Abstract
Autoimmune associated congenital heart block (CHB) may result from pathogenic cross-talk between inflammatory and profibrosing pathways. Incubation of macrophages with immune complexes (IC) composed of Ro60, a target of the pathologic maternal autoantibodies necessary for CHB, hY3 ssRNA, and affinity-purified anti-Ro60 antibody induces the Toll-like receptor 7 (TLR7)-dependent generation of supernatants that provoke a fibrosing phenotype in human fetal cardiac fibroblasts. We show herein that these cells are a major source of TGFβ and that endothelin-1 (ET-1) is one of the key components responsible for the profibrosing effects generated by stimulated macrophages. Supernatants from macrophages incubated with IC induced the fibroblast secretion of TGFβ, which was inhibited by treating the macrophages with an antagonist of TLR7. Under the same conditions, the induced fibroblast secretion of TGFβ was decreased by inhibitors of the ET-1 receptors ETa or ETb or by an anti-ET-1 antibody but not by an isotype control. Exogenous ET-1 induced a profibrosing phenotype, whereas fibroblasts transfected with either ETa or ETb siRNA were unresponsive to the profibrosing effects of the IC-generated macrophage supernatants. Immunohistochemistry of the hearts from two fetuses dying with CHB revealed the presence of ET-1-producing mononuclear cells in the septal region in areas of calcification and fibrosis. In conclusion, these data support a novel role of ET-1 in linking TLR7 inflammatory signaling to subsequent fibrosis and provide new insight in considering therapeutics for CHB.
Keywords: Endothelin, Fibrosis, Inflammation, Toll-like receptors (TLR), Transforming Growth Factor beta (TGFbeta)
Introduction
Cardiac conduction defects detected before or at birth, in the absence of structural abnormalities, are strongly associated with maternal autoantibodies to SSA/Ro and/or SSB/La ribonucleoproteins, independent of whether the mother has systemic lupus erythematosus or Sjögren's syndrome or is asymptomatic (1, 2). The mechanism by which maternal anti-SSA/Ro-SSB/La antibodies initiate and perpetuate inflammation with consequent scarring of the atrioventricular node (the signature lesion of CHB)2 and endocardium (3) has not been fully defined. One proposed pathologic cascade centers apoptosis of the fetal cardiocytes as the cellular event that accounts for the surface expression of the otherwise intracellular target antigens (4–6), facilitating subsequent opsonization by circulating maternal autoantibodies. Experimental data suggest that hY3 ssRNA associated with the SSA/Ro protein bound by affinity-purified anti-Ro60 antibody (AP60) gains access to the macrophage endosome via FcγR uptake with subsequent ligation of the Toll-like receptor 7 (TLR7). In clearing the opsonized apoptotic cardiomyocytes, infiltrating macrophages secrete factors that promote a scarring phenotype of the resident cardiac fibroblasts, as evidenced by their transdifferentiation to myofibroblasts and production of collagen (7, 8).
Immunohistochemical studies (5) of hearts from several fetuses dying with CHB demonstrate abundant TGFβ in the septal region. TGFβ probably contributes to scarring, as supported by its promotion of fetal cardiac fibroblast transdifferentiation and the partial inhibition of this scarring phenotype when fibroblasts are exposed to macrophage supernatants generated by ssRNA containing immune complexes (IC) in the presence of anti-TGFβ neutralizing antibodies (8). The partial inhibition by neutralizing antibodies and the absence of detectable TGFβ in the macrophage supernatants generated by TLR7 ligation collectively point to the fibroblast as the source of TGFβ and reinforce that the macrophage supernatants contain profibrosing factors as yet undiscovered.
Fibrosis is generally conceptualized as the result of a persistent tissue repair program characterized by myofibroblast tenacity and excessive production and remodeling of the extracellular matrix. Although traditionally characterized as a potent vasoconstrictor secreted from endothelial cells (9, 10), ET-1 can induce extracellular matrix production and myofibroblast differentiation (11, 12). The source of ET-1 is not restricted to endothelial cells. Human macrophages have been shown to produce ET-1 in response to lipopolysaccharide (LPS) (13), and human monocyte-derived dendritic cells secrete ET-1 in response to TLR2 and TLR4 agonists (14). ET-1 acts through the stimulation of two subtypes of receptors, ET-1 receptor subtype A (ETa) and ET-1 receptor subtype B (ETb). Of the three isoforms of ET, ET-1 is the significant isoform in humans. ET-1 is initially generated as a precursor of 212 amino acids, which is twice cleaved to a biologically active peptide of 21 amino acids (15). Of potential relevance to the pathogenesis of anti-SSA/Ro-SSB/La-associated cardiac diseases, several lines of evidence support a profibrotic involvement of ET-1 in heart diseases. ET-1 can stimulate proliferation of neonatal and adult rat cardiac fibroblasts via activation of the ETa receptor (16–18). ET-1 induces neonatal rat cardiac fibroblasts to express α-smooth muscle actin (α-SMA), a marker of myofibroblast differentiation (19). ET-1 stimulates the synthesis of collagen by cardiac fibroblasts from different species, including humans, albeit only from adults (18–22). Further profibrotic effects of ET-1 occur at the level of matrix metalloproteinases, with evidence that ET-1, acting via the ETa receptor, can reduce collagenase activity (23). The connection between TGFβ, whose expression is elevated in response to injury (24, 25), and ET-1 has been established, and several lines of evidence indicate that transdifferentiation of fibroblasts occurs in response to the concerted actions of TGFβ, ET-1, and angiotensin (11).
Accordingly, this study was initiated to test two hypotheses. The first is that the fibroblast is a source of TGFβ. The second is that ET-1 is secreted by the macrophage via TLR7 ligation following ingestion of IC comprised of the Ro60 antigen, hY3 ssRNA, and affinity-purified anti-Ro60, a surrogate for apoptotic cardiocytes opsonized by anti-SSA/Ro-SSB/La antibodies. After the release of TGFβ by fibroblasts exposed to TLR7-dependent macrophage supernatants was established, the contribution of ET-1 was then explored. This was approached by addressing the effect of ET-1 on TGFβ secretion and transdifferentiation of fibroblasts and subsequently exploiting receptor antagonists, blocking antibodies, and siRNA-mediated down-regulation of ETa and ETb in the fibroblasts. The source of ET-1 was established by evaluating the EDN1 (gene encoding ET-1) mRNA expression, protein expression, and ET-1 secretion by macrophages stimulated with hY3 or IC, and both conditions after pretreatment with IRS661 (antagonist of TLR7) (26). In vivo support for the contribution of ET-1 was sought by immunohistologic evaluation of the hearts from two fetuses dying at 29 and 40 weeks of gestation with CHB.
