Abstract
Among the eukaryotes only plants and a number of fungi are able to synthesize biotin. Although initial events leading to the biosynthesis of biotin remain largely unknown, the final steps are known to occur in the mitochondria. Here we deleted the Aopex5 and Aopex7 genes encoding the receptors for peroxisomal targeting signals PTS1 and PTS2, respectively, in the filamentous fungus Aspergillus oryzae. In addition to exhibiting defects in the peroxisomal targeting of either PTS1 or PTS2 proteins, the deletion strains also displayed growth defects on minimal medium containing oleic acid as the sole carbon source. Unexpectedly, these peroxisomal transport-deficient strains also exhibited growth defects on minimal medium containing glucose as the sole carbon source that were remediated by the addition of biotin and its precursors, including 7-keto-8-aminopelargonic acid (KAPA). Genome database searches in fungi and plants revealed that BioF protein/KAPA synthase, one of the biotin biosynthetic enzymes, has a PTS1 sequence at the C terminus. Fungal ΔbioF strains expressing the fungal and plant BioF proteins lacking PTS1 still exhibited growth defects in the absence of biotin, indicating that peroxisomal targeting of KAPA synthase is crucial for the biotin biosynthesis. Furthermore, in the plant Arabidopsis thaliana, AtBioF localized to the peroxisomes through recognition of its PTS1 sequence, suggesting involvement of peroxisomes in biotin biosynthesis in plants. Taken together we demonstrate a novel role for peroxisomes in biotin biosynthesis and suggest the presence of as yet unidentified peroxisomal proteins that function in the earlier steps of biotin biosynthesis.
Keywords: Biotin, Fungi, Mitochondria, Peroxisomes, Plant
Introduction
Peroxisomes are ubiquitous organelles in eukaryotic cells and typically contain enzymes involved in β-oxidation of fatty acids and detoxification of reactive oxygen species. In addition, as peroxisomal matrix enzymes vary depending on the cellular environment and tissue, these organelles display diverse functions in different eukaryotic groups. For example, mammalian peroxisomes participate in the biosynthesis of lipids, such as ether phospholipids and cholesterol, and in the oxidation of amino acids and polyamines (1). In plants, peroxisomes are involved in the glyoxylate cycle (2), photorespiration (3), and hormone biosynthesis of jasmonic acid and auxin (4, 5). The peroxisomes of methylotrophic yeasts are required for methanol metabolism (6), and those of filamentous fungi are involved in penicillin biosynthesis, plant pathogenicity, and sexual development (7–11). Peroxisomes are also required for the formation of the Woronin body, an organelle specific to filamentous ascomycetes and functions in wound healing (12, 13). Peroxisomes are known to play important roles during growth of higher organisms, which is exemplified by the fact that peroxisomal deficiency causes lethal neurological disorder in humans (14) and embryonic defects in plants (15–19). Evidenced by delayed germination and abnormal nuclear and mitochondrial morphology, several reports on peroxisome-deficient mutants in filamentous fungi have confirmed fundamental roles for peroxisomes during growth (9, 20, 21). However, the molecular mechanisms responsible for the severe effects of peroxisomal deficiency during growth remain unknown.
Peroxins encoded by PEX genes are the proteins required for peroxisomal biogenesis (22). Most peroxins are engaged in the import of peroxisomal matrix proteins from the cytosol into the peroxisome lumen. In general, peroxisomal matrix proteins have either the peroxisomal targeting signal PTS1 or PTS2 sequences, which are typically located at the C and N termini, respectively. PTS1 sequence is a tripeptide motif with the consensus sequence (S/A)(K/R)(L/M) (23), whereas PTS2 is defined by the motif (R/K)(L/V/I)-X5-(H/Q)(L/A/F/I) (24). The receptors of PTS1 and PTS2 are Pex5 and Pex7, respectively, and serve to load their cargoes to a large multiprotein complex at the peroxisomal membrane, which finally translocates the peroxisomal matrix proteins into the lumen (25). Because Pex5 and Pex7 transport different subsets of enzymes into the peroxisomes to initiate a complex network of peroxisomal metabolic pathways, any defect in either of the PTS receptors is expected to result in different metabolic defects and phenotypes.
Biotin is an essential cofactor involved in a number of carboxylation and decarboxylation reactions (26). Although numerous bacteria, plants and a number of fungi are capable of biotin biosynthesis, this process has mainly been analyzed in bacteria and plants. These studies have revealed that the last four reactions, which convert pimeloyl-CoA to biotin, are conserved between bacteria and plants (27) (supplemental Fig. S1). In plants, AtBioF, Bio1, Bio3, and Bio2 represent the homologs of bacterial BioF, BioA, BioD, and BioB, respectively, and are involved in the last four steps of biotin biosynthesis. In Arabidopsis thaliana, the flanking BIO3 and BIO1 genes is aligned unidirectionally and expressed as a chimeric transcript; the resultant Bio3-Bio1 product acts as a bifunctional protein (28). Pinon et al. (29) reported that AtBioF, which is responsible for the first step of the conserved biotin biosynthesis reactions in plants, localized to the cytoplasm. The last three steps occur in mitochondria as Bio3-Bio1 is predicted to have a putative mitochondrial targeting signal at its N terminus (28), and Bio2 requires mitochondrial targeting for activity (30). It was therefore suggested that plant biotin biosynthesis occurs in the cytoplasm and mitochondria (31). However, the upstream reactions of the eukaryotic biotin biosynthesis have never been investigated.
Here, in the filamentous fungus Aspergillus oryzae, we found that growth defects of the peroxisomal transport-deficient strains are a result of biotin auxotrophy. Among the biotin biosynthetic enzymes, BioF proteins from fungi and plants have peroxisomal targeting sequences. We demonstrate that BioF proteins localize to peroxisomes, and its peroxisomal targeting is required for the biotin biosynthesis. For the first time we provide evidence supporting peroxisomal function in biotin biosynthesis of eukaryotic organisms and suggest a role for peroxisomes early in its biosynthesis pathway.
