 nm thrombin around plastic inoculation loops. After incubation at 23 °C for 45 min, clots were counted for radioactivity and incubated in tubes containing 5 ml of 2 m NaCl and 2 mm of EDTA (triangles), 2 mm CaCl2 and 10 μm diethyldithiocarbamate trihydrate (squares), or 2 mm CaCl2 and 20 μm ZnCl2 (circles). At intervals, clots were removed, and residual radioactivity was used to determine the percent of 125I-HRG that remained clot-associated. The symbols represent the mean ± S.D. of three experiments, whereas the lines represent nonlinear regression analyses of the data using two-component exponential decay model.
Effect of FPRck-thrombin on HRG Binding to the γ′-Chain
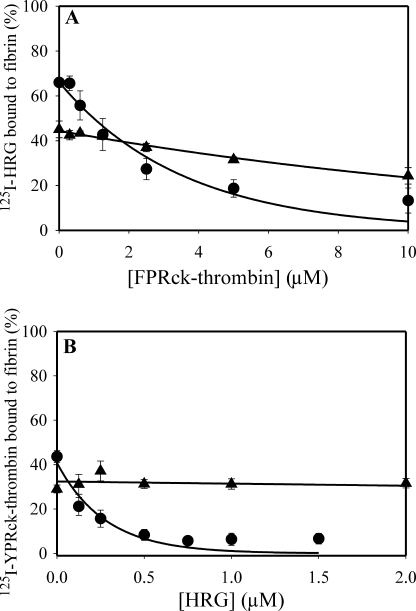
In addition to binding HRG, the γ′-chain COOH extension binds thrombin (5, 9). SPR was used to determine whether the two proteins compete for binding to this region. This approach exploits the fact that the dissociation rate of HRG from immobilized γ′-peptide is much slower than that of thrombin. HRG (1 μm) was injected into flow cells containing immobilized biotinylated γ′-peptide, and the subsequent dissociation phase was monitored in the absence or presence of FPRck-thrombin or prothrombin in concentrations up to 8.0 μm. Prothrombin was used as a negative control because it does not bind to the γ′-peptide (33). Whereas FPRck-thrombin displaced HRG from the γ′-peptide in a concentration-dependent manner, prothrombin did not (Fig. 7). These data confirm that thrombin and HRG compete for binding to the γ′-peptide.
FIGURE 7.
Effect of FPRck-thrombin or prothrombin on the interaction of HRG with γ′-peptide. Biotinylated γ′-peptide was immobilized on a streptavidin-modified flow cell to 200 RU, and an unmodified flow cell served as a control. Arrows indicate injection of 1 μm HRG for 200 s followed by injections of FPRck-thrombin (panel A) or prothrombin (panel B) at the concentrations (μm) indicated. Injections were carried out in the presence of 20 μm Zn2+. These are data from a single experiment, which was performed three times.
To determine whether the same was true with fibrin clots, we next examined the effect of increasing concentrations of FPRck-thrombin on 125I-HRG binding to γA/γA- or γA/γ′-fibrin clots. At 10 μm, FPRck-thrombin reduced the amount of HRG bound to γA/γ′-fibrin clots by 90% but only reduced HRG bound to γA/γA-fibrin clots by 15% (Fig. 8A). Similar results were obtained in the reciprocal competition experiments using varying concentrations of HRG and a fixed concentration of 125I-YPRck-thrombin (Fig. 8B). Collectively, these data confirm that HRG and thrombin compete for binding to the γ′-chain on γA/γ′-fibrin(ogen) in a mutually exclusive fashion.
FIGURE 8.
