Abstract
Endothelial NOS (eNOS)-derived NO is a key factor in regulating microvascular permeability. We demonstrated previously that eNOS translocation from the plasma membrane to the cytosol is required for hyperpermeability. Herein, we tested the hypothesis that eNOS activation in the cytosol is necessary for agonist-induced hyperpermeability. To study the fundamental properties of endothelial cell monolayer permeability, we generated ECV-304 cells that stably express cDNA constructs targeting eNOS to the cytosol or plasma membrane. eNOS-transfected ECV-304 cells recapitulate the eNOS translocation and permeability properties of postcapillary venular endothelial cells (Sánchez, F. A., Rana, R., Kim, D. D., Iwahashi, T., Zheng, R., Lal, B. K., Gordon, D. M., Meininger, C. J., and Durán, W. N. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 6849–6853). We used platelet-activating factor (PAF) as a proinflammatory agonist. PAF activated eNOS by increasing phosphorylation of Ser-1177 and inducing dephosphorylation of Thr-495, increasing NO production, and elevating permeability to FITC-dextran 70 in monolayers of cells expressing wild-type and cytosolic eNOS. PAF failed to increase permeability to FITC-dextran 70 in monolayers of cells transfected with eNOS targeted to the plasma membrane. Interestingly, this occurred despite eNOS Ser-1177 phosphorylation and production of comparable amounts of NO. Our results demonstrate that the presence of eNOS in the cytosol is necessary for PAF-induced hyperpermeability. Our data provide new insights into the dynamics of eNOS and eNOS-derived NO in the process of inflammation.
Keywords: Endothelium, Guanylate Cyclase (Guanylyl Cyclase), Inflammation, Nitric Oxide, Nitric-oxide Synthase, Endothelial Nitric-oxide Synthase, Endothelial Permeability
Introduction
Increased microvascular permeability occurs in response to inflammatory agents, bacterial infection, angiogenesis, and migration of tumor cells. Endothelial NOS (eNOS)2 and eNOS-derived NO are fundamental factors in eliciting the hyperpermeability response in vivo and in vitro (1–3). eNOS is tightly regulated by protein-protein interactions and by phosphorylation (4–8); however, the physiological and pathophysiological significance of this change in NO output is poorly understood. We are pursuing the hypothesis that translocation of eNOS to specific subcellular compartments is a determinant of eNOS-regulated microvascular function.
Platelet-activating factor (PAF) is a potent proinflammatory agent that increases microvascular endothelial permeability in vivo and in vitro (2, 9, 10). We have demonstrated a strong correlation between PAF-induced eNOS activation and hyperpermeability such that depletion of endogenous eNOS by siRNA abolished PAF-stimulated hyperpermeability in bovine coronary venular endothelial cells (3). Furthermore, we demonstrated that eNOS internalization via caveolar endocytosis is an absolute requirement to increase permeability in response to PAF and VEGF, a finding that suggests that eNOS has to be located properly so that NO can reach its targets to induce its microvascular function (11).
In this study, we employed PAF as an agonist to address the question of whether or not eNOS location in the cytosol is necessary to stimulate the onset of hyperpermeability. Specific location of eNOS was achieved by transfecting cells with eNOS cDNA constructs that encode mutant proteins fused to GFP and target eNOS to specific subcellular compartments. Our results demonstrate that the presence of eNOS in the cytosol is necessary for PAF-induced hyperpermeability.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
ECV-304 and ECV-eNOS-GFP cells (a gift from W. C. Sessa, Yale University, New Haven, CT) were grown in Dulbecco's modified Eagle's medium supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 1 mm l-glutamine, and 100 IU/ml penicillin. ECV-eNOS-GFP cells were additionally supplemented with 400 μg/ml Geneticin (Invitrogen). ECV-304 cells were transfected with 5 μg of cDNA by electroporation according to the manufacturer's instructions (Amaxa Biosystems, Gaithersburg, MD). We transfected ECV-304 cells with cDNA constructs GFP-eNOS-G2A (cytosolic) (12) and GFP-eNOS-CAAX (plasma membrane) (13).