EXPERIMENTAL PROCEDURES
Preparation of hY3 ssRNAs
As previously described (8), with minor modifications, for obtaining Ro60-associated hY3 ssRNA, hY3 plasmid (27), kindly provided by Dr. Sandra Wolin (Yale University, New Haven, CT), was digested with DraI restriction enzyme for linearization. In brief, 1 μg of template was subjected to transcription with the TranscriptAid transcription kit (Fermentas Life Sciences, Burlington, Ontario, Canada) using 4 μl of 5× reaction buffer; 8 μl of an equimolar mixture of ATP, CTP, GTP, and UTP; and 2 μl of enzyme mix. The reaction mixture was incubated at 37 °C for 2 h. After the reaction, 2 μl of RNase-free DNase I was added, and the mixture was further incubated at 37 °C for 15 min. The DNase reaction was stopped by the addition of 2 μl of EDTA, pH 8.0, and incubation at 65 °C for 10 min. The transcripts were purified by phenol/chloroform extraction and resuspended in water at 2.5 μg/μl, and the quality was evaluated by RNAQQNANO Technologies (Genomics Facility, New York University Medical Center). hY3 A/U RNA (8) was used as a negative control.
AP60
As previously described (6, 8), AP60 was generated from the serum of an SSA/Ro-positive mother of a child with CHB by affinity column chromatography using Ro60 recombinant protein coupled to cyanogen bromide-activated Sepharose 4B. Protein concentrations of the AP60 were assessed by a protein quantification kit (Pierce).
Preparation of the Immune Complexes (IC) Containing Ro60, hY3 ssRNA, and Anti-Ro60 Antibody
As described previously (8), with minor modifications, IC were prepared by reaction for 1 h at 22 °C on rotation of endotoxin-free native Ro60 (4.7 μg; GenWay Biotech, San Diego, CA) with equimolar amounts of hY3 ssRNA (2.5 μg; earlier subjected to a sequence of heating-cooling (95 °C for 2 min, ice for 2 min) in RNA-protein reaction buffer (20 mm Hepes, pH 7.9, 2 mm MgCl2, 10 μm ZnCl2, 0.02% Nonidet P-40, 70 mm NH4Cl, and 0.05 μg/μl yeast RNA). AP60 was added to achieve a final concentration of 15 μg/ml, and the mixture was further incubated for 1 h under the same conditions. IC were then added to cultured, IFNγ-primed macrophages (see below).
Isolation and Preparation of Macrophages
Human macrophages derived from peripheral blood mononuclear cells were isolated from white blood cell concentrate (Leukopak; New York Blood Center, New York, NY) by centrifugation on Ficoll-Hypaque gradients and purified by positive selection using CD14 microbeads (Miltenyi Biotech, Auburn, CA) and LS columns (Miltenyi Biotech). As described previously (8), with some modifications, the resulting monocytes were then cultured in Teflon beakers (RPMI 1640, 10% FCS plus 10 ng/ml GM-CSF; Invitrogen) for 1 week. Monocyte-derived macrophages (5 × 105 cells) were plated on growth medium containing 10% serum and incubated at 37 °C. After 48 h, attached macrophages were incubated with serum-free medium containing INFγ (10 nm) for 6 h. After a double wash with HBSS buffer, macrophages were DOTAP-transfected (DOTAP Liposomal Transfection Reagent, Roche Applied Science) with 2.5 μg of hY3 or hY3 A/U ssRNAs (which represents a substitution of the U nucleotides with A nucleotides throughout the entire sequence of hY3, synthesized on a ribose-based backbone by Thermo Scientific (Chicago, IL)) or treated with the Ro60-containing IC, in the absence or presence of 32 ng/ml endotoxin-free oligonucleotide IRS661 (26) for 30 min prior to the addition of hY3 or IC, followed by a 16-h incubation at 37 °C. As previously described, IRS661 specifically blocks TLR7 in a model of R848-stimulated dendritic cells (26). Macrophage supernatants were collected for evaluation of TNFα (as a marker of macrophage activation) and ET-1 (see below) secretion and subsequent incubation with human fetal cardiac fibroblasts (see below). Total mRNA was isolated from attached cells for evaluation of gene expression by qRT-PCR (see below).
Isolation, Preparation, and Treatment of Cardiac Fibroblasts
Fibroblasts were isolated from the hearts of abortuses aged 16–24 weeks, as described previously (7). Fibroblasts were plated at a density of 5 × 104 cells/well (on 12-well plates or glass coverslips). For experiments described in the legends to Figs. 1–4, fibroblasts were incubated for 16 h with the following: 1) supernatants generated from untreated macrophages or macrophages transfected with hY3 or treated with IC in the presence or absence of IRS661, as indicated above (Fig. 1); 2) macrophage supernatants preincubated in the absence or presence of anti-ET-1 antibody or isotype control normal rabbit IgG (10 μg/ml; Santa Cruz Biotechnology, Inc., Santa Cruz, CA) (after a 30-min preincubation of fibroblasts in the absence or presence of selective ETa and ETb receptor antagonists, BQ-123 or BQ-788 respectively (100 nm; Tocris Bioscience)) (Fig. 2); 3) exogenous ET-1 (Sigma) at 100 nm (a dose known to induce cardiac myofibroblast formation (19, 28)) with or without anti-ET-1 antibody or normal rabbit IgG (as above) (after a 30-min preincubation of fibroblasts in the absence and presence of BQ-123 or BQ-788 (as above)) (Fig. 3); and 4) supernatants generated from macrophages treated with IC (after transfection of fibroblasts with siRNAs (see below)). Supernatants collected from fibroblasts were assessed for the quantity of secreted TGFβ and collagen (see below). Immunofluorescence with anti α-SMA antibody (1:200; Sigma-Aldrich), anti-Col1A antibody (collagen; 1:200; Santa Cruz Biotechnology, Inc.), or normal mouse IgG (Santa Cruz Biotechnology, Inc.; isotype control) as primary antibodies and anti-mouse IgG-FITC as secondary antibody was also employed to assess profibrosing readouts in fibroblasts cultured on glass coverslips, treated, and then washed, fixed with 4% paraformaldehyde, and permeabilized with 0.1% Triton X-100.
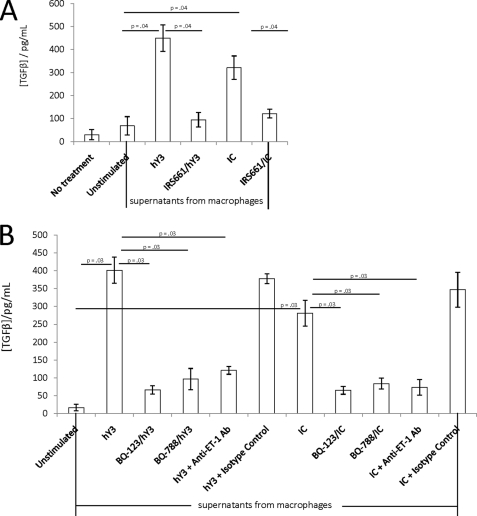
FIGURE 1.
Macrophage supernatants generated following TLR7 ligation induce TGFβ secretion by fibroblasts dependent on ET-1, ETa, and ETb. A, additions to cultured human fetal cardiac fibroblasts (5 × 104 cells) included supernatants obtained from macrophages transfected with hY3 in the presence and absence of IRS661 (32 ng/ml) or treated with IC composed of affinity-purified anti-Ro60 antibody, native Ro60, and hY3, in the presence and absence of IRS661. After 16 h, TGFβ levels (pg/ml) were determined from fibroblast supernatants by ELISA. Statistical significances (p) are indicated (n = 4 for each condition). B, human fetal cardiac fibroblasts (5 × 104 cells) were incubated with supernatants generated from macrophages transfected with hY3 or treated with IC as described in A, in the absence or presence of BQ-123 (100 nm), BQ-788 (100 nm), anti-ET-1 antibody (10 μg/ml), or normal rabbit IgG (isotype control, 10 μg/ml). After 16 h, TGFβ levels (pg/ml) were determined from the fibroblast supernatants by ELISA. Statistical significances (p) are indicated (n = 4 for each condition). Error bars, S.E.