Effect of competitors on the binding of 125I-HRG or 125I-YPRck-thrombin to γA/γA- or γA/γ′-fibrin clots. A, 125I-HRG (20 nm) was added to microcentrifuge tubes containing 2 μm γA/γA-fibrinogen (triangles) or γA/γ′-fibrinogen (circles) in the presence of FPRck-thrombin (0–10 μm). B, 125I-YPRck-thrombin (20 nm) was added to microcentrifuge tubes containing 2 μm γA/γA-fibrinogen (triangles) or 0.25 μm γA/γ′-fibrinogen (circles) in the presence of HRG (0–2 μm). In both experiments 2 mm CaCl2 plus 20 μm ZnCl2 were present and clotting was initiated with 10 nm thrombin. After incubation at 23 °C for 45 min, fibrin was pelleted by centrifugation, and free 125I-HRG or 125I-YPRck-thrombin in the supernatant was used to calculate the bound fraction. The percent of fibrin-bound 125I-HRG or 125I-YPRck-thrombin is plotted versus the FPRck-thrombin or HRG concentration, respectively. The symbols represent the mean ± S.D. of two experiments, each performed in duplicate, whereas the lines represent nonlinear regression analyses of the data.
Detection of HRG-Fibrinogen Complexes in Plasma
Because HRG binds fibrinogen with high affinity, it was of interest to determine whether HRG-fibrinogen complexes can be detected in plasma by immunoassay. Plasma was first dialyzed to remove citrate and then reconstituted with 18 μm ZnCl2. The concentration of HRG-fibrinogen complexes detected in normal plasma was 1 μm, whereas no complexes were detected in HRG-deficient plasma. Because the plasma concentration of HRG ranges from 1.6 to 2 μm (12, 13, 24), our findings suggest that in the presence of Zn2+ ∼50–60% of HRG in plasma circulates in complex with fibrinogen.
DISCUSSION
Despite increasing evidence that the interaction of HRG with fibrin(ogen) plays an important role in innate immunity, inflammation, and coagulation, little is known about this interaction. To address this gap, we characterized the binding of HRG to γA/γA- and γA/γ′-forms of fibrinogen and fibrin. In the presence of physiological concentrations of Zn2+, HRG binds γA/γA- and γA/γ′-fibrinogen with similar affinities (Kd values of ∼9 nm). The affinities of HRG for both isoforms of fibrin are comparable to those for fibrinogen, suggesting that the conversion of fibrinogen to fibrin does not alter the binding of HRG. In addition to its interaction with the unique COOH terminus of the γ′-chain of γA/γ′-fibrin(ogen), HRG binds to fragments D and E, suggesting that there are several HRG binding sites on fibrinogen. In support of these observations, HRG-fibrinogen complexes were detected in plasma. Taken together, our findings suggest that HRG circulates in plasma bound to fibrinogen and that the complex remains intact when fibrinogen is converted to fibrin.
The absolute requirement for Zn2+ to promote the HRG-fibrinogen interaction underscores the regulatory role this cation may have in hemostasis. Zn2+ is important for the binding of HRG to bacteria, cells, and hemostatic factors, such as glycosaminoglycans, plasminogen, and factor XIIa (12, 19, 24, 34). It is likely that there are still other HRG interactions that have been overlooked because of the widespread use of citrate as an anticoagulant. The total Zn2+ concentration in plasma is ∼20 μm, and the majority of Zn2+ is bound to albumin (35). Although the concentration of Zn2+ that is not bound to proteins is only 0.5–1 μm (31, 36), the free Zn2+ concentration can increase under a variety of conditions. For example, platelets can secrete Zn2+ when they are activated at sites of vascular injury (37). Furthermore, when fatty acids bind to albumin, they displace Zn2+ from the protein, thereby providing another mechanism whereby the concentration of free Zn2+ in plasma can be augmented (35). In addition to alterations in the Zn2+ concentration, the activity of HRG can also be modulated by changes in pH, with optimal binding to ligands observed under more acidic conditions. Consequently, the decrease in pH that occurs with reduced tissue perfusion may also enhance the affinity of HRG for both Zn2+ and its ligands (14). Therefore, the current data extend the concept that Zn2+ serves as a dynamic switch that regulates HRG activity and directs it to various pathways involved in hemostasis (14, 34).