EXPERIMENTAL PROCEDURES
Strains and Growth Media
A. oryzae strains used in this study are listed in supplemental Table S1, and the methods for their construction are described in the supplemental Methods. The routine liquid cultivation and growth analyses of the A. oryzae strains were performed with DPY medium (2% dextrin, 1% polypeptone, 0.5% yeast extract, 0.5% KH2PO4, 0.05% MgSO4·7H2O, pH 5.5) at 30 °C. Czapek Dox (CD)2 + Met (0.3% NaNO3, 0.2% KCl, 0.1% KH2PO4, 0.05% MgSO4·7H2O, 0.002% FeSO4·7H2O, 0.0015% methionine, and either 2% glucose or another carbon source (2% acetate or 10 mm oleic acid), pH 5.5) and M + Met (0.2% NH4Cl, 0.1% (NH4)2SO4, 0.05% KCl, 0.05% NaCl, 0.1% KH2PO4, 0.05% MgSO4·7H2O, 0.002% FeSO4·7H2O, 0.15% methionine, and either 2% glucose or another carbon source (2% acetate or 10 mm oleic acid), pH 5.5) media were used for transformation and growth analyses of A. oryzae. Transformation of A. oryzae was carried out as previously described (32). Escherichia coli DH5α was used for DNA manipulation.
Chemicals
Dethiobiotin (DTB) and biotin were purchased from Sigma (St. Louis, MO). Pimelic acid was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). 7-Keto-8-aminopelargonic acid (KAPA) was kindly provided by Ajinomoto Pharmaceuticals Co., Ltd. Pimeloyl-CoA was chemically synthesized and purified as described by Ploux and Marquet (33). For the growth analyses, the concentrations of KAPA, DTB, and biotin were 8.2 nm, and those of pimelic acid and pimeloyl-CoA were 8.2 μm and 82 μm, respectively. One gram of oleic acid (Sigma) was mixed with 100 μl of IGEPAL CA-630 (ICN Biomedicals, Costa Mesa, CA), added into 10 ml of autoclaved water, and then stored at 4 °C.
Fluorescence Microscopy
Conidia (1 × 103) of the A. oryzae strains were inoculated into 100 μl of liquid minimal medium on a glass-bottom dish (Asahi Techno Glass, Chiba, Japan) and incubated at 30 °C for 18 h. Confocal microscopy was performed using an IX71 inverted microscope (Olympus, Tokyo, Japan) equipped with a CSU22 confocal scanning system (Yokogawa Electronics, Tokyo, Japan), an iXon cooled digital charged-coupled device camera (Andor Technology PLC, Belfast, UK), semi-conductor lasers at 488 nm (Furukawa Electric, Tokyo, Japan) and 561 nm (Melles Griot, Carlsbad, CA), and GFP, DsRed, and DualView filters (Nippon Roper, Chiba, Japan). Images were analyzed with iQ software (Andor Technology PLC).
Mitochondrial Staining
Conidia were inoculated in 100 μl of liquid medium and incubated in glass-bottom dishes at 30 °C for 24 h. Mycelia were transferred into a medium containing 1 μm MitoTracker Red CMXRos (Molecular Probes, Eugene, OR) and incubated for 15 min at 30 °C. The mycelia were then washed twice with medium and observed by fluorescence microscopy.
Intracellular Localization Experiments of AtBioF in A. thaliana
AtbioF cDNA was amplified using A. thaliana cDNA as a template and the following primer pairs (supplemental Table S2): GFP-FL-F and GFP-FL-R for EGFP-AtBioF; GFP-ΔPKL-F and GFP-ΔPKL-R for EGFP-AtBioFΔPKL; and FL-GFP-F and FL-GFP-R for AtBioF-EGFP. The resulting PCR fragments were cloned into pK7WGF2 or pK7FWG2 (34) by Gateway recombination (Invitrogen, Carlsbad, CA). The resultant vectors were introduced into leaf epidermal cells of A. thaliana (background Columbia) using a Model PDS-1000/He Biolistic particle delivery system (Bio-Rad, Hercules, CA). Fluorescence was visualized 20–24 h later. EGFP fluorescence of the fusion proteins was excited at 488 nm with an argon laser, and the emitted light was collected through a 500 nm to 530 nm filter and acquired using a Nikon Digital Eclipse C1si confocal laser scanning microscope system with a 60× Plan Apo oil-immersion lens (numerical aperture = 1.4). Peroxisomes were visualized by introducing the PTS2-RFP vector (35) into Arabidopsis leaf epidermal cells as described above. The fluorescence of PTS2-mRFP1 was excited at 543 nm with an HeNe laser, and the emitted light was collected through a 565 nm to 615 nm filter. Images were processed using a Nikon EZ-C1 Gold version 3.60 and Adobe Photoshop CS4 version 11.0.1.