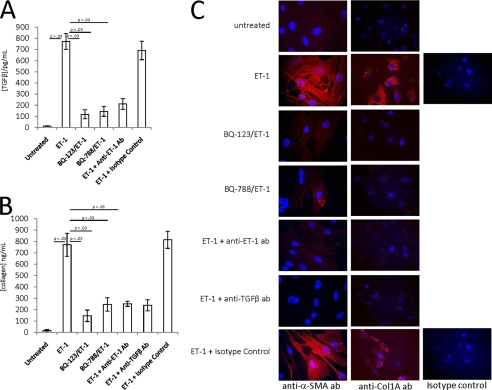
FIGURE 2.
Exogenous ET-1 induces a profibrosing phenotype in cultured human fetal cardiac fibroblasts. A and B, TGFβ (pg/ml) (A) and collagen (ng/ml) (B) were measured in the supernatants generated from human fetal cardiac fibroblasts incubated with ET-1 (100 nm) alone or in the presence of BQ-123 (100 nm), BQ-788 (100 nm), anti-ET-1 antibody (10 μg/ml), or normal rabbit IgG (isotype control; 10 μg/ml). ET-1 was also incubated with anti-TGFβ antibody (10 μg/ml) in the collagen assay. TGFβ was measured 16 h postincubation. Soluble collagen was measured after 16 h by the soluble collagen binding assay (C). In parallel, human fetal cardiac fibroblasts (5 × 104 cells) cultured under identical conditions were fixed, permeabilized, and incubated with anti-α-SMA antibody, anti-Col1A antibody (to assess collagen), or normal mouse IgG (isotype control) for immunofluorescence analysis; red color indicates the presence of α-SMA (left column) or collagen (middle column). Images (×20 magnification) are representative of three experiments. Bar, 40 μm. Error bars, S.E.
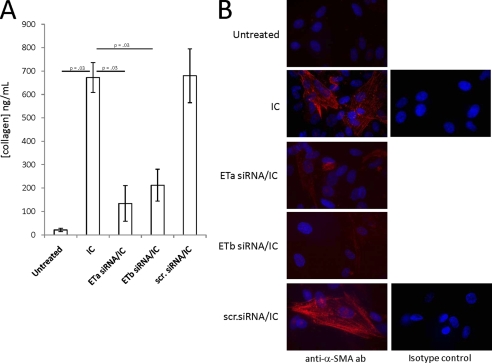
FIGURE 3.
Supernatants from IC-stimulated macrophages do not induce a profibrosing phenotype in ETa or ETb siRNA-transfected fibroblasts. A and B, human fetal cardiac fibroblasts (5 × 104 cells) were transfected with siRNA targeting either ETa or ETb or control scrambled siRNA. After 72 h, expression levels of ETa and ETb were verified by qRT-PCR. siRNA-treated and control cells were incubated with supernatants from untreated or IC-incubated macrophages (as described in the legend to Fig. 1). After 16 h, collagen released (ng/ml) into the fibroblast supernatants was measured (A), and α-SMA production was analyzed by immunofluorescence (B). Images (×40 magnification) are representative of three experiments. Error bars, S.E.
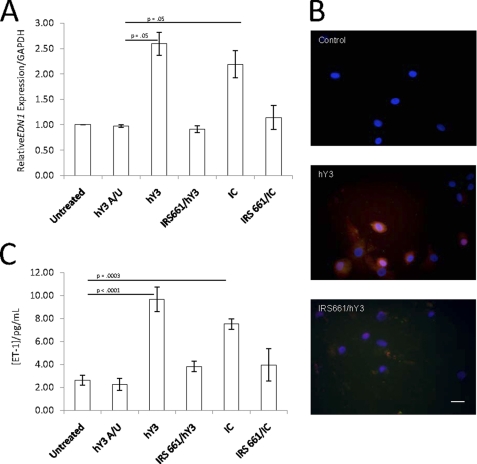
FIGURE 4.
TLR7 ligation increases macrophage EDN1 gene mRNA expression and protein expression and secretion of ET-1. A and B, macrophages (5 × 105 cells) were transfected with hY3 or incubated with IC (as described in the legend to Fig. 1) for 16 h in the absence or presence of IRS661. Transfection with hY3 A/U was evaluated as a negative control. The mRNA expression of EDN1 relative to GAPDH was measured by qRT-PCR after isolation of total mRNA (A). Macrophages (5 × 105 cells) were transfected with hY3 for 16 h in the absence or presence of IRS661 (B). The next day cells were fixed, stained with mouse monoclonal ET-1 antibody, and analyzed by fluorescence microscopy (original magnification, ×40). Results are representative of three experiments. Supernatants were evaluated for ET-1 (pg/ml) by ELISA (C). Error bars, S.E.
The Tetrazolium Salt Assay
For determination of the potential toxic effect of the compounds on fibroblast viability, the CellTiter 96® non-radioactive cell proliferation assay kit (Promega, Madison, WI) was used according to the manufacturer's instructions. Briefly, 1 × 104 fibroblasts were plated on a 96-well dish in a volume of 100 μl of culture medium in the presence or absence of the appropriate compounds (BQ-123 (100 nm), BQ-123 (1000 nm), BQ-788 (100 nm), BQ-788 (1000 nm), anti-ET-1 (10 μg/ml), and anti-TGFβ (10 μg/ml)). After an overnight incubation at 37 °C, 15 μl of Dye Solution was added to each well, and the plate was further incubated for 4 h in a humidified CO2 incubator. 100 μl of solubilization/stop solution was added to each well, and the absorbance was measured at 570 nm using a 96-well plate reader.
Fluorescence-activated Cell Sorting, Immunofluorescence, and SDS-PAGE/Western Blot
Cultured fibroblasts were subjected to fluorescence-activated cell sorting analysis, as described previously (8), and immunofluorescence (as above) with anti-ETa or anti-ETb antibodies or normal rabbit IgG as an isotype control (Santa Cruz Biotechnology, Inc.) as primary antibodies to detect expression of ETa and ETb. Anti-rabbit-FITC antibody was used as secondary antibody. The same primary antibodies were used in SDS-PAGE/Western blot (carried out as described elsewhere) after total protein was extracted from cultured fibroblasts for further confirmation of ETa and ETb expression.
qRT-PCR
Total mRNA was isolated from macrophages either untreated or treated with hY3, IRS661/hY3, IC, IRS661/IC, or hY3 A/U for 16 h at 37 °C using the Absolutely RNA® Miniprep kit (Stratagene). For real-time quantitative qRT-PCR analysis, levels of ET-1, ETa, and ETb gene expression were quantified by amplifying a segment of their respective mRNA with Santa Cruz Biotechnology, Inc. primers sc-45394-PR for ET-1, sc-39960-PR for ETa, and sc-39962-PR for ETb. Levels of expression were normalized by parallel amplification and quantification of the GAPDH mRNA level with primers 5′-ACCACAGTCCATGCCATCAC-3′ and 5′-TCCACCACCCTGTTGCTGTA-3′. Brilliant® SYBR® Green RT-PCR (Invitrogen) was used as RT-PCR reaction mix.