The eNOS-G2A construct contains a mutation in which glycine at position 2 is exchanged for alanine. This prevents the co-translational attachment of myristic acid to glycine 2 and also the post-translational attachment of palmitic acid to cysteines 15 and 26. This modification does not modify enzyme activity but greatly diminishes the ability of eNOS to target intracellular membranes (14). eNOS-CAAX is a fusion protein that combines eNOS-G2A with the C-terminal plasma membrane-targeting domain from K-ras (GKKKKKKSKTKCVIM). This modification targets the cytosolic eNOS-G2A construct to the plasma membrane (13).
Immunofluorescence Microscopy
Cells were grown on glass coverslips to form a confluent monolayer. Cells were fixed and permeabilized in methanol at −20 °C for 10 min and observed under a Zeiss Axiovert 100 microscope using a 63×/numerical aperture 1.4 oil objective. The fluorescence of GFP was excited at 488 nm. Image acquisition and basic image processing were performed with AxioVision Release 4.5 software (Carl Zeiss, Inc.).
Western Blot Analysis
Cells were grown to confluence in 100-mm plates. Proteins were extracted on ice with lysis buffer containing 1% Triton X-100, 50 mm Tris (pH 7.4), 150 mm NaCl, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm PMSF, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mm Na3VO4, and 50 mm NaF (2, 15). Protein concentration was determined by the bicinchoninic acid protein assay. Mouse anti-eNOS, mouse anti-phospho-Ser-1177 eNOS, and mouse anti-phospho-Thr-495 eNOS antibodies were from BD Transduction Laboratories. Mouse anti-β-actin, rabbit anti-α1-soluble guanylyl cyclase, and rabbit anti-pan-cadherin antibodies were from Sigma. Rabbit anti-GFP antibody was from Clontech. The corresponding secondary antibodies coupled to horseradish peroxidase were from Pierce and Sigma. Proteins of interest were detected by ECL (Pierce). We analyzed Western blots densitometrically using the NIH ImageJ program.
Immunoprecipitation
Cell lysates (500 μg) were incubated and precleared with 5 μl of protein A/G-agarose (Santa Cruz Biotechnology) for 30 min at 4 °C on a rotator. The supernatant from a 14,000 × g centrifugation was collected and incubated with 10 μg of anti-eNOS antibody for 2 h at 4 °C on a rotator. 10 μg of nonspecific mouse IgG served as a negative control. Immediately afterward, 20 μl of protein A/G-agarose was added, and the mixture was incubated at 4 °CC for 1 h on the rotator. Samples were subsequently centrifuged for 1 min at 14,000 × g, washed four times with lysis buffer, and prepared for Western blotting.
Subcellular Fractionation
To separate total membrane from cytosolic fractions, two confluent 100-mm plates were used per point. Cell pellets were resuspended in 1 ml of homogenization buffer (50 mm Tris-HCl, 0.1 mm EDTA, 0.1 mm EGTA, antiprotease mixture (pH 7.5)). The cell suspension was sonicated at 4 °C and then centrifuged at 55,000 rpm for 90 min in a Beckman Coulter Optima ultracentrifuge at 4 °C. The supernatant was saved as cytosolic fractions, and the pellet was saved as total membrane fractions.
NO Measurements
NO production was measured using NO-sensitive recessed-tip microelectrodes (11, 16). A calibration range for NO of 0–600-1200 nm was established using nitrogen-equilibrated saline as a zero reference for NO and 400 and 800 ppm NO in nitrogen. Coverslips containing confluent cells were placed in a perfusion chamber. The cells were superfused at a rate of 1 ml/min. This rate produced a shear stress of 1.0 × 10−4 dyne/cm2. PAF (Sigma) was added through a side port in the perfusion line to achieve a concentration of 100 nm.
Permeability Measurements
Transfected ECV-304 cells were grown on fibronectin-coated polycarbonate membranes (12-mm diameter, 0.4-μm pore size; Costar) for 5–6 days to achieve confluence. Upon confluence, cells were serum-starved for at least 3 h. On the day of the experiment, the membranes were placed in a diffusion chamber (NaviCyte Scientific, San Diego, CA). The permeability of the monolayer to FITC-dextran 70 was determined according to the Fick equation (2, 11, 15). Samples for base-line permeability were obtained every 15 min for a period of 60 min. After the addition of 100 nm PAF, samples were obtained for an additional 60 min (supplemental Fig. S1).