RESULTS
Fungal Peroxisome-deficient Strains Show Growth Defects on Minimal Medium Containing Glucose as the Sole Carbon Source
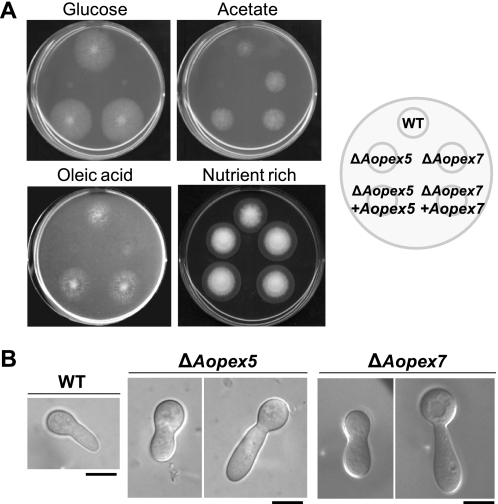
Genome database searches (available online in the website of the National Research Institute of Brewing, Japan) revealed that A. oryzae AO080509000034 (Aopex5) and AO080511000135 (Aopex7) encode proteins homologous to Pex5 and Pex7, respectively. We therefore deleted the entire ORF regions of the Aopex5 and Aopex7 genes and confirmed that the ΔAopex5 and ΔAopex7 strains were impaired in PTS1 and PTS2 targeting, respectively, to peroxisomes (supplemental Fig. S2). To examine whether these strains exhibited peroxisomal deficiency, their growth was assayed on minimal medium containing either glucose or oleic acid or acetate as the sole carbon source (Fig. 1A). Growth of the ΔAopex5 and ΔAopex7 strains on oleic acid minimal medium was hardly detectable, as previously reported for other fungal phenotypes of peroxisomal deficiency (21, 36). However, their growth was significantly decreased on glucose minimal medium (Fig. 1A). Furthermore, although the ΔAopex7 strain grew normally on acetate minimal medium (Fig. 1A), growth of the ΔAopex5 strain was significantly impaired, a result that was consistent with the Aspergillus nidulans Δpex5 (pexE) strain (21). The growth defects of the ΔAopex5 and ΔAopex7 strains on minimal media were restored by the introduction of the wild-type Aopex5 and Aopex7 genes, respectively. On nutrient-rich medium, the growth of the ΔAopex5 and ΔAopex7 strains was comparable with the wild-type strain (Fig. 1A).
FIGURE 1.
Growth impairment of strains defective in peroxisomal targeting signal receptors on glucose minimal medium. A, growth of the A. oryzae ΔAopex5 and ΔAopex7 strains on minimal media (CD + Met) containing either glucose or acetate or oleic acid as the sole carbon source, and nutrient-rich medium (DPY). Conidial solutions (103 conidia/5 μl) of the wild-type, ΔAopex5, and ΔAopex7 strains and the complemented strains were spotted on each medium (as indicated to the right) and incubated at 30 °C for 3 days. B, swollen morphology of germinated conidia of the ΔAopex5 and ΔAopex7 strains in glucose minimal medium. Conidia of the wild-type, ΔAopex5, and ΔAopex7 strains were inoculated in minimal medium (CD + Met) containing glucose at a concentration of 103 conidia/100 μl. The cultures were incubated at 30 °C for 7 h (wild-type) or 15 h (ΔAopex5 and ΔAopex7) and observed by microscopy. Note that a longer incubation time was required for the ΔAopex5 and ΔAopex7 strains to germinate. Bars: 5 μm.
Hyphal morphology of the ΔAopex5 and ΔAopex7 strains during germination in glucose minimal medium was observed microscopically (Fig. 1B). In both mutants the germinated conidia exhibited a swollen morphology. Taken together, these results indicated that the loss of the two PTS receptors caused abnormal polarity.
Peroxisome-deficient Strains Show Biotin Auxotrophy on Glucose Minimal Medium
We next attempted to identify compounds that could restore the defective growth phenotypes of the peroxisome-deficient strains. A complete growth recovery of the mutants was observed in rich medium, leading to the assumption that the peroxisome-deficient strains may require vitamins present in the yeast extract for their growth. To verify this possibility, glucose minimal medium was supplemented with a mixture of vitamins, including biotin, pantothenate, folic acid, riboflavin, and thiamine, which revealed growth recovery of the ΔAopex5 and ΔAopex7 strains. After a combination of vitamin supplementation experiments we found that supplementation with biotin alone was sufficient to restore their growth defects on glucose minimal medium (Fig. 2). Moreover abnormal polarity of the ΔAopex5 and ΔAopex7 strains was also suppressed by the addition of biotin (supplemental Fig. S3). However, addition of biotin in the oleic acid minimal medium did not restore the growth defects of the ΔAopex5 and ΔAopex7 strains (data not shown).
FIGURE 2.
Biotin and its biosynthetic precursors restore the growth defects of the peroxisome-deficient strains on glucose minimal medium. Conidial solutions (103 conidia/5 μl) of the wild-type, ΔAopex5, and ΔAopex7 strains were spotted on minimal medium (CD + Met) containing glucose supplemented with biotin or its biosynthetic precursors, pimelic acid (Pim), pimeloyl-CoA (Pim-CoA), 7-keto-8-aminopelargonic acid (KAPA), and dethiobiotin (DTB). Photographs of the cultures were taken after incubation at 30 °C for 3 days.
To determine the cause of biotin auxotrophy of the peroxisome-deficient strains, we also examined whether the growth defects were restored by the addition of biotin biosynthetic precursors (Fig. 2). Although biotin is commonly synthesized from pimeloyl-CoA in bacteria and plants, the biosynthetic pathway leading to this precursor has not been clearly elucidated in eukaryotes. In our growth assays, three common precursors, pimeloyl-CoA, KAPA, and DTB (supplemental Fig. S1), and pimelic acid, the precursor of pimeloyl-CoA in Gram-positive bacteria (37), were used. On glucose minimal medium supplemented with either KAPA or DTB, growth of both the mutants was nearly identical to the wild-type strain. The growth defect of the ΔAopex7 strain was also restored on the medium supplemented with pimelic acid or pimeloyl-CoA; however, these supplements only caused formation of small colonies by the ΔAopex5 strain (Fig. 2). These results suggested that loss of the PTS1 receptor limits the biotin biosynthetic pathway up to the KAPA synthase, whereas the PTS2 receptor contributes to earlier steps of the pathway before the conversion of pimelic acid to pimeloyl-CoA.