Determination of TGFβ, Collagen, and ET-1
The release of TGFβ and collagen by fibroblasts was determined using the human TGFβ1 ELISA kit (Cell Sciences, Canton, MA) and the Sircol soluble collagen assay (Biocolor, Belfast, Ireland), respectively, following the manufacturer's instructions. Secretion of active ET-1 (ET-1 released when big ET-1 is cleaved by endothelin-converting enzyme) by the macrophages was determined by the commercially available Endothelin-1 enzyme immunometric assay kit (Assay Designs).
Immunofluorescence of Macrophages
Macrophages were seeded in 4-well chamber slides in complete RPMI. After 48 h, attached macrophages were incubated with serum-free medium containing IFNγ (10 nm) for 6 h. After a double wash with HBSS buffer, macrophages were DOTAP-transfected with 2.5 μg of hY3 in the absence or presence of 32 ng/ml of the endotoxin-free oligonucleotide IRS661 (26) for 30 min prior to the addition of hY3, followed by a 16-h incubation at 37 °C. Immunodetection of ET-1 was performed on cells after fixation with 3% paraformaldehyde (Fisher) in PBS (pH 7.4) for 15 min at room temperature. After nonspecific binding was blocked with 3% normal goat serum (Jackson ImmunoResearch, West Grove, PA), cells were incubated with mouse monoclonal antibody to ET-1 (ab20940) (AbCam) and then with secondary antibody, anti-mouse Alexa Fluor dye 568 (Molecular Probes) at room temperature. Cells were then stained with 2 μg/ml Hoechst 33258 for 30 min at 37 °C and embedded in Vectashield mounting medium (Vector Laboratories, Burlingame, CA). For each sample, at least six images from three independent experiments were analyzed. Cells were viewed with Axioplan microscope (Carl Zeiss Meditec, Thornwood, NY), as indicated. For the Axioplan, images were captured with a charge-coupled device camera (SPOT-2; Diagnostic Instruments, Sterling Heights, MI), and processed by PhotoShop (Adobe Systems, Mountain View, CA). The images were subsequently combined and processed with ImageJ software (National Institutes of Health, Bethesda, MD).
Tissue Sections from Fetal Hearts
Formalin-fixed paraffin sections were obtained from the hearts of two fetuses dying with CHB (29 and 40 weeks of gestation; clinical description and gross anatomy previously published in the fetus dying at 40 weeks (5)) and a 24-week heart from a fetus with non-cardiac disease (control). For immunostaining, 6-mm sections of the fetal hearts were prepared as described previously (5). Endogenous peroxide activity was blocked with 3% hydrogen peroxide for 5 min. Anti-ET1/2/3 antibody (Santa Cruz Biotechnology, Inc.) or normal rabbit IgG (Alpha Diagnostic International, San Antonio, TX) was applied to the slides for 30 min at room temperature. Secondary labeling and visualization was performed using the SuperPicTureTM polymer detection system (Invitrogen) with ImmPACT DAB (Vector Laboratories, Burlingame, CA) as the chromagen. Slides were counterstained with hematoxylin (Richard-Allen, Kalamazoo, MI).
Fibroblast Transfection with siRNA
In a 6-well plate, 2 × 105 cells (subconfluent) were plated in 2 ml of antibiotic-free growth medium containing 10% FBS and incubated at 37 °C for 16 h. 1 μg of ETa, ETb, or scrambled siRNA (Santa Cruz Biotechnology, Inc.) was diluted in 100 μl of siRNA transfection medium (Santa Cruz Biotechnology, Inc.), and combined with 100 μl of transfection medium containing 8 μl of siRNA. The mixture was incubated and 0.8 ml of transfection medium was added for 30 min at 22 °C. Prior to transfection, fibroblasts were washed once with 2 ml of siRNA transfection medium and treated with the diluted mix. Transfection was allowed for 7 h at 37 °C, followed by the addition of 1 ml of growth serum containing 2 times the normal serum and antibiotic concentration and incubation for an additional 16 h. Growth medium was then replaced with fresh growth medium containing a normal concentration of serum and antibiotics. Fibroblasts were further incubated for 48 h prior to ETa/ETb mRNA expression assessment and treatment with supernatants generated from macrophages treated with IC (see above).
Statistical Analysis
The Mann-Whitney test was used as appropriate to compare TGFβ and collagen released by fibroblasts and ET-1 released by macrophages between the different groups. The same test was used to compare EDN1 gene relative expression in macrophages between the different groups. Values of p < 0.05 were considered significant.
RESULTS
Supernatants from Macrophages Stimulated with either hY3 or IC in a TLR7-dependent Manner Induce TGFβ Secretion by Human Fetal Fibroblasts
Based on in vivo and in vitro evidence implicating TGFβ in fibrosis of the atrioventricular node and endocardium (5, 24, 25), fibroblasts were examined as the direct source of this cytokine. Exposure of cultured human fetal cardiac fibroblasts to macrophage supernatants generated following transfection with hY3 significantly increased TGFβ release compared with supernatants from unstimulated macrophages (450 ± 57 versus 69 ± 40 pg/ml, respectively, p = 0.04, n = 4; Fig. 1A). Similarly, supernatants generated from macrophages treated with IC also significantly increased TGFβ secretion compared with supernatants from unstimulated macrophages (321 ± 51 pg/ml, p = 0.04 versus unstimulated, n = 4; Fig. 1A). Support for TLR7 dependence was obtained by the demonstration that the secretion of TGFβ was markedly decreased when fibroblasts were treated with supernatants generated from macrophages either transfected with hY3 or treated with IC in the presence of 32 ng/ml IRS661 (95 ± 31 pg/ml for IRS661/hY3; 122 ± 19 pg/ml for IRS661/IC, p = 0.04, n = 4 for each versus the respective macrophage treatments in the absence of IRS661; Fig. 1A).