Statistical Analysis
Data are presented as means ± S.E. Groups were analyzed for differences by one-way analysis of variance, followed by Tukey's test. Significance was accepted at p < 0.05.
RESULTS
Targeting of eNOS Constructs
ECV-304 cells were used for this study because they resemble endothelial cells morphologically and functionally but do not express eNOS (2, 17). ECV-304 cells, which were based originally on human umbilical vein endothelial cells, have been greatly modified, including loss of eNOS and contamination with epithelial cells from a human urinary bladder carcinoma (18–20). ECV-304 cells were transfected with eNOS-GFP and with eNOS-GFP constructs that target eNOS to either the plasma membrane (GFP-eNOS-CAAX) or the cytosol (GFP-eNOS-G2A) (12, 13). These conditions allowed us to activate eNOS and to study the functional impact of its intracellular location.
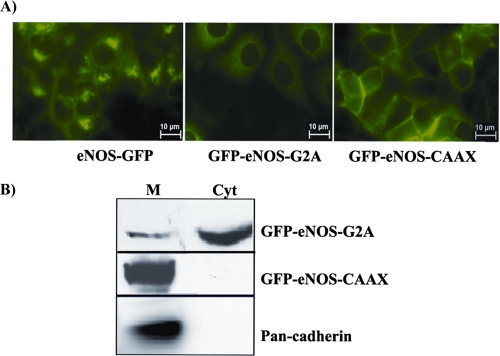
The expression of eNOS constructs in ECV-304 cells was verified by Western blotting and immunofluorescence. Human umbilical vein endothelial cells were used as a positive control for eNOS. As shown in Fig. 1A, eNOS-GFP was present primarily in the plasma membrane and in the perinuclear/Golgi region, which is in agreement with the distribution of endogenous eNOS in endothelial cells. GFP-eNOS-G2A was distributed throughout the cytosol, and GFP-eNOS-CAAX was seen clearly in the plasma membrane of the transfected ECV-304 cells. Additionally, we verified the location of eNOS constructs by subcellular fractionation (Fig. 1B). Total membrane and cytosolic fractions were separated by sonication in hypotonic buffer and ultracentrifugation. We used pan-cadherin as a plasma membrane marker. Fig. 1B shows cytosolic eNOS (G2A) in total membrane and cytosolic fractions; however, it was more concentrated in the cytosolic fraction. Plasma membrane eNOS (CAAX) was observed only in the total membrane fraction. These observations confirmed that these constructs effectively target eNOS to specific subcellular compartments (12, 13). We also confirmed that PAF does not translocate GFP-eNOS-CAAX away from the plasma membrane (supplemental Fig. S2).
FIGURE 1.
Expression and location of eNOS constructs in ECV-304 cells. A, immunofluorescence images showing the targeting of the eNOS constructs. B, subcellular fractionation of ECV-GFP-eNOS-G2A and ECV-GFP-eNOS-CAAX cells. M, membrane; Cyt, cytosol.
PAF Induces Phosphorylation in Targeted eNOS Constructs
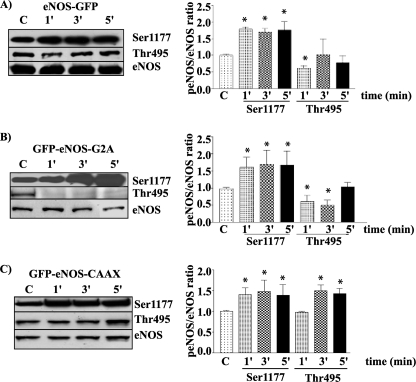
We measured the time course of eNOS phosphorylation as an index of enzyme activation. We stimulated ECV-304 cells stably transfected with eNOS-GFP, GFP-eNOS-G2A, and GFP-eNOS-CAAX with 100 nm PAF for 1, 3, and 5 min and evaluated the phosphorylation of eNOS at Ser-1177 and its dephosphorylation at Thr-495 by Western blotting. Western blot data were analyzed by the densitometric ratio of phosphorylated eNOS to total eNOS. Fig. 2A shows that eNOS-GFP was phosphorylated by 100 nm PAF at Ser-1177 as early as at 1 min and stayed phosphorylated at all times tested, whereas it was significantly dephosphorylated at Thr-495 at 1 min and returned toward base-line levels thereafter. Fig. 2B shows that 100 nm PAF induced significant phosphorylation of cytosolic GFP-eNOS-G2A at Ser-1177 at 1 and 5 min and significant dephosphorylation at Thr-495 at 1 and 3 min. Fig. 2C shows that 100 nm PAF significantly phosphorylated GFP-eNOS-CAAX at Ser-1177 at 1 and 3 min. Interestingly, PAF did not dephosphorylate GFP-eNOS-CAAX at Thr-495 at 1 min but caused significant phosphorylation at this site when applied for 3 and 5 min.