Biotin Biosynthetic Enzyme BioF Protein/KAPA Synthase from Fungi and Plants Has Peroxisomal Targeting Signal
To further investigate the cause of biotin auxotrophy in the peroxisome-deficient strains, we analyzed the biotin biosynthetic pathway in A. oryzae. Genome database searches revealed that A. oryzae has an identical gene cluster for the last four steps of biotin biosynthesis as described in A. nidulans (28). The cluster consists of three genes: AO080518000021, AO080518000022, and AO080518000023, which were designated AobioF, AobioD/A, and AobioB, respectively (supplemental Fig. S1). Moreover, AoBioF shared 23.2% identity with A. thaliana AtBioF (KAPA synthase) and possessed a PTS1 motif (ARL) at its C terminus, which is consistent with the fact that addition of KAPA restored the growth defect caused by loss of the PTS1 receptor (Fig. 2). Genome database searches in other organisms revealed that BioF proteins from fungi and plants also have the PTS1 sequence at their C termini (supplemental Fig. S4). This raised the possibility that the BioF protein/KAPA synthase has a function in the peroxisomes.
Peroxisomal Targeting of BioF Proteins Is Required for Biotin Biosynthesis in Glucose Minimal Medium
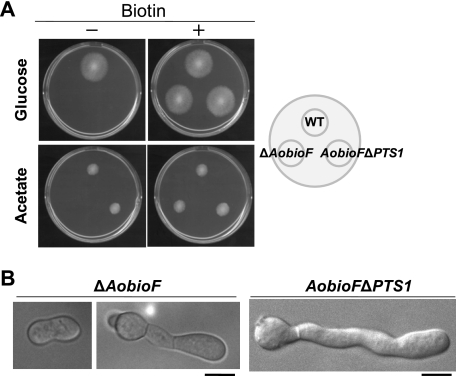
To examine the function of fungal BioF protein, the entire coding sequence of the AobioF gene was deleted in A. oryzae. Although the resulting ΔAobioF strain was unable to grow on glucose minimal medium, the growth defect was restored in the presence of biotin (Fig. 3A) and its biosynthetic precursors (KAPA and DTB) but not by the upstream precursors (pimelic acid and pimeloyl-CoA) (supplemental Fig. S5). To further evaluate biotin auxotrophy of the ΔAobioF strain, A. thaliana AtBioF, which has KAPA synthase activity (29), was expressed in the A. oryzae ΔAobioF strain (supplemental Fig. S6). Expression of AtBioF fused with mDsRed immediately upstream of its PTS1 motif (PKL) partially restored biotin auxotrophy of the ΔAobioF strain. Furthermore, replacement of the AtBioF PTS1 with the AoBioF PTS1 motif (ARL) and expression of the resulting AtBioF-mDsRed fusion protein in the ΔAobioF strain resulted in full restoration of the biotin auxotrophy. These data suggest that AoBioF functions as a KAPA synthase in the biotin biosynthetic pathway of A. oryzae.
FIGURE 3.
Deletion of PTS1 from BioF protein leads to biotin auxotrophy on glucose minimal medium. A, growth of the ΔAobioF and AobioFΔPTS1 strains on minimal medium (M + Met) containing either glucose or acetate as the sole carbon source supplemented with biotin. Conidia solutions (103 conidia/5 μl) of the strains were spotted on plates (as indicated to the right) and incubated at 30 °C for 3 days. B, swollen morphology of germinated conidia of the ΔAobioF and AobioFΔPTS1 strains in glucose minimal medium. Conidia of these strains were inoculated in minimal medium (CD + Met) containing glucose and 0.5% casamino acids at a concentration of 103 conidia/100 μl. Photographs of germinated conidia were taken after the cultures were incubated at 30 °C for 20 h. Bars: 5 μm.
Next, to investigate the role for peroxisomal targeting of BioF protein in biotin biosynthesis, we constructed an A. orzyae strain endogenously expressing AoBioFΔPTS1, in which the genomic DNA region encoding PTS1 in the AobioF gene was deleted. The AobioFΔPTS1 strain was unable to grow on glucose minimal medium, and the growth defects could be restored by the addition of biotin (Fig. 3A) and its precursors (KAPA and DTB) but not by pimelic acid and pimeloyl-CoA (supplemental Fig. S5). Similarly, expression of A. thaliana AtBioF lacking the PTS1 motif did not restore biotin auxotrophy of the ΔAobioF strain (supplemental Fig. S6). This result indicated that the PTS1 sequence is required for the BioF proteins to function during biotin biosynthesis in glucose minimal medium.
Microscopic observation of the ΔAobioF and AobioFΔPTS1 strains during germination in glucose minimal medium revealed that these strains displayed a swollen morphology of germinated conidia (Fig. 3B) similar to the ΔAopex5 and ΔAopex7 strains (Fig. 1B). This indicated that biotin auxotrophy due to the loss of the BioF protein function resulted in abnormal polar growth as observed in the peroxisome-deficient strains.
We also analyzed growth of the ΔAobioF and AobioFΔPTS1 strains on acetate minimal medium. Although growth of the ΔAobioF strain was severely impaired, the AobioFΔPTS1 strain grew normally (Fig. 3A). This indicated that peroxisomal targeting of AoBioF is not required for biotin biosynthesis in acetate minimal medium.