Although we show that Zn2+ is essential for the interaction of HRG with fibrin(ogen), the mechanism by which Zn2+ mediates this binding is unknown. Both HRG and fibrin(ogen) bind Zn2+ (37, 38). Therefore, Zn2+ may act as a cofactor that simultaneously binds HRG and fibrin(ogen) in a coordinated fashion (39). Alternatively, Zn2+ binding to HRG may induce conformational changes that facilitate its interaction with fibrin(ogen), a concept supported by the observation that Zn2+ alters the conformation of a synthetic His-Pro-rich peptide (16). Furthermore, the intrinsic fluorescence of HRG decreases upon Zn2+ titration (data not shown), providing additional support for the notion that Zn2+ alters HRG conformation. Binding of Zn2+ to the HRR domain of HRG indirectly promotes the interaction of heparan sulfate or plasminogen with the NH2-terminal cystatin domains of HRG, suggesting that Zn2+ binding to the HRR domain modulates other domains (15, 40). These data point to a mechanism whereby Zn2+ modulates the structure and function of HRG.
Because HRG binds to the γ′-chain on γA/γ′-fibrin(ogen), novel roles of HRG can be envisioned. In addition to binding HRG, the COOH terminus of the γ′-chain also binds thrombin and factor XIIIa and, by so doing, may modulate coagulation and fibrinolysis (10, 41). The importance of fibrin as a reservoir of thrombin is highlighted by the observation that thrombi harvested at autopsy contain abundant amounts of active thrombin (42). Fibrin-bound thrombin has been postulated to be an important mediator of thrombus expansion because of its capacity to locally activate platelets and to promote its own generation through activation of factor V and factor VIII (43, 44). The procoagulant activity of thrombin bound to γA/γ′-fibrin appears to be greater than that of thrombin bound to γA/γA-fibrin because the γ′-chain mediates the high affinity interaction of thrombin with fibrin, and γA/γ′-fibrin affords bound thrombin more protection from inhibition by the antithrombin-heparin complex than γA/γA-fibrin (10). Further support for this concept comes from epidemiological studies that suggest that higher circulating levels of γA/γ′-fibrinogen are associated with an increased risk of cardiovascular disease (45, 46). HRG competes with thrombin for binding to the γ′-chain as evidenced by its capacity to displace FPRck-thrombin from γ′-peptide or from γA/γ′-fibrin clots. Consequently, by displacing fibrin-bound thrombin, HRG may have antithrombotic properties. HRG may also compete with factor XIII, which is proposed to bind the γ′-chain (47), thereby attenuating fibrin cross-linking and endowing HRG with pro-fibrinolytic activity. Studies in HRG-deficient mice support the concept that HRG affects hemostasis. HRG-deficient mice have a shorter prothrombin time and a longer bleeding time than their wild-type counterparts. In addition, thrombi formed in HRG-deficient mice are more susceptible to fibrinolysis than those generated in control mice (17). The contribution of the HRG interaction with the γ′-chain to the anticoagulant and anti-fibrinolytic activities of HRG remains to be determined.
In addition to its role in hemostasis, fibrinogen appears to be an important mediator of inflammation and innate immunity because fibrinogen and fibrin stimulate peripheral blood mononuclear cells and vascular smooth muscle cells to synthesize proinflammatory cytokines (48–50). Furthermore, bacteria trapped within fibrin clots are protected from host defenses and the action of antibiotics (51). However, because HRG has antimicrobial properties, the HRG-fibrin interaction promotes bacterial entrapment and killing (21). The capacity of HRG to enhance bacterial killing has been localized to its NH2-terminal and HRR domains. Thus, in the presence of Zn2+ or when the pH is low, HRG induces lysis of the bacterial cell wall (19, 20). These conditions can occur at sites of injury or wound-healing where activated platelets release Zn2+ and local ischemia lowers pH (13, 52). Activated platelets also release HRG (13) and, by so doing, may amplify the antimicrobial effect. Our observation that HRG binds fibrinogen and fibrin provides a regulatory mechanism by which HRG may mediate bacterial killing within a clot. The importance of HRG in modulating the inflammatory response has been confirmed in mouse models. Thus, compared with wild-type mice, HRG-deficient mice given subcutaneous injections of S. pyogenes exhibit attenuated abscess formation and reduced recruitment of neutrophils and macrophages to the site of infection (21), suggesting that HRG modulates the inflammatory response. In support of this concept, a synthetic peptide analog of the HRR of HRG attenuated the secretion of interleukin-8 from lipopolysaccharide-stimulated, CD14-transfected monocytes (53). These observations raise the possibility that HRG plays a part in the inflammatory response to infection.