FIGURE 2.
PAF modulates the patterns of phosphorylation in ECV-304 cells transfected with different targeted eNOS constructs. Left panels, Western blots; right panels, quantification. A, phosphorylation of ECV-eNOS-GFP cells at Ser-1177 and Thr-495 (n = 3). B, phosphorylation (p) of ECV-GFP-eNOS-G2A cells at Ser-1177 and Thr-495 (n = 4). C, phosphorylation of ECV-GFP-eNOS-CAAX cells at Ser-1177 and Thr-495 (n = 3). *, p < 0.05 compared with the control (C).
NO Production by the Different Targeted eNOS Constructs
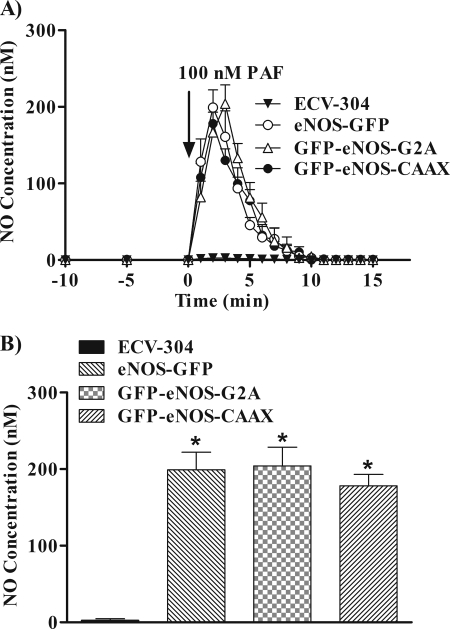
To test further activation of the enzyme, we measured PAF-stimulated NO production as a functional index of eNOS activation. Fig. 3A shows that 100 nm PAF significantly induced NO production in ECV-304 cells transfected with eNOS-GFP, GFP-eNOS-G2A, and GFP-eNOS-CAAX. Production of NO increased immediately after PAF application and exhibited the same kinetics in all ECV-304 cells transfected with targeted eNOS. PAF did not increase NO production in naive ECV-304 cells (Fig. 4A) and in ECV-304 cells transfected with the empty vector (data not shown). We chose the peak value of NO production for the statistical analysis of PAF action as a function of eNOS location. Fig. 3B displays the mean peak NO values elicited by PAF. For eNOS-GFP, the mean ± S.E. was 199.0 ± 23.2 nm; for GFP-eNOS-G2A, the mean peak was 204.2 ± 24.4 nm; and for GFP-eNOS-CAAX, it was 178 ± 15.1 nm. The mean ± S.E. for ECV-304 cells was 2.5 ± 1.8, i.e. not different from base line.
FIGURE 3.
NO production stimulated by 100 nm PAF in naive ECV-304 cells and in ECV-304 cells transfected with different targeted eNOS constructs. The different cell lines grown on glass coverslips were challenged with 100 nm PAF, and NO production was measured as a function of time. A, time course of PAF-induced changes in NO concentration. Base-line production of NO was undetectable. ECV-304 cells transfected with the control empty vector behaved as naive ECV-304 cells and showed no changes in base-line NO concentration and no changes in NO production in response to PAF (not shown). B, statistical analysis of base-line and PAF-induced peak NO concentrations for each of the different cell lines (mean ± S.E., n = 4). *, p < 0.05.