BioF Proteins Localize to Peroxisomes in Fungal and Plant Cells
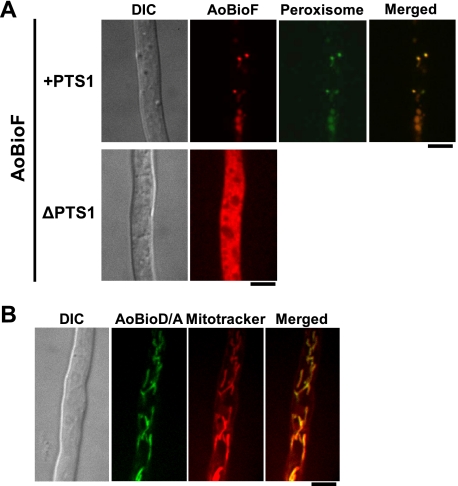
To examine the localization of fungal BioF protein, AoBioF-mDsRed-PTS1 and AoBioF-mDsRed-ΔPTS1 fusion proteins were expressed in the ΔAobioF strain. Growth assay on glucose minimal medium indicated that, although the expression of AoBioF-mDsRed-PTS1 restored biotin auxotrophy of the ΔAobioF strain, AoBioF-mDsRed-ΔPTS1 did not rescue this defective phenotype (supplemental Fig. S7). These growth phenotypes were consistent with those of the wild-type and AobioFΔPTS1 strains expressing wild-type AoBioF and AoBioFΔPTS1, respectively (Fig. 3A). Fluorescence microscopic observation revealed that AoBioF-mDsRed-PTS1 localized to the peroxisomes, whereas AoBioF-mDsRed-ΔPTS1 localized throughout the cytoplasm (Fig. 4A). These results indicated that AoBioF is transported to peroxisomes by recognition of its PTS1 sequence.
FIGURE 4.
Localization analysis of AoBioF and its downstream enzyme. A, AoBioF localization in peroxisomes. Conidia of strains expressing either AoBioF-mDsRed-PTS1 or AoBioF-mDsRed-ΔPTS1 were inoculated in minimal medium (CD + Met) containing glucose and 0.5% casamino acids. After a 20-h incubation at 30 °C, the hyphae were observed by fluorescence microscopy. To visualize peroxisomes, EGFP-PTS1 was also expressed in the A. oryzae strain expressing AoBioF-mDsRed-PTS1. Bars: 5 μm. B, AoBioD/A localization in mitochondria. The strain expressing AoBioD/A-EGFP endogenously was grown at 30 °C for 24 h in minimal medium (CD + Met) containing glucose and 0.5% casamino acids. Mitochondria were stained with MitoTracker Red CMXRos and observed by fluorescence microscopy. DIC, differential interference contrast microscopy. Bar: 5 μm.
We also investigated localization of the AoBioF-mDsRed fusion proteins expressed in the ΔAobioF strain when cultured in acetate minimal medium. In the growth assay, not only AoBioF-mDsRed-PTS1 but also AoBioF-mDsRed-ΔPTS1 restored the biotin auxotrophy of the ΔAobioF strain (supplemental Fig. S7). The complementation by AoBioF-mDsRed-ΔPTS1 in acetate minimal medium coincided with the result of the AobioFΔPTS1 strain (Fig. 3A). Fluorescence microscopic analysis revealed that the localizations of AoBioF-mDsRed-PTS1 and AoBioF-mDsRed-ΔPTS1 are peroxisomal and cytoplasmic, respectively (supplemental Fig. S8), which are similar to those when cultured in glucose minimal medium (Fig. 4A).
AobioD and AobioA are predicted to encode a chimeric protein that functions downstream of AoBioF (supplemental Fig. S1) and is similar to the case for A. nidulans, Ustilago maydis, and Cryptococcus neoformans (28). Because the AoBioD/A chimeric protein has a putative mitochondrial targeting signal at its N terminus, we investigated the localization of AoBioD/A by integrating the egfp gene at the 3′-end of the AobioD/A ORF. The strain expressing AoBioD/A-EGFP endogenously grew normally in the absence of biotin (data not shown), suggesting that the fusion protein was functional. Fluorescence microscopic observation revealed that AoBioD/A localized to mitochondria (Fig. 4B).
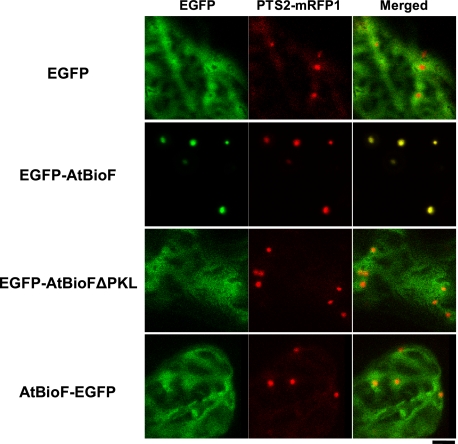
Finally, we examined whether peroxisomal localization of BioF was also observed in plants. Although Pinon et al. (29) reported that A. thaliana AtBioF localized to the cytoplasm, we thought that this result may have been due to impaired PTS1 recognition caused by the C-terminal GFP fusion. Therefore, we evaluated AtBioF localization by fusing EGFP to either its N (EGFP-AtBioF) or C termini (AtBioF-EGFP) (Fig. 5). EGFP-AtBioF localized to the peroxisomes, whereas localizations of both AtBioF-EGFP and EGFP-AtBioF lacking PTS1 were observed throughout the cytoplasm. This result confirmed that AtBioF localizes to the peroxisomes of A. thaliana and requires an accessible PTS1 motif at the C terminus for proper targeting.
FIGURE 5.
Plant BioF localizes to peroxisomes by utilizing the PTS1 sequence at the C terminus. Confocal images of EGFP (EGFP) and PTS2-mRFP1 fluorescence (PTS2-mRFP1) and their merged images (Merged) are shown in Arabidopsis leaf epidermal cells transiently expressing the cDNA constructs of EGFP (EGFP) and the full-length (EGFP-AtBioF) and C-terminal proline-lysine-leucine-deleted mutant proteins of AtBioF (EGFP-AtBioFΔPKL) fused to the C terminus of EGFP. The full-length AtBioF fused to the N terminus of EGFP (AtBioF-EGFP) was also examined. Bar: 5 μm.