TGFβ Secretion by Fibroblasts is ET-1-dependent
Due to the well known inherent capacity of ET-1 to induce a profibrotic fibroblast phenotype (11, 12), experiments were focused on evaluation of this peptide. As determined by Western blot, immunofluorescence, and fluorescence-activated cell sorting analyses, both ETa and ETb receptors were expressed on the fetal cardiac fibroblasts (data not shown). The addition of the cyclic peptide BQ-123 (100 nm), a specific ETa inhibitor, to the fibroblasts significantly attenuated the release of TGFβ induced by incubation with supernatants from macrophages transfected with hY3-generated (402 ± 37 versus 66 ± 12 pg/ml, in the absence and presence of BQ-123, p = 0.03, n = 4; Fig. 1B) or IC-generated macrophage supernatants (281 ± 36 versus 65 ± 11 pg/ml, in the absence and presence of BQ-123, p = 0.03, n = 3; Fig. 1B). ETb-specific inhibitory peptide BQ-788 (100 nm) also significantly reduced TGFβ release after incubation with macrophage supernatants generated under the same conditions (402 ± 37 versus 96 ± 30 pg/ml, in the absence and presence of BQ-788 for hY3, p = 0.03, n = 4 (Fig. 1B) and 281 ± 36 versus 83 ± 15 pg/ml, in the absence and presence of BQ-788 for IC, p = 0.03, n = 4 (Fig. 1B)). Based on the tetrazolium salt assay (described under “Experimental Procedures”), the inhibitory effects of these two peptides were not secondary to cell death (data not shown). The addition of a neutralizing anti-ET-1 antibody (10 μg/ml) to the fibroblasts significantly attenuated the release of TGFβ induced by incubation with supernatants from macrophages transfected with hY3 (402 ± 37 versus 121 ± 11 pg/ml, in the absence and presence of anti-ET-1 antibody, respectively, p = 0.03, n = 4; Fig. 1B) or IC (281 ± 36 versus 72 ± 22 pg/ml, in the absence and presence of anti-ET-1 antibody, respectively, p = 0.03, n = 4; Fig. 1B). No inhibition was observed with normal rabbit IgG isotype control (10 μg/ml).
Exogenous ET-1 Induces a Profibrotic Phenotype of the Cardiac Fibroblasts
The addition of exogenous ET-1 (100 nm) to the fibroblasts mimicked the results obtained from the macrophage supernatants in that TGFβ secretion was significantly increased (13 ± 3 versus 772 ± 71 pg/ml, in the absence and presence of ET-1, respectively, p = 0.03, n = 4; Fig. 2A). Moreover, ET-1 at 100 nm also significantly increased collagen synthesis (18 ± 8 versus 772 ± 101 ng/ml, in the absence and presence of ET-1, p = 0.03, n = 4; Fig. 2B). Pretreatment of ET-1-treated fibroblasts with BQ-123 or BQ-788 (100 nm) or co-treatment with anti-ET-1 antibody (10 μg/ml) significantly reduced TGFβ secretion (from 772 ± 71 to 119 ± 41, 145 ± 43, and 211 ± 48 pg/ml, respectively, p = 0.03, n = 4; Fig. 2A) and collagen secretion (from 772 ± 101 to 147 ± 50, 247 ± 59, and 253 ± 23 ng/ml, respectively, p = 0.03, n = 4; Fig. 2B). The addition of an inhibitory anti-TGFβ antibody (8) also significantly reduced ET-1-mediated collagen secretion (from 772 ± 101 to 239 ± 49 ng/ml, p = 0.03, n = 4; Fig. 2B).
Consistent with a profibrosing effect, incubation of cultured fibroblasts with ET-1 (100 nm) increased α-SMA as well as collagen type I (Col1A) expression, as evidenced by the increased staining with either anti-α-SMA or anti-Col1A antibodies (Fig. 2C), compared with untreated cells. A marked decrease of both α-SMA and collagen staining was observed when fibroblasts were pretreated with either BQ-123 or BQ-788 (each 100 nm) or co-treated with either anti-ET-1 antibody (10 μg/ml) or an anti-TGFβ antibody (10 μg/ml) (8) but not normal mouse IgG isotype control (10 μg/ml).
Fibroblasts Transfected with Either ETa or ETb siRNA Are Unresponsive to Supernatants Generated from IC-stimulated Macrophages
Further support for the profibrosing effects of ET-1 was provided by transfection of the fibroblasts with either ETa or ETb siRNA. As measured by qRT-PCR, ETa siRNA down-regulated ETa expression ∼8-fold, but not ETb expression, whereas ETb siRNA down-regulated ETb expression ∼5 fold, but not ETa expression. A scrambled siRNA did not down-regulate either ETa or ETb (data not shown). The levels of collagen secretion (Fig. 3A) induced by supernatants from IC-stimulated macrophages were significantly higher in untransfected fibroblasts (673 ± 64 ng/ml) than in fibroblasts transfected with either ETa siRNA (134 ± 76 ng/ml, p = 0.03, n = 4) or ETb siRNA (212 ± 68 ng/ml, p = 0.03, n = 4) but not fibroblasts transfected with control scrambled siRNA (680 ± 115 ng/ml). Likewise, the α-SMA staining induced by supernatants from IC-stimulated macrophages was greater in untransfected fibroblasts or fibroblasts transfected with scrambled siRNA compared with fibroblasts transfected with either ETa or ETb siRNA (Fig. 3B).
Both hY3 ssRNA and IC Induce Overexpression of EDN1 mRNA and ET-1 Protein Secretion by Human Macrophages
To directly address whether ET-1 is generated and secreted in a TLR7-dependent manner by macrophages, both mRNA expression and protein expression/secretion were evaluated. Based on qRT-PCR, the mean -fold induction of EDN1 expression was ∼2.60 when macrophages were transfected with hY3 and ∼2.19 when cells were treated with IC (p = 0.05, n = 4, Fig. 4A). The control ssRNA, hY3 A/U, did not increase EDN1 expression. Treatment of cells with IRS661 prior to hY3 transfection or treatment with IC decreased EDN1 gene expression to levels comparable with that of untreated cells.
Protein expression of ET-1, as assessed by anti-ET-1 antibody staining, was increased in macrophages stimulated with hY3 in a TLR7-dependent manner (Fig. 4B). The level of ET-1 in supernatants from macrophages transfected with hY3 was significantly greater than that measured in untreated macrophages (9.7 ± 1.1 versus 2.6 ± 0.5 pg/ml, p < 0.0001, n = 10) or macrophages treated with hY3 A/U ssRNA, negative control (2.2 ± 0.5 pg/ml; Fig. 4C). ET-1 in supernatants from IC-stimulated macrophages was also significantly greater than base line (7.5 ± 0.5 pg/ml versus 2.6 ± 0.5 pg/ml, p = 0.0003, n = 10). Pretreatment of macrophages with IRS661 decreased the ET-1 secretion induced by hY3 or IC (3.8 ± 0.5 versus 9.7 ± 1.1 pg/ml for hY3; 4.0 ± 1.4 versus 7.5 ± 0.5 pg/ml).
ET-1 Is Detected in the Hearts of Two Fetuses Dying with CHB
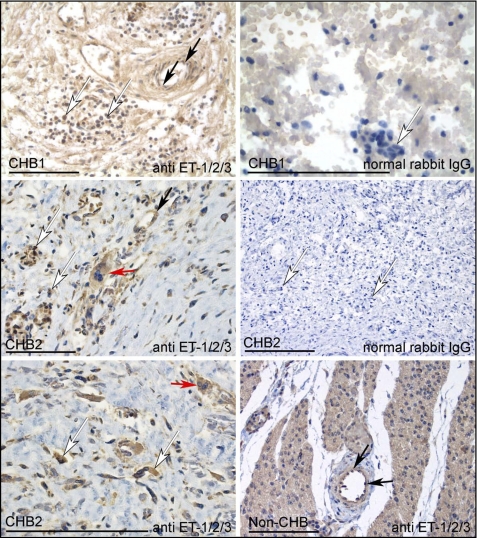
In vivo proof of concept was sought by immunohistochemical evaluation of the hearts from two fetuses, one diagnosed with CHB at 19 weeks and dying at 40 weeks (described previously (5)) and one diagnosed at 22 weeks and electively terminated at 29 weeks. As assessed by staining with anti-ET-1/2/3 antibody, ET-1-positive mononuclear cells were readily identified in the diseased heart (Fig. 5, white arrows, top, middle, and lower left panels). The isotype control antibody (normal rabbit IgG) did not stain these mononuclear cells (Fig. 5, top and middle right panels). Histological evaluation of this region revealed granular deposits of calcification and fibrosis, as well as a mononuclear cell infiltrate. These cells were not observed in tissue sections from the heart of a neonate who died of noncardiac causes (Fig. 5, bottom right panel). As expected, in both the CHB and non-CHB hearts, anti-ET-1/2/3 antibody stained endothelial cells lining the blood vessels (Fig. 5, black arrows).