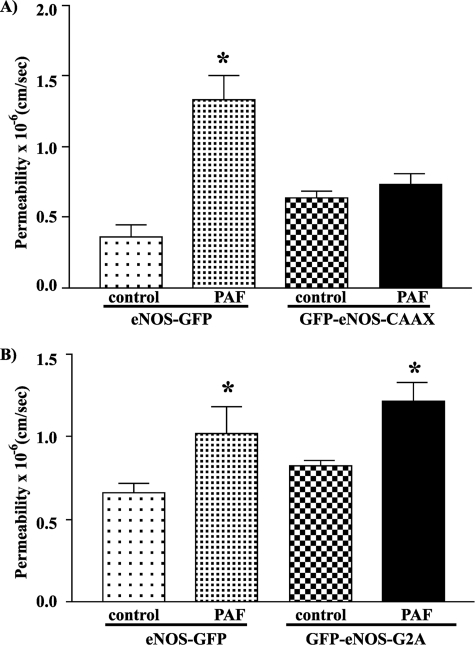
FIGURE 4.
Permeability measurements in ECV-304 cells transfected with different targeted eNOS constructs. Permeability data are expressed as paired bars expressing mean permeability ± S.E. for the base line and after 100 nm PAF. A and B illustrate independent experiments. A, ECV-GFP-eNOS-CAAX cells did not increase permeability in response to PAF (n = 3). B, ECV-GFP-eNOS-G2A cells showed a significant increase in permeability in response to PAF (n = 6). *, p < 0.05.
Importance of eNOS Location for PAF-stimulated Hyperpermeability
To test the relevance of eNOS location to the microvascular functions of the enzyme, we measured the monolayer permeability to FITC-dextran 70 in cells expressing the various targeted eNOS constructs. PAF does not increase permeability to FITC-dextran 70 in untransfected ECV-304 cells, as these cells lack eNOS (2).
PAF produced a nearly 3-fold increment in permeability to FITC-dextran 70 in eNOS-GFP monolayers (Fig. 4A), which served as the reference control. PAF at 100 nm failed to elicit hyperpermeability in monolayers of ECV-304 cells transfected with GFP-eNOS-CAAX (Fig. 4A). The mean permeability values for ECV-GFP-eNOS-CAAX cells (i.e. transfected with eNOS targeted to the plasma membrane) were (0.64 ± 0.05) × 10−6 cm/s (base-line control) and (0.74 ± 0.08) × 10−6 cm/s after application of PAF (p > 0.05).
In contrast to the preceding observations, PAF significantly stimulated hyperpermeability to FITC-dextran 70 in GFP-eNOS-G2A, in which eNOS is targeted to the cytosol (Fig. 4B). The data showed a significant increase in permeability to FITC-dextran 70 from (0.82 ± 0.03) × 10−6 to (1.21 ± 0.11) × 10−6 cm/s (Fig. 4B), which is comparable with the results obtained in ECV-304 cells transfected with eNOS-GFP.
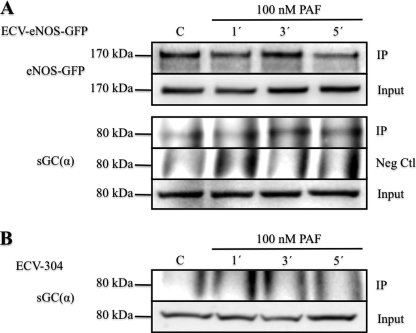
Because NO production was comparable in the eNOS-targeted cells and yet stimulation of hyperpermeability was different, we reasoned that eNOS probably interacts with another key protein in the cytosol to elicit hyperpermeability. Because soluble guanylyl cyclase (sGC) is the most common NO receptor, we tested for association between eNOS and sGC. Fig. 5 shows by co-immunoprecipitation that PAF stimulated association of eNOS with sGC in a time-dependent fashion in ECV-eNOS-GFP cells, which peaked at 3 min. We tested the specificity of this association through a traditional negative control and in ECV-304 cells that lack eNOS. Fig. 5 shows that sGC was not detected in the pulldown assay with anti-eNOS antibody in ECV-304 cells. We confirmed by immunofluorescence microscopy that GFP-eNOS-G2A colocalized with sGC in the cytosol under control and PAF-stimulated conditions (supplemental Fig. S3).
FIGURE 5.