DISCUSSION
In this study, we discovered a novel peroxisomal function: its involvement in biotin biosynthesis. Previous studies on biotin biosynthesis in the yeasts have provided limited information, because the commonly used yeast isolates, including Saccharomyces cerevisiae laboratory strain S288c, are biotin auxotrophs (38). In A. nidulans, a model filamentous fungus, the biA1 mutant allele causes biotin auxotrophy (39). However, most of the previous studies on A. nidulans have used strains containing this mutant allele in the presence of biotin, which may have impeded the analysis of biotin biosynthesis. Furthermore, another model filamentous fungus, Neurospora crassa, is auxotrophic for biotin (40), and according to the genome database it does not possess any BioF homologs. Consequently, our investigation on the industrial fungus A. oryzae revealed the involvement of peroxisomes in biotin biosynthesis for the first time.
Peroxisomal targeting of AoBioF is required for biotin biosynthesis in minimal medium containing glucose as the sole carbon source (Figs. 3 and 4). Our genome database searches revealed that the BioF proteins from fungi and plants have a PTS1 motif at the C terminus (supplemental Fig. S4). In the human-pathogenic basidiomycete C. neoformans, peroxisome-deficient mutants are defective for growth in medium containing glucose as a carbon source, but not in nutrient-rich medium (20). C. neoformans has a BioF homolog containing PTS1 (supplemental Fig. S4), which suggests that the reported growth defect of peroxisomal mutants may also be due to biotin auxotrophy. We demonstrated that, in A. thaliana, AtBioF localizes to the peroxisomes by the PTS1 motif at the C terminus (Fig. 5, EGFP-AtBioF). Deletion of the PTS1 motif eliminated the peroxisomal localization of AtBioF (Fig. 5, EGFP-AtBioFΔPKL). Pinon et al. (29) reported that AtBioF localized to the cytoplasm, however, this was based on the observations using AtBioF fused to GFP at its C terminus, as we also showed in this study (Fig. 5, AtBioF-EGFP). Because PTS1 is a conserved signal motif found at the extreme C terminus of peroxisomal proteins (23), such a GFP fusion may prevent proper recognition of PTS1 motif by its receptor Pex5, resulting in mislocalization of AtBioF to the cytoplasm. Furthermore, our study demonstrates the functional requirement of BioF peroxisomal targeting for the biotin biosynthesis. Taken together, these results suggest that the KAPA synthase in the biotin biosynthetic pathway is a peroxisomal enzyme in both fungi and plants.
Our present study provides a new model of biotin biosynthesis in eukaryotes, indicating that a biotin precursor KAPA is synthesized in peroxisomes (Fig. 6). Following its synthesis, KAPA may then be transported from the peroxisomes to mitochondria, which are the location of AoBioD/A, the next enzyme in the pathway (Fig. 4B). Although the later steps of biotin biosynthesis are established, the earlier steps in the biosynthetic pathway have not been investigated in eukaryotes. In bacteria, two different pathways for the formation of pimeloyl-moiety have been reported; one utilizes pimelic acid as a precursor for pimeloyl-CoA synthesis (37), and the other involves a modified fatty acid synthetic pathway for synthesis of the pimeloyl-acyl carrier protein as recently reported in E. coli (41). A previous study in yeasts demonstrated that pimelic acid could be synthesized from long chain fatty acids such as oleic acid (42). Here, the growth defect of the ΔAopex7 strain but not the ΔAopex5 strain was restored by the addition of pimelic acid and pimeloyl-CoA to glucose minimal medium (Fig. 2). Based on this result, we propose a model that a PTS2-dependent protein participates in the supply of pimelic acid and a PTS1-dependent protein then synthesizes pimeloyl-CoA from pimelic acid in peroxisomes (Fig. 6).
FIGURE 6.
Schematic model of eukaryotic biotin biosynthesis. BioF is transported into peroxisomes through the recognition of the PTS1 motif by Pex5, and it converts pimeloyl-CoA to KAPA. Our study suggests that a PTS2-dependent protein participates in the supply of pimelic acid and a PTS1-dependent protein then synthesizes pimeloyl-CoA from pimelic acid. Note that in acetate minimal medium BioFΔPTS1 is not transported to peroxisomes; it synthesizes KAPA from pimeloyl-CoA supplied in the cytoplasm (see “Discussion”).
We also found that the AobioFΔPTS1 strain grew normally on acetate minimal medium (Fig. 3A), in which AoBioFΔPTS1 was cytoplasmic (supplemental Fig. S8). It is presumed that pimeloyl-CoA is supplied in the cytoplasm when A. oryzae is cultured in acetate minimal medium. A similar phenomenon was reported for a peroxisomal enzyme, malate synthase (Mls1p); a S. cerevisiae strain expressing a PTS1-less Mls1p in the cytoplasm grows normally with acetate as a sole carbon source but displays reduced growth with oleic acid that requires peroxisomal β-oxidation (43). When yeast cells utilize acetate and oleic acid, acetyl-CoA is supplied in the cytoplasm and peroxisomes, respectively (44). However, the abovementioned assumption appears contradictory to the fact that the wild-type AoBioF targeted to the peroxisomes is also able to function in biotin biosynthesis in acetate minimal medium (supplemental Figs. S7 and S8). This raises one possibility that the wild-type AoBioF might act on pimeloyl-CoA in the cytoplasm before its peroxisomal targeting when A. oryzae is cultured in acetate minimal medium. To address this issue, further stringent analyses of the enzyme localization are required. On the other hand, the ΔAopex7 strain grew normally on acetate minimal medium in contrast to its growth defect on glucose minimal medium (Fig. 2), which is consistent with the report of C. neoformans peroxisome-deficient mutants (20). This suggests that PTS2-dependent proteins are dispensable for biotin biosynthesis in acetate minimal medium.