Although HRG is an abundant plasma protein with multiple ligands, its physiological role remains unknown (for review, see Ref. 18). HRG is hypothesized to be an important effector of hemostasis and immunity. The ability of HRG to bind fibrinogen and displace thrombin from fibrin may provide an important link between these two systems and reveals a potential mechanism by which this could occur. Additional regulation may result from variations in the local pH and/or Zn2+ concentration.
This work was supported in part by the Canadian Institutes of Health Research (MOP 3992, MOP 102735, and CTP 79846), the Heart and Stroke Foundation of Ontario (T4792 and T4730), the Heart and Stroke Foundation of Canada (a Novel and Exploratory Research Fund grant), and the Ontario Research and Development Challenge Fund.
- HRG
- histidine-rich glycoprotein
- HRR
- histidine-rich region
- Fab
- fragment antibody binding
- FPRck
- d-Phe-Pro-Arg chloromethyl ketone
- YPRck
- d-Tyr-Pro-Arg chloromethyl ketone
- HBS
- Hepes-buffered saline
- γ′-peptide
- synthetic analog of the COOH-terminal portion of the γ′-chain of γA/γ′-fibrinogen
- RU
- response units
- Req
- amount of ligand bound to immobilized protein at equilibrium
- SPR
- surface plasmon resonance.
REFERENCES
- 1. Henschen A., Lottspeich F., Kehl M., Southan C. (1983) Ann. N.Y. Acad. Sci. 408, 28–43 [DOI] [PubMed] [Google Scholar]
- 2. Wolfenstein-Todel C., Mosesson M. W. (1981) Biochemistry 20, 6146–6149 [DOI] [PubMed] [Google Scholar]
- 3. Chung D. W., Davie E. W. (1984) Biochemistry 23, 4232–4236 [DOI] [PubMed] [Google Scholar]
- 4. Fornace A. J., Jr., Cummings D. E., Comeau C. M., Kant J. A., Crabtree G. R. (1984) J. Biol. Chem. 259, 12826–12830 [PubMed] [Google Scholar]
- 5. Meh D. A., Siebenlist K. R., Mosesson M. W. (1996) J. Biol. Chem. 271, 23121–23125 [DOI] [PubMed] [Google Scholar]
- 6. Weitz J. I., Hudoba M., Massel D., Maraganore J., Hirsh J. (1990) J. Clin. Invest. 86, 385–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jackman M. P., Parry M. A., Hofsteenge J., Stone S. R. (1992) J. Biol. Chem. 267, 15375–15383 [PubMed] [Google Scholar]
- 8. Liu L. W., Vu T. K., Esmon C. T., Coughlin S. R. (1991) J. Biol. Chem. 266, 16977–16980 [PubMed] [Google Scholar]
- 9. Pospisil C. H., Stafford A. R., Fredenburgh J. C., Weitz J. I. (2003) J. Biol. Chem. 278, 21584–21591 [DOI] [PubMed] [Google Scholar]
- 10. Fredenburgh J. C., Stafford A. R., Leslie B. A., Weitz J. I. (2008) J. Biol. Chem. 283, 2470–2477 [DOI] [PubMed] [Google Scholar]
- 11. Leung L. L. (1986) J. Clin. Invest. 77, 1305–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lijnen H. R., Hoylaerts M., Collen D. (1980) J. Biol. Chem. 255, 10214–10222 [PubMed] [Google Scholar]