Association of eNOS and sGC. A, experiments in ECV-eNOS-GFP cells. Samples were immunoprecipitated with either anti-eNOS antibody (IP) or nonspecific IgG (negative control (Neg Ctl)) and subsequently blotted for sGC. IP blots correspond to samples obtained after the pulldown process, whereas Input blots correspond to samples before immunoprecipitation and serve as a loading reference. PAF stimulated the time-dependent association of eNOS and sGC in ECV-eNOS-GFP cells. B, experiments in ECV-304 cells that lack eNOS and served as a second negative control for eNOS and as a test for the specificity of anti-eNOS antibody. Samples were immunoprecipitated with anti-eNOS antibody and blotted against sGC. C, control without PAF. n = 3 in both panels.
DISCUSSION
Using differentially targeted eNOS constructs in ECV-304 cells that do not express endogenous eNOS, we have demonstrated that the presence of eNOS in the cytosol is necessary to cause an increase in permeability in response to PAF. We used ECV-304 cells in our study as a model to investigate fundamental processes in endothelial cells for two principal reasons. 1) Their lack of eNOS allows for transfection of eNOS constructs that target eNOS to specific subcellular locations and report on the functional consequence of the specific location; 2) even though not identical to endothelial cells (19–21), as they do not express eNOS (2, 17), they serve as models for functional studies of endothelial cells. In fact, ECV-304 cells were our second choice for this research. We attempted first to deplete eNOS in postcapillary venular endothelial cells (3, 11) with siRNA and reconstitute eNOS in the depleted or knockdown endothelial cells. Despite the inherent advantages of using true endothelial cells engaged normally in microvascular transport, transfection is fraught with problems in endothelial cells, and the problems are compounded in double-transfection experiments. In addition, the rate of growth of double-transfected endothelial cells does not allow them to reach a level of monolayer confluence compatible with measurements of transport across the monolayer while endogenous eNOS is knockdown. Because we have shown previously that ECV-304 cells transfected with eNOS-GFP reproduce the molecular biology associated with the regulation of microvascular transport as compared with in vivo experiments (2), ECV-304 cells represent an adequate model to investigate the functional significance of eNOS location in our study.
Our report is one of the first demonstrations that the location of eNOS is a major determinant for the onset of PAF-induced hyperpermeability. We showed previously that internalization of eNOS via caveolae was required to stimulate hyperpermeability in response to PAF and VEGF (11). Here, we provide strong support for the concept that eNOS must be located in the cytosol to modulate, via NO production, the effector(s) that initiates the development of hyperpermeability. The requirement for NO production by cytosolic eNOS for the onset of hyperpermeability is supported by the observations that PAF and VEGF fail to elicit a hyperpermeability response in tissues of eNOS knock-out mice (1, 22), which express other NOS isoforms. We report here evidence that eNOS associates with sGC in the cytosol. Association with sGC agrees with the role of cGMP and ERK1/2 in promoting hyperpermeability (23).
We confirmed the phosphorylation of eNOS at Ser-1177 in response to stimulating agonists (2, 4, 6) and have shown that the ability of PAF to induce eNOS phosphorylation at Ser-1177 is independent of the location of the enzyme (Fig. 2, A–C). Importantly, our findings suggest that the intracellular location of the enzyme may play an important role in the usually observed dephosphorylation of eNOS at Thr-495. PAF-induced activation of eNOS-GFP and cytosolic eNOS (G2A) resulted in the dephosphorylation of eNOS at Thr-495 (Fig. 2, A and B). However, PAF failed to dephosphorylate eNOS at Thr-495 in plasma membrane-targeted GFP-eNOS-CAAX at the tested times. Instead, exposure to PAF for 3 and 5 min led to significant phosphorylation of eNOS at Thr-495 in these cells (Fig. 2C). Whether this unusual finding in GFP-eNOS-CAAX is caused exclusively by eNOS location to the plasma membrane or is due to unforeseen modifications induced by the targeting process remains to be elucidated.