The A. oryzae peroxisome-deficient strains showing biotin auxotrophy exhibited abnormal polar growth (Fig. 1B). In plant A. thaliana, mutants deficient in biotin biosynthesis have defective embryos (28), and peroxisomes are essential for embryo development (15–19). These similarities in the fungal and plant phenotypes indicate that plant embryo lethality due to peroxisomal deficiency may be partially or entirely attributed to biotin auxotrophy. Consistently, RT-PCR analysis revealed that the A. oryzae AobioF gene was up-regulated after germination compared with conidia (supplemental Fig. S9), and the expression level of A. thaliana AtbioF gene is significantly higher in embryo than in other tissues (supplemental Fig. S10). These data support the idea that the BioF protein is important for early developmental stages in filamentous fungi and plants. For example, biotin is incorporated into a number of biotin-dependent carboxylases, such as acetyl-CoA carboxylase (ACC1), which is essential for embryo development in plants (45). In fungi deletion of the ACC1 gene is lethal (46, 47), and ACC1 mutant alleles affect various cellular processes, such as the function of the nuclear pore complex, vacuolar morphology, and cell cycle regulation (48–50). Important roles for biotin have also been identified in mammals, including cell signaling, gene expression, and chromatin structure (51). Taken together, biotin auxotrophy resulting from peroxisomal deficiency likely causes pleiotropic effects on many cellular processes.
Peroxisomes are a multipurpose organelle with diverse functions, but they play important roles in the growth as peroxisomal deficiency causes lethal phenotypes. Our present study demonstrates the peroxisomal function in biotin biosynthesis and, concurrently, provides new evidence for the biologically important role of this organelle in eukaryotic production of an essential vitamin. In the future, our findings will focus attention on unidentified peroxisomal proteins as candidate enzymes required for biotin biosynthesis, providing clues to unknown upstream reactions of the eukaryotic biotin biosynthetic pathway.
Supplementary Material
Acknowledgments
We thank Ryusuke Hirama (Ajinomoto Pharmaceuticals Co., Ltd.) and Nami Nakamura (Ajinomoto Co., Inc.) for providing biotin precursors. We also thank Dr. Masatoshi Nakajima (The University of Tokyo) for providing A. thaliana cDNA and Dr. Shoji Mano (National Institute for Basic Biology) for providing PTS2-RFP vector. We are thankful to Dr. Hiroshi Kitagaki (Saga University) and Dr. Praveen Rao Juvvadi (Duke University Medical Center) for helpful discussions and critical reading of our manuscript.
This study was supported by Grant-in-Aid for Young Scientist (to J. M. and S. Y.) and Research Fellowship for Young Scientist (to S. Y.) from the Japan Society for the Promotion of Science.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Methods, Tables S1 and S2, and Figs. S1–S10.
- CD
- Czapek Dox
- PTS1
- peroxisomal targeting signal 1
- PTS2
- peroxisomal targeting signal 2
- KAPA
- 7-keto-8-aminopelargonic acid
- DTB
- dethiobiotin.
REFERENCES
- 1. Wanders R. J., Waterham H. R. (2006) Annu. Rev. Biochem. 75, 295–332 [DOI] [PubMed] [Google Scholar]
- 2. Mano S., Nishimura M. (2005) Vitam. Horm. 72, 111–154 [DOI] [PubMed] [Google Scholar]
- 3. Reumann S., Weber A. P. (2006) Biochim. Biophys. Acta 1763, 1496–1510 [DOI] [PubMed] [Google Scholar]
- 4. Weber H. (2002) Trends Plant Sci. 7, 217–224 [DOI] [PubMed] [Google Scholar]
- 5. Woodward A. W., Bartel B. (2005) Ann. Bot. 95, 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Klei I. J., Yurimoto H., Sakai Y., Veenhuis M. (2006) Biochim. Biophys. Acta 1763, 1453–1462 [DOI] [PubMed] [Google Scholar]
- 7. Kiel J. A., van der Klei I. J., van den Berg M. A., Bovenberg R. A., Veenhuis M. (2005) Fungal Genet. Biol. 42, 154–164 [DOI] [PubMed] [Google Scholar]
- 8. Kimura A., Takano Y., Furusawa I., Okuno T. (2001) Plant Cell 13, 1945–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonnet C., Espagne E., Zickler D., Boisnard S., Bourdais A., Berteaux-Lecellier V. (2006) Mol. Microbiol. 62, 157–169 [DOI] [PubMed] [Google Scholar]
- 10. Asakura M., Okuno T., Takano Y. (2006) Appl. Environ. Microbiol. 72, 6345–6354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peraza-Reyes L., Zickler D., Berteaux-Lecellier V. (2008) Traffic 9, 1998–2009 [DOI] [PubMed] [Google Scholar]
- 12. Liu F., Ng S. K., Lu Y., Low W., Lai J., Jedd G. (2008) J. Cell Biol. 180, 325–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Escaño C. S., Juvvadi P. R., Jin F. J., Takahashi T., Koyama Y., Yamashita S., Maruyama J., Kitamoto K. (2009) Eukaryot. Cell 8, 296–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gould S. J., Valle D. (2000) Trends Genet. 16, 340–345 [DOI] [PubMed] [Google Scholar]
- 15. Hu J., Aguirre M., Peto C., Alonso J., Ecker J., Chory J. (2002) Science 297, 405–409 [DOI] [PubMed] [Google Scholar]