- 13. Leung L. L., Harpel P. C., Nachman R. L., Rabellino E. M. (1983) Blood 62, 1016–1021 [PubMed] [Google Scholar]
- 14. Jones A. L., Hulett M. D., Parish C. R. (2005) Immunol. Cell Biol. 83, 106–118 [DOI] [PubMed] [Google Scholar]
- 15. Jones A. L., Hulett M. D., Parish C. R. (2004) J. Biol. Chem. 279, 30114–30122 [DOI] [PubMed] [Google Scholar]
- 16. Jancsó A., Kolozsi A., Gyurcsik B., Nagy N. V., Gajda T. (2009) J. Inorg. Biochem. 103, 1634–1643 [DOI] [PubMed] [Google Scholar]
- 17. Tsuchida-Straeten N., Ensslen S., Schäfer C., Wöltje M., Denecke B., Moser M., Gräber S., Wakabayashi S., Koide T., Jahnen-Dechent W. (2005) J. Thromb. Haemost. 3, 865–872 [DOI] [PubMed] [Google Scholar]
- 18. Poon I. K., Patel K. K., Davis D. S., Parish C. R., Hulett M. D. (2011) Blood 117, 2093–2101 [DOI] [PubMed] [Google Scholar]
- 19. Rydengård V., Andersson, Nordahl E., Schmidtchen A. (2006) FEBS J. 273, 2399–2406 [DOI] [PubMed] [Google Scholar]
- 20. Rydengård V., Olsson A. K., Mörgelin M., Schmidtchen A. (2007) FEBS J. 274, 377–389 [DOI] [PubMed] [Google Scholar]
- 21. Shannon O., Rydengård V., Schmidtchen A., Mörgelin M., Alm P., Sørensen O. E., Björck L. (2010) Blood 116, 2365–2372 [DOI] [PubMed] [Google Scholar]
- 22. Lovely R. S., Moaddel M., Farrell D. H. (2003) J. Thromb. Haemost. 1, 124–131 [DOI] [PubMed] [Google Scholar]
- 23. Schaefer A. V., Leslie B. A., Rischke J. A., Stafford A. R., Fredenburgh J. C., Weitz J. I. (2006) Biochemistry 45, 4257–4265 [DOI] [PubMed] [Google Scholar]
- 24. MacQuarrie J. L., Stafford A. R., Yau J. W., Leslie B. A., Vu T. T., Fredenburgh J. C., Weitz J. I. (2011) Blood 117, 4134–4141 [DOI] [PubMed] [Google Scholar]
- 25. Kaczmarek E., McDonagh J. (1988) J. Biol. Chem. 263, 13896–13900 [PubMed] [Google Scholar]
- 26. Olman M. A., Williams W. F., Strickland J. H., Jr., Hagood J. S., Simmons W. L., Rivera K. E. (1998) Protein Expr. Purif. 14, 71–78 [DOI] [PubMed] [Google Scholar]
- 27. Marder V. J., Shulman N. R., Carroll W. R. (1969) J. Biol. Chem. 244, 2111–2119 [PubMed] [Google Scholar]
- 28. Nikula T. K., Bocchia M., Curcio M. J., Sgouros G., Ma Y., Finn R. D., Scheinberg D. A. (1995) Mol. Immunol. 32, 865–872 [DOI] [PubMed] [Google Scholar]
- 29. Fredenburgh J. C., Stafford A. R., Weitz J. I. (2001) J. Biol. Chem. 276, 44828–44834 [DOI] [PubMed] [Google Scholar]
- 30. Petrera N. S., Stafford A. R., Leslie B. A., Kretz C. A., Fredenburgh J. C., Weitz J. I. (2009) J. Biol. Chem. 284, 25620–25629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gorgani N. N., Altin J. G., Parish C. R. (1999) Immunology 98, 456–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lakomaa E. L., Sato S., Goldberg A. M., Frazier J. M. (1982) Toxicol. Appl. Pharmacol. 65, 286–290 [DOI] [PubMed] [Google Scholar]