Our results also confirmed the ability of eNOS to produce NO independent of its subcellular location (24, 25), as we found no significant differences in production of NO among the three different targeted eNOS constructs. Our results are at variance with reports indicating that eNOS produces more NO when located in the cell membrane or the Golgi than when located in the cytosol (12, 25–28). Those studies, which also used non-endothelial cells as the primary models for fundamental investigations, measured basal NO (with different methods) and NO released in response to different agents (ionomycin or ATP, for instance); thus, the comparison for reconciliation of results is not simple. However, our data indicate that the location of the enzyme when it releases NO may be more important than the amount of NO released for development of significant microvascular functions.
Our observation that PAF induces NO production in GFP-eNOS-CAAX despite its failure to dephosphorylate eNOS at Thr-495 suggests that this step is not a mandatory requirement for activation of eNOS, at least under our experimental conditions. Earlier reports considered dephosphorylation of eNOS at Thr-495 an important regulator of Ca2+/calmodulin-dependent eNOS activity (29, 30). Our results and the earlier reports on dephosphorylation of eNOS at Thr-495 led to the speculation that internalization of eNOS to the cytosol is necessary for its dephosphorylation at Thr-495.
The cytosolic target of eNOS-derived NO remains to be firmly identified. Our data suggest that one of the cytosolic steps is an association between eNOS and sGC. We speculate that eNOS and sGC form a molecular complex that enhances the subsequent steps that lead to hyperpermeability. The location of proteins that regulate the availability of cGMP and cAMP should also be considered in this context.
Our report is one of the few studies that directly establish a correlation between molecular events and functional outcomes in cell monolayers. In summary, our collective results demonstrate that eNOS location in the cytosol is necessary to stimulate the onset of hyperpermeability. This conclusion is based on the ability of PAF to stimulate activation of eNOS, production of NO, and an increase in permeability to FITC-dextran 70 exclusively in monolayers of cells transfected with eNOS targeted to the cytosol.
Supplementary Material
This work was supported, in whole or in part, by National Institutes of Health Grants 5R01 HL070634 and 5R01 HL088479. This work was also supported by Fondo Nacional de Desarrollo Científico Technológico (FONDECYT) Grant 1100569.

The on-line version of this article (available at http://www.jbc.org) contains supplemental “Materials and Methods,” “Results,” and Figs. S1–S3.
- eNOS
- endothelial NOS
- PAF
- platelet-activating factor
- sGC
- soluble guanylyl cyclase.
REFERENCES
- 1. Hatakeyama T., Pappas P. J., Hobson R. W., 2nd, Boric M. P., Sessa W. C., Durán W. N. (2006) J. Physiol. 574, 275–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sánchez F. A., Savalia N. B., Durán R. G., Lal B. K., Boric M. P., Durán W. N. (2006) Am. J. Physiol. Heart Circ. Physiol. 291, H1058–H1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sánchez F. A., Kim D. D., Durán R. G., Meininger C. J., Durán W. N. (2008) Am. J. Physiol. Heart Circ. Physiol. 295, H1642–H1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fulton D., Gratton J. P., McCabe T. J., Fontana J., Fujio Y., Walsh K., Franke T. F., Papapetropoulos A., Sessa W. C. (1999) Nature 399, 597–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fulton D., Gratton J. P., Sessa W. C. (2001) J. Pharmacol. Exp. Ther. 299, 818–824 [PubMed] [Google Scholar]
- 6. Sessa W. C. (2004) J. Cell Sci. 117, 2427–2429 [DOI] [PubMed] [Google Scholar]