- 16. Schumann U., Wanner G., Veenhuis M., Schmid M., Gietl C. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 9626–9631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sparkes I. A., Brandizzi F., Slocombe S. P., El-Shami M., Hawes C., Baker A. (2003) Plant Physiol. 133, 1809–1819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tzafrir I., Pena-Muralla R., Dickerman A., Berg M., Rogers R., Hutchens S., Sweeney T. C., McElver J., Aux G., Patton D., Meinke D. (2004) Plant Physiol. 135, 1206–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fan J., Quan S., Orth T., Awai C., Chory J., Hu J. (2005) Plant Physiol. 139, 231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Idnurm A., Giles S. S., Perfect J. R., Heitman J. (2007) Eukaryot. Cell 6, 60–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hynes M. J., Murray S. L., Khew G. S., Davis M. A. (2008) Genetics 178, 1355–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Distel B., Erdmann R., Gould S. J., Blobel G., Crane D. I., Cregg J. M., Dodt G., Fujiki Y., Goodman J. M., Just W. W., Kiel J. A., Kunau W. H., Lazarow P. B., Mannaerts G. P., Moser H. W., Osumi T., Rachubinski R. A., Roscher A., Subramani S., Tabak H. F., Tsukamoto T., Valle D., van der Klei I., van Veldhoven P. P., Veenhuis M. (1996) J. Cell Biol. 135, 1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heiland I., Erdmann R. (2005) FEBS J. 272, 2362–2372 [DOI] [PubMed] [Google Scholar]
- 24. Brocard C., Hartig A. (2006) Biochim. Biophys. Acta 1763, 1565–1573 [DOI] [PubMed] [Google Scholar]
- 25. Girzalsky W., Platta H. W., Erdmann R. (2009) Biol. Chem. 390, 745–751 [DOI] [PubMed] [Google Scholar]
- 26. Knowles J. R. (1989) Annu. Rev. Biochem. 58, 195–221 [DOI] [PubMed] [Google Scholar]
- 27. Streit W. R., Entcheva P. (2003) Appl. Microbiol. Biotechnol. 61, 21–31 [DOI] [PubMed] [Google Scholar]
- 28. Muralla R., Chen E., Sweeney C., Gray J. A., Dickerman A., Nikolau B. J., Meinke D. (2008) Plant Physiol. 146, 60–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pinon V., Ravanel S., Douce R., Alban C. (2005) Plant Physiol. 139, 1666–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arnal N., Alban C., Quadrado M., Grandjean O., Mireau H. (2006) Plant Mol. Biol. 62, 471–479 [DOI] [PubMed] [Google Scholar]
- 31. Rébeillé F., Alban C., Bourguignon J., Ravanel S., Douce R. (2007) Photosynth. Res. 92, 149–162 [DOI] [PubMed] [Google Scholar]
- 32. Kitamoto K. (2002) Adv. Appl. Microbiol. 51, 129–153 [DOI] [PubMed] [Google Scholar]
- 33. Ploux O., Marquet A. (1992) Biochem. J. 283, 327–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karimi M., De Meyer B., Hilson P. (2005) Trends Plant Sci. 10, 103–105 [DOI] [PubMed] [Google Scholar]
- 35. Mano S., Nakamori C., Nito K., Kondo M., Nishimura M. (2006) Plant J. 47, 604–618 [DOI] [PubMed] [Google Scholar]
- 36. Erdmann R., Veenhuis M., Mertens D., Kunau W. H. (1989) Proc. Natl. Acad. Sci. U.S.A. 86, 5419–5423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bower S., Perkins J., Yocum R. R., Serror P., Sorokin A., Rahaim P., Howitt C. L., Prasad N., Ehrlich S. D., Pero J. (1995) J. Bacteriol. 177, 2572–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hall C., Dietrich F. S. (2007) Genetics 177, 2293–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Roper J. A. (1950) Nature 166, 956–957 [DOI] [PubMed] [Google Scholar]
- 40. Vogel H. J. (1956) Microb. Genet. Bull. 13, 42–43 [Google Scholar]
- 41. Lin S., Hanson R. E., Cronan J. E. (2010) Nat. Chem. Biol. 6, 682–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ohsugi M., Miyauchi K., Tachibana K., Nakao S. (1988) J. Nutr. Sci. Vitaminol. 34, 343–352 [DOI] [PubMed] [Google Scholar]
- 43. Kunze M., Kragler F., Binder M., Hartig A., Gurvitz A. (2002) Eur. J. Biochem. 269, 915–922 [DOI] [PubMed] [Google Scholar]
- 44. Kunze M., Pracharoenwattana I., Smith S. M., Hartig A. (2006) Biochim. Biophys. Acta 1763, 1441–1452 [DOI] [PubMed] [Google Scholar]
- 45. Baud S., Guyon V., Kronenberger J., Wuillème S., Miquel M., Caboche M., Lepiniec L., Rochat C. (2003) Plant J. 33, 75–86 [DOI] [PubMed] [Google Scholar]
- 46. Hasslacher M., Ivessa A. S., Paltauf F., Kohlwein S. D. (1993) J. Biol. Chem. 268, 10946–10952 [PubMed] [Google Scholar]
- 47. Bailey A., Keon J., Owen J., Hargreaves J. (1995) Mol. Gen. Genet. 249, 191–201 [DOI] [PubMed] [Google Scholar]
- 48. Schneiter R., Hitomi M., Ivessa A. S., Fasch E. V., Kohlwein S. D., Tartakoff A. M. (1996) Mol. Cell Biol. 16, 7161–7172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schneiter R., Guerra C. E., Lampl M., Tatzer V., Zellnig G., Klein H. L., Kohlwein S. D. (2000) Mol. Cell Biol. 20, 2984–2995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Al-Feel W., DeMar J. C., Wakil S. J. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 3095–3100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zempleni J. (2005) Annu. Rev. Nutr. 25, 175–196 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.