FIGURE 5.
ET-1 expression in fetal heart. Sections from the septal region of a 40-week fetus (CHB1) dying with CHB, a 29-week fetus dying with CHB (CHB2), and a 24-week non-CHB fetus (control) were stained with anti-ET-1/2/3 antibody or normal rabbit IgG (isotype control for anti-ET-1/2/3 antibody). Stains were visualized using anti-rabbit IgG peroxidase (brown) and counterstained with hematoxylin. Locations of mononuclear cells in CHB hearts and endothelial cells (lining the vessel wall) are indicated by white and black arrows, respectively. Multinucleated giant cells are indicated by red arrows. Anti-ET-1/2/3 strongly stained mononuclear cells in both CHB hearts. Anti-ET-1/2/3 antibody also stained endothelial cells in both CHB hearts and the non-CHB heart. Bar, 100 μm.
DISCUSSION
Although it is widely acknowledged that maternal anti-SSA/Ro antibodies are clinically associated with fetal conduction disease, preventative strategies can only be formulated by understanding the molecular events that link the culprit antibody to fibrotic replacement of the atrioventricular node. Given the rarity of the disease, it is likely that a series of events involving an imbalance of physiologic and pathologic responses generate persistent myofibroblast differentiation and scar. TNFα, mRNA, and TGFβ protein are expressed in the septal region of fetal hearts dying with CHB (29). In vitro experiments demonstrate that IC comprised of the Ro60 antigen and hY3 ssRNA bound by an affinity-purified antibody against Ro60 induce macrophage activation via TLR7 signaling after uptake by an FcγR-dependent pathway with consistent release of TNFα (8) yet variable and limited detection of TGFβ. The supernatants from stimulated macrophages promote the fetal cardiac fibroblasts to undergo myofibroblast differentiation and secrete collagen. Based on antibody neutralizing experiments, both TNFα and TGFβ were considered potential mediators of fibrosis, but neither antibody completely abolished the fibrosing effects of the macrophage supernatants. Thus, additional factors were sought. TGFβ, but not TNFα, directly stimulates myofibroblast differentiation (7). However, TNFα is a plausible contributory candidate because it stimulates type I collagen, induces tissue inhibitor of metalloproteinase-1 expression, and reduces matrix metalloproteinase-2 activity and collagen degradation in intestinal myofibroblasts (30).
In an attempt to clarify the source of TGFβ, data generated herein identify the human fetal cardiac fibroblast per se as the cell largely responsible for the secretion of TGFβ. This cytokine plays a central role in fibroblast activation (12) as specifically exemplified by the demonstration of an overproduction of TGFβ by fibroblasts in the skin of patients with fibrosing diseases, such as systemic sclerosis (31, 32). The fibroblast reactivity in the CHB model appears to be indirectly TLR7-dependent in that the macrophage supernatants inducing this reactivity were the products of hY3 transfection or IC containing hY3 ssRNA inhibited by IRS661. Moreover, it is notable that TNFα secretion in these same macrophage supernatants was consistently higher following transfection with hY3 than following stimulation with IC. However, the levels of TGFβ released by the fibroblasts incubated with these same supernatants were equivalent across every experiment. These observations suggest that, although uptake via FcγR and subsequent TLR7 ligation may induce less TNFα secretion than direct transfection with hY3, the macrophage promotion of a scarring fibroblast phenotype is similar, thus supporting translation of these observations to the human disease, in which anti-SSA/Ro antibodies drive the uptake of the ssRNA and subsequent fibrosing injury.
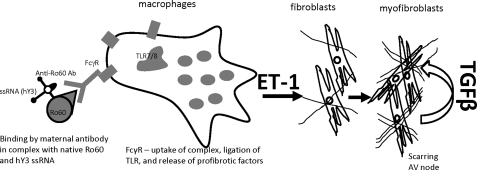
Based on the increasing number of reports strongly associating ET-1 with profibrotic phenotypes observed in various organs, including the heart (11, 12, 16–22, 33), we hypothesized that ET-1 is one of the profibrosing factors linking the inflammatory component (macrophages activated via FcγR/TLR7) to the fibrosis component (fibroblast transdifferentiation) in our CHB model (summarized pictorially in Fig. 6). The experiments herein demonstrate a novel role for ET-1. IC composed of Ro60 and hY3 ssRNA bound by anti-Ro60 affinity antibodies up-regulates EDN1 gene expression, up-regulates ET-1 protein expression, and increases ET-1 secretion by cultured human macrophages. EDN1 is among a number of up-regulated genes recently observed in mRNA microarray experiments, in which macrophages were treated under identical conditions.3 Both ET-1 and supernatants collected from IC-stimulated macrophages promote fibroblast transdifferentiation in an ETa- and ETb-dependent manner with release of TGFβ. A similar observation was reported by Shi-Wen et al. (34), in which the readout was collagen production by ET-1 stimulated fibroblasts. Accordingly, these observations position ET-1 as a bridge between Ro60-ssRNA IC and cardiac fibrosis in the context of CHB.
FIGURE 6.
Schematic representation of the proposed role of ET-1 as one of the profibrosing factors linking TLR7 inflammatory signaling to fibrosis. Binding and uptake of IC composed of the maternal anti-Ro60 antibody, hY3 ssRNA, and the antigen Ro60 by FcγR present on macrophages induces TLR7-dependent release of ET-1 as one of the key profibrotic factors of the inflammatory signaling cascade. ET-1 subsequently induces the resident cardiac fibroblasts to release TGFβ and promote its transdifferentiation and subsequent scarring.
There is precedent for a TGFβ-ET-1 axis in fibrosis. Lagares et al. (35) recently demonstrated TGFβ-induced ET-1 expression in human dermal fibroblasts and showed that the TGFβ1-induced expression of profibrotic genes was dependent on ET-1. Similarly, Shi-Wen et al. (35) showed that ET-1 mediates the induction of profibrotic genes, including type I collagen, fibronectin, and CCN2 from human lung fibroblasts in the presence of exogenous TGFβ (36). Although these experiments support a linear directionality distinct from that reported herein, for the fetal cardiac fibroblasts, it is likely that TGFβ can act in an autocrine manner to amplify the production of ET-1 (39). Specifically, fetal cardiac fibroblasts may secrete TGFβ in response to ET-1 secreted by infiltrating macrophages, which may in turn induce the further production and secretion of ET-1. This effect exaggerates the ET-1 signal initiated by TLR7-activated macrophages, setting forth an autocrine loop aimed at producing excessive extracellular matrix leading to scar.