- 7. Zimmermann K., Opitz N., Dedio J., Renne C., Muller-Esterl W., Oess S. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 17167–17172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bauer P. M., Fulton D., Boo Y. C., Sorescu G. P., Kemp B. E., Jo H., Sessa W. C. (2003) J. Biol. Chem. 278, 14841–14849 [DOI] [PubMed] [Google Scholar]
- 9. Dillon P. K., Durán W. N. (1988) Circ. Res. 62, 732–740 [DOI] [PubMed] [Google Scholar]
- 10. Ramírez M. M., Quardt S. M., Kim D., Oshiro H., Minnicozzi M., Durán W. N. (1995) Microvasc. Res. 50, 223–234 [DOI] [PubMed] [Google Scholar]
- 11. Sánchez F. A., Rana R., Kim D. D., Iwahashi T., Zheng R., Lal B. K., Gordon D. M., Meininger C. J., Durán W. N. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 6849–6853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Church J. E., Fulton D. (2006) J. Biol. Chem. 281, 1477–1488 [DOI] [PubMed] [Google Scholar]
- 13. Fulton D., Babbitt R., Zoellner S., Fontana J., Acevedo L., McCabe T. J., Iwakiri Y., Sessa W. C. (2004) J. Biol. Chem. 279, 30349–30357 [DOI] [PubMed] [Google Scholar]
- 14. Sessa W. C., García-Cardeña G., Liu J., Keh A., Pollock J. S., Bradley J., Thiru S., Braverman I. M., Desai K. M. (1995) J. Biol. Chem. 270, 17641–17644 [DOI] [PubMed] [Google Scholar]
- 15. Breslin J. W., Pappas P. J., Cerveira J. J., Hobson R. W., 2nd, Durán W. N. (2003) Am. J. Physiol. Heart Circ. Physiol. 284, H92–H100 [DOI] [PubMed] [Google Scholar]
- 16. Bohlen H. G. (1998) Am. J. Physiol. 275, H542–H550 [DOI] [PubMed] [Google Scholar]
- 17. Sowa G., Liu J., Papapetropoulos A., Rex-Haffner M., Hughes T. E., Sessa W. C. (1999) J. Biol. Chem. 274, 22524–22531 [DOI] [PubMed] [Google Scholar]
- 18. Brown J., Reading S. J., Jones S., Fitchett C. J., Howl J., Martin A., Longland C. L., Michelangeli F., Dubrova Y. E., Brown C. A. (2000) Lab. Invest. 80, 37–45 [DOI] [PubMed] [Google Scholar]
- 19. Drexler H. G., Quentmeier H., Dirks W. G., MacLeod R. A. (2002) In Vitro Cell. Dev. Biol. Anim 38, 185–186; author reply 187 [DOI] [PubMed] [Google Scholar]
- 20. Gil'yano N. Y., Semenova E. G., Fedortseva R. F., Konevega L. V. (2009) Cell Tissue Biol. 3, 274–282 [Google Scholar]
- 21. Suda K., Rothen-Rutishauser B., Günthert M., Wunderli-Allenspach H. (2001) In Vitro Cell. Dev. Biol. Anim. 37, 505–514 [DOI] [PubMed] [Google Scholar]
- 22. Fukumura D., Gohongi T., Kadambi A., Izumi Y., Ang J., Yun C. O., Buerk D. G., Huang P. L., Jain R. K. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 2604–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Varma S., Breslin J. W., Lal B. K., Pappas P. J., Hobson R. W., 2nd, Durán W. N. (2002) Microvasc. Res. 63, 172–178 [DOI] [PubMed] [Google Scholar]
- 24. Fulton D., Fontana J., Sowa G., Gratton J. P., Lin M., Li K. X., Michell B., Kemp B. E., Rodman D., Sessa W. C. (2002) J. Biol. Chem. 277, 4277–4284 [DOI] [PubMed] [Google Scholar]
- 25. Zhang Q., Church J. E., Jagnandan D., Catravas J. D., Sessa W. C., Fulton D. (2006) Arterioscler. Thromb. Vasc. Biol. 26, 1015–1021 [DOI] [PubMed] [Google Scholar]
- 26. Iwakiri Y., Satoh A., Chatterjee S., Toomre D. K., Chalouni C. M., Fulton D., Groszmann R. J., Shah V. H., Sessa W. C. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 19777–19782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin M. I., Fulton D., Babbitt R., Fleming I., Busse R., Pritchard K. A., Jr., Sessa W. C. (2003) J. Biol. Chem. 278, 44719–44726 [DOI] [PubMed] [Google Scholar]
- 28. Liu J., Hughes T. E., Sessa W. C. (1997) J. Cell Biol. 137, 1525–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Fleming I., Fisslthaler B., Dimmeler S., Kemp B. E., Busse R. (2001) Circ. Res. 88, E68–E75 [DOI] [PubMed] [Google Scholar]
- 30. Lenasi H., Kohlstedt K., Fichtlscherer B., Mülsch A., Busse R., Fleming I. (2003) Cardiovasc. Res. 59, 844–853 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.