- 33. Kretz C. A., Stafford A. R., Fredenburgh J. C., Weitz J. I. (2006) J. Biol. Chem. 281, 37477–37485 [DOI] [PubMed] [Google Scholar]
- 34. Borza D. B., Morgan W. T. (1998) J. Biol. Chem. 273, 5493–5499 [DOI] [PubMed] [Google Scholar]
- 35. Stewart A. J., Blindauer C. A., Sadler P. J. (2009) Biochimie 91, 1518–1522 [DOI] [PubMed] [Google Scholar]
- 36. Foote J. W., Delves H. T. (1984) J. Clin. Pathol. 37, 1050–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marx G., Korner G., Mou X., Gorodetsky R. (1993) J. Cell. Physiol. 156, 437–442 [DOI] [PubMed] [Google Scholar]
- 38. Morgan W. T. (1978) Biochim. Biophys. Acta 535, 319–333 [DOI] [PubMed] [Google Scholar]
- 39. Maret W. (2004) J. Anal. At. Spectrom. 19, 15–19 [Google Scholar]
- 40. Jones A. L., Hulett M. D., Altin J. G., Hogg P., Parish C. R. (2004) J. Biol. Chem. 279, 38267–38276 [DOI] [PubMed] [Google Scholar]
- 41. Lorand L. (2001) Ann. N.Y. Acad. Sci. 936, 291–311 [DOI] [PubMed] [Google Scholar]
- 42. Mutch N. J., Robbie L. A., Booth N. A. (2001) Thromb. Haemost. 86, 1028–1034 [PubMed] [Google Scholar]
- 43. Kumar R., Béguin S., Hemker H. C. (1995) Thromb. Haemost. 74, 962–968 [PubMed] [Google Scholar]
- 44. Weitz J. I., Bates E. R. (2003) Cardiovasc. Toxicol. 3, 13–25 [DOI] [PubMed] [Google Scholar]
- 45. Lovely R. S., Kazmierczak S. C., Massaro J. M., D'Agostino R. B., Sr., O'Donnell C. J., Farrell D. H. (2010) Clin. Chem. 56, 781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. van den Herik E. G., Cheung E. Y., de Lau L. M., den Hertog H. M., Leebeek F. W., Dippel D. W., Koudstaal P. J., de Maat M. P. (2011) Thromb. Haemost. 105, 430–434 [DOI] [PubMed] [Google Scholar]
- 47. Siebenlist K. R., Meh D. A., Mosesson M. W. (1996) Biochemistry 35, 10448–10453 [DOI] [PubMed] [Google Scholar]
- 48. Jensen T., Kierulf P., Sandset P. M., Klingenberg O., Joø G. B., Godal H. C., Skjønsberg O. H. (2007) Thromb. Haemost. 97, 822–829 [DOI] [PubMed] [Google Scholar]
- 49. Guo F., Liu J., Wang C., Liu N., Lu P. (2009) Biochem. Biophys. Res. Commun. 390, 942–946 [DOI] [PubMed] [Google Scholar]
- 50. Lu P. P., Liu J. T., Liu N., Guo F., Ji Y. Y., Pang X. (2011) Life Sci. 88, 839–845 [DOI] [PubMed] [Google Scholar]
- 51. Turcotte A., Bergeron M. G. (1992) Antimicrob. Agents Chemother. 36, 2211–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. LaManna J. C. (1996) Adv. Exp. Med. Biol. 388, 283–292 [DOI] [PubMed] [Google Scholar]
- 53. Bosshart H., Heinzelmann M. (2003) FEBS Lett. 553, 135–140 [DOI] [PubMed] [Google Scholar]