Although ET-1 and its related peptides ET-2 and ET-3 have been traditionally regarded as vasoconstrictors, in the past 2 decades, ET-1 has gained attention as a trophic agent. Pertinent to the pathogenesis of CHB, ET-1 has been established as a key mediator of fibrosis in heart diseases. For example, the persistently high levels of ET-1 in diabetes patients have been strongly associated with cardiac fibrosis (37), and elevated atrial ET-1 content is associated with atrial dilatation, fibrosis, and hypertrophy (38). Consistent with the data herein, several reports directly implicate ET-1 in cardiac fibroblast transdifferentiation (reviewed in Ref. 33). The cardiac tissues of patients with congestive heart failure have increased levels of ET-1 (40), suggesting a role for ET-1 in heart remodeling.
In humans, ET-1 is normally produced by endothelial cells but is also expressed by epithelial cells, bone marrow mast cells, macrophages, polymorphonuclear leukocytes, cardiomyocytes, and fibroblasts (15, 41). In the CHB model, ET-1 is secreted by the infiltrating macrophages. The levels of ET-1 secreted by human macrophages approximate those reported for other immune cells, including canine macrophages (42), human dendritic cells (14), and human neutrophils (43). Previous publications support the notion that activation of macrophages induces ET-1 production and release. Specifically, it was recently reported that the canine macrophage cell line, DH82, constitutively secretes both ET-1 and its biologically inactive precursor big ET-1 and that the production of both peptides is increased after either stimulation with LPS from Gram-negative bacteria or with intact and viable Gram-positive and Gram-negative bacteria (42). The secretion of ET-1 by polymorphonuclear neutrophils, macrophages, and mast cells increases during inflammation (43, 44). Down-regulation of ET-1 mRNA expression and protein secretion by macrophages treated with either hY3 or IC in the presence of IRS661, a specific antagonist of TLR7, indicates that elevated ET-1 expression is TLR7-dependent. It has been recently demonstrated that stimulation of human monocyte-derived dendritic cells with exogenous as well as endogenous selective TLR4 and TLR2 agonists induces the production of ET-1 (14).
Increased collagen synthesis and expression of α-SMA by fibroblasts in the presence of either exogenous ET-1 or supernatants from macrophages stimulated by hY3 or IC is ETa- and ETb-dependent. There was no difference between the effect of BQ-123 (inhibitor of ETa) or BQ-788 (inhibitor of ETb), suggesting that ETa and ETb may be equally distributed on fetal fibroblasts. It has been reported, however, that the distribution of ETa and ETb is highly variable from tissue to tissue and from species to species and is related to stage of development, growth, and health conditions. Hafizi et al. (22) showed that the profibrotic effects of ET-1 in cultured adult human cardiac fibroblasts occur via ETa, suggesting that during development, ETa and ETb may be equally expressed and that this balance may shift during growth. Furthermore, there is evidence in humans for a shift away from a balanced mixture of ETa (60%) and ETb (40%) subtypes in non-failing hearts to a predominance of ETa (80%) in failing hearts (45). It is acknowledged that a limitation in the interpretation of the experiments presented herein is that the cardiac fibroblasts are isolated from hearts of healthy abortuses. Because most of the affected hearts are from fetuses dying in utero, tissue is less amenable to cellular isolation and culturing. Moreover, these hearts are generally prioritized to autopsy evaluation.
In summary, these data support a novel role for ET-1 in the profound profibrotic responses that characterize the pathology of anti-SSA/Ro-associated fetal cardiac disease. TLR7-dependent ET-1 secretion by infiltrating macrophages attempting clearance of IC bound apoptotic cardiocytes may be positioned as a direct inducer of the fibroblast secretion of TGFβ, with subsequent transdifferentiation and collagen production. Disruption of the anti-SSA/Ro antibody-generated TGFβ-ET-1 axis may provide a novel strategy for the prevention of conduction disease and endocardial fibroelastosis.
This work was supported, in whole or in part, by National Institutes of Health Grants AR-42455 and N01-AR-4-2271 and a grant from the Mary Kirkland Center for Lupus Research.
P. Briassouli, manuscript in preparation.
- CHB
- congenital heart block
- IC
- immune complexe(s)
- SMA
- smooth muscle actin
- AP60
- affinity-purified anti-Ro60 antibody
- qRT-PCR
- quantitative RT-PCR.
REFERENCES
- 1. Brucato A., Frassi M., Franceschini F., Cimaz R., Faden D., Pisoni M. P., Muscarà M., Vignati G., Stramba-Badiale M., Catelli L., Lojacono A., Cavazzana I., Ghirardello A., Vescovi F., Gambari P. F., Doria A., Meroni P. L., Tincani A. (2001) Arthritis Rheum. 44, 1832–1835 [DOI] [PubMed] [Google Scholar]
- 2. Buyon J. P., Clancy R. M. (2007) Dubois' Lupus Erythematosus (Wallace D. J., Hahn B. H. eds) pp. 1058–1080, Lippincott Williams & Wilkins, Philadelphia [Google Scholar]
- 3. Nield L. E., Silverman E. D., Smallhorn J. F., Taylor G. P., Mullen J. B., Benson L. N., Hornberger L. K. (2002) J. Am. Coll. Cardiol. 40, 796–802 [DOI] [PubMed] [Google Scholar]
- 4. Miranda-Carús M. E., Boutjdir M., Tseng C. E., DiDonato F., Chan E. K., Buyon J. P. (1998) J. Immunol. 161, 5886–5892 [PubMed] [Google Scholar]
- 5. Clancy R. M., Kapur R. P., Molad Y., Askanase A. D., Buyon J. P. (2004) Arthritis Rheum. 50, 173–182 [DOI] [PubMed] [Google Scholar]
- 6. Clancy R. M., Neufing P. J., Zheng P., O'Mahony M., Nimmerjahn F., Gordon T. P., Buyon J. P. (2006) J. Clin. Invest. 116, 2413–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clancy R. M., Askanase A. D., Kapur R. P., Chiopelas E., Azar N., Miranda-Carus M. E., Buyon J. P. (2002) J. Immunol. 169, 2156–2163 [DOI] [PubMed] [Google Scholar]
- 8. Clancy R. M., Alvarez D., Komissarova E., Barrat F. J., Swartz J., Buyon J. P. (2010) J. Immunol. 184, 2148–2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Inoue A., Yanagisawa M., Kimura S., Kasuya Y., Miyauchi T., Goto K., Masaki T. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 2863–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Levin E. R. (1995) N. Engl. J. Med. 333, 356–363 [DOI] [PubMed] [Google Scholar]
- 11. Leask A. (2008) Cell. Signal. 20, 1409–1414 [DOI] [PubMed] [Google Scholar]
- 12. Leask A. (2010) Circ. Res. 106, 1675–1680 [DOI] [PubMed] [Google Scholar]
- 13. Ehrenreich H., Anderson R. W., Fox C. H., Rieckmann P., Hoffman G. S., Travis W. D., Coligan J. E., Kehrl J. H., Fauci A. S. (1990) J. Exp. Med. 172, 1741–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spirig R., Potapova I., Shaw-Boden J., Tsui J., Rieben R., Shaw S. G. (2009) Mol. Immunol. 46, 3178–3182 [DOI] [PubMed] [Google Scholar]
- 15. Teder P., Noble P. W. (2000) Am. J. Respir. Cell Mol. Biol. 23, 7–10 [DOI] [PubMed] [Google Scholar]
- 16. Piacentini L., Gray M., Honbo N. Y., Chentoufi J., Bergman M., Karliner J. S. (2000) J. Mol. Cell Cardiol. 32, 565–576 [DOI] [PubMed] [Google Scholar]
- 17. Ogata T., Miyauchi T., Irukayama-Tomobe Y., Takanashi M., Goto K., Yamaguchi I. (2004) J. Cardiovasc. Pharmacol. 44, S279–S282 [DOI] [PubMed] [Google Scholar]
- 18. Kuruvilla L., Nair R. R., Umashankar P. R., Lal A. V., Kartha C. C. (2007) Cell Biochem. Biophys. 47, 65–72 [DOI] [PubMed] [Google Scholar]
- 19. Nishida M., Onohara N., Sato Y., Suda R., Ogushi M., Tanabe S., Inoue R., Mori Y., Kurose H. (2007) J. Biol. Chem. 282, 23117–23128 [DOI] [PubMed] [Google Scholar]
- 20. Katwa L. C. (2003) Am. J. Physiol. Heart Circ Physiol. 285, H1132–H1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chintalgattu V., Katwa L. C. (2004) J. Pharmacol. Exp. Ther. 311, 691–699 [DOI] [PubMed] [Google Scholar]
- 22. Hafizi S., Wharton J., Chester A. H., Yacoub M. H. (2004) Cell Physiol. Biochem. 14, 285–292 [DOI] [PubMed] [Google Scholar]
- 23. Guarda E., Katwa L. C., Myers P. R., Tyagi S. C., Weber K. T. (1993) Cardiovasc. Res. 27, 2130–2134 [DOI] [PubMed] [Google Scholar]
- 24. Kane C. J., Hebda P. A., Mansbridge J. N., Hanawalt P. C. (1991) J. Cell. Physiol. 148, 157–173 [DOI] [PubMed] [Google Scholar]
- 25. Buyon J. P., Clancy R. M. (2005) Autoimmun. Rev. 4, 1–7 [DOI] [PubMed] [Google Scholar]
- 26. Barrat F. J., Meeker T., Gregorio J., Chan J. H., Uematsu S., Akira S., Chang B., Duramad O., Coffman R. L. (2005) J. Exp. Med. 202, 1131–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. O'Brien C. A., Wolin S. L. (1994) Genes Dev. 8, 2891–2903 [DOI] [PubMed] [Google Scholar]
- 28. Shi-Wen X., Denton C. P., Dashwood M. R., Holmes A. M., Bou-Gharios G., Pearson J. D., Black C. M., Abraham D. J. (2001) J. Invest. Dermatol. 116, 417–425 [DOI] [PubMed] [Google Scholar]
- 29. Clancy R. M., Backer C. B., Yin X., Kapur R. P., Molad Y., Buyon J. P. (2003) J. Immunol. 171, 3253–3261 [DOI] [PubMed] [Google Scholar]
- 30. Theiss A. L., Simmons J. G., Jobin C., Lund P. K. (2005) J. Biol. Chem. 280, 36099–36109 [DOI] [PubMed] [Google Scholar]
- 31. Gruschwitz M., Müller P. U., Sepp N., Hofer E., Fontana A., Wick G. (1990) J. Invest. Dermatol. 94, 197–203 [DOI] [PubMed] [Google Scholar]
- 32. Peltonen J., Kähäri L., Jaakkola S., Kähäri V. M., Varga J., Uitto J., Jimenez S. A. (1990) J. Invest. Dermatol. 94, 365–371 [DOI] [PubMed] [Google Scholar]
- 33. Porter K. E., Turner N. A. (2009) Pharmacol. Ther. 123, 255–278 [DOI] [PubMed] [Google Scholar]
- 34. Shi-Wen X., Rodríguez-Pascual F., Lamas S., Holmes A., Howat S., Pearson J. D., Dashwood M. R., du Bois R. M., Denton C. P., Black C. M., Abraham D. J., Leask A. (2006) Mol. Cell. Biol. 26, 5518–5527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lagares D., García-Fernández R. A., Jiménez C. L., Magán-Marchal N., Busnadiego O., Lamas S., Rodríguez-Pascual F. (2010) Arthritis Rheum. 62, 878–889 [DOI] [PubMed] [Google Scholar]
- 36. Shi-wen X., Kennedy L., Renzoni E. A., Bou-Gharios G., du Bois R. M., Black C. M., Denton C. P., Abraham D. J., Leask A. (2007) Arthritis Rheum. 56, 4189–4194 [DOI] [PubMed] [Google Scholar]
- 37. Widyantoro B., Emoto N., Nakayama K., Anggrahini D. W., Adiarto S., Iwasa N., Yagi K., Miyagawa K., Rikitake Y., Suzuki T., Kisanuki Y. Y., Yanagisawa M., Hirata K. (2010) Circulation 121, 2407–2418 [DOI] [PubMed] [Google Scholar]
- 38. Mayyas F., Niebauer M., Zurick A., Barnard J., Gillinov A. M., Chung M. K., Van Wagoner D. R. (2010) Circ. Arrhythm. Electrophysiol. 3, 369–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shephard P., Hinz B., Smola-Hess S., Meister J. J., Krieg T., Smola H. (2004) Thromb. Haemost. 92, 262–274 [DOI] [PubMed] [Google Scholar]
- 40. Zolk O., Quattek J., Sitzler G., Schrader T., Nickenig G., Schnabel P., Shimada K., Takahashi M., Böhm M. (1999) Circulation 99, 2118–2123 [DOI] [PubMed] [Google Scholar]
- 41. Rubanyi G. M., Polokoff M. A. (1994) Pharmacol. Rev. 46, 325–415 [PubMed] [Google Scholar]
- 42. Divino J. N., Chawla K. S., da Silva C. M., Bjorge A. M., Brittingham A. (2010) Vet. Immunol. Immunopathol. 136, 127–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cambiaggi C., Mencarelli M., Muscettola M., Grasso G. (2001) Cytokine 14, 230–233 [DOI] [PubMed] [Google Scholar]
- 44. Massai L, Carbotti P., Cambiaggi C., Mencarelli M., Migliaccio P., Muscettola M., Grasso G. (2003) Am. J. Physiol. Gastrointest. Liver Physiol. 284, G340–G348 [DOI] [PubMed] [Google Scholar]
- 45. Asano K., Bohlmeyer T. J., Westcott J. Y., Zisman L., Kinugawa K., Good M., Minobe W. A., Roden R., Wolfel E. E., Lindenfeld J., David Port J., Perryman M. B., Clevel J., Lowes B. D., Bristow M. R. (2002) J. Mol. Cell Cardiol. 34, 833–846 [DOI] [PubMed] [Google Scholar]