Abstract
ATP-binding cassette transporter A1 (ABCA1) mediates the rate-limiting step in high density lipoprotein (HDL) particle formation, and its expression is regulated primarily by oxysterol-dependent activation of liver X receptors. We previously reported that ABCA1 expression and HDL formation are impaired in the lysosomal cholesterol storage disorder Niemann-Pick disease type C1 and that plasma HDL-C is low in the majority of Niemann-Pick disease type C patients. Here, we show that ABCA1 regulation and activity are also impaired in cholesteryl ester storage disease (CESD), caused by mutations in the LIPA gene that result in less than 5% of normal lysosomal acid lipase (LAL) activity. Fibroblasts from patients with CESD showed impaired up-regulation of ABCA1 in response to low density lipoprotein (LDL) loading, reduced phospholipid and cholesterol efflux to apolipoprotein A-I, and reduced α-HDL particle formation. Treatment of normal fibroblasts with chloroquine to inhibit LAL activity reduced ABCA1 expression and activity, similar to that of CESD cells. Liver X receptor agonist treatment of CESD cells corrected ABCA1 expression but failed to correct LDL cholesteryl ester hydrolysis and cholesterol efflux to apoA-I. LDL-induced production of 27-hydroxycholesterol was reduced in CESD compared with normal fibroblasts. Treatment with conditioned medium containing LAL from normal fibroblasts or with recombinant human LAL rescued ABCA1 expression, apoA-I-mediated cholesterol efflux, HDL particle formation, and production of 27-hydroxycholesterol by CESD cells. These results provide further evidence that the rate of release of cholesterol from late endosomes/lysosomes is a critical regulator of ABCA1 expression and activity, and an explanation for the hypoalphalipoproteinemia seen in CESD patients.
Keywords: ABC Transporter, Cholesterol Metabolism, Gene Expression, High Density Lipoprotein (HDL), Lysosomal Storage Disease, ABCA1
Introduction
High density lipoproteins (HDL) are thought to protect against atherosclerosis by diverse mechanisms, including mediating reverse cholesterol transport and anti-inflammatory effects (1). The rate-limiting step in HDL particle formation is the lipidation of apolipoprotein A-I (apoA-I) and other HDL apolipoproteins by the membrane lipid transporter ATP-binding cassette transporter A1 (ABCA1) (2). Expression of ABCA1 is induced by increased cell cholesterol content, through oxysterol-dependent activation of the nuclear liver X receptors (LXRs)4 on the promoter region of ABCA1 (3, 4). Further cholesterol efflux to HDL can be mediated via ABCG1, also regulated transcriptionally by LXR (5, 6). However, the roles of different intracellular cholesterol pools, including de novo synthesized cholesterol, late endosomal/lysosomal cholesterol, and plasma membrane cholesterol, in the regulation of oxysterol production and ABCA1 expression are poorly understood.
We have previously demonstrated that regulation of ABCA1 is impaired in the lysosomal cholesterol storage disorder Niemann-Pick disease type C1 (NPC1), leading to reduced HDL particle formation by human NPC1 disease fibroblasts (7). The defect in the lipidation of apoA-I was overcome by addition of an exogenous non-oxysterol synthetic ligand for LXR to up-regulate ABCA1, suggesting ABCA1 might be able to mobilize cholesterol from late endosomes/lysosomes even in the presence of NPC1 deficiency (8). We also determined that plasma HDL-C was low in 17/21 patients studied with NPC disease (7), further confirming a recent report of a larger cohort of NPC1 subjects (9). Low plasma HDL-C in patients with NPC1 disease occurs independent of their plasma triglyceride levels (9), further suggesting that impaired ABCA1 regulation as a consequence of reduced flux of cholesterol out of lysosomes is the cause of the hypoalphalipoproteinemia in this disease.
In this study, we have extended these findings to another lysosomal cholesterol storage disorder, cholesteryl ester storage disease (CESD), caused by deficiency of lysosomal acid lipase (LAL) activity (10). CESD and its more severe form Wolman disease are autosomal recessive diseases caused by mutations in the LIPA gene that encodes for LAL, the sole enzyme responsible for acidic hydrolysis of cholesteryl esters and triglycerides delivered from lipoproteins to lysosomes (11, 12). In contrast to Wolman disease, in which complete absence of LAL activity results in death usually in the first 6 months of life, splice mutations of LIPA that result in the 5–10% residual LAL activity in CESD allow patients to survive usually to adulthood. In addition to hepatosplenomegaly, individuals with CESD exhibit premature atherosclerosis and plasma HDL-C levels that are approximately half the normal levels (10). The reason for low HDL-C in CESD has not previously been known. Here, we describe impaired regulation of ABCA1 in human CESD fibroblasts, reduced lipid efflux to apoA-I and HDL particle formation, induction of the same defects by inhibition of LAL activity in normal fibroblasts, and correction of ABCA1 expression and HDL particle formation in CESD cells treated with LAL-containing conditioned medium from normal fibroblasts or recombinant human LAL. We also demonstrate impaired oxysterol generation in CESD fibroblasts, as previously shown in NPC1−/− and NPC2−/− fibroblasts (13), and correction of oxysterol formation in CESD cells following incubation with LAL-containing conditioned medium or purified LAL enzyme. Together with our findings in NPC1 disease, these results provide a likely reason for the low HDL-C in CESD-impaired regulation of ABCA1, and they further demonstrate the critical importance of the rate of flux of cholesterol out of lysosomes in the regulation of ABCA1 expression and HDL particle formation.
EXPERIMENTAL PROCEDURES
Materials
Cholesteryl oleate, unesterified cholesterol, 1-monooleoyl-rac-glycerol, 1,2-distearoyl-rac-glycerol, triolein, oleic acid, 4-methylumbelliferyl oleate. Phosphatidylcholine, sphingomyelin, LXR agonist TO-901317, chloroquine diphosphate, and fatty acid-free bovine serum albumin (BSA) were purchased from Sigma. Purified oxysterols were purchased from Avanti Polar Lipids. [cholesteryl-1,2,6,7-3H]cholesteryl linoleate (60–100 Ci/mmol) was purchased from American Radiolabeled Chemicals, and [methyl-3H]choline chloride (66.7 Ci/mmol) was from PerkinElmer Life Sciences. Dulbecco's modified Eagle's medium (DMEM) was purchased from BioWhittaker, and fetal bovine serum and lipoprotein-deficient serum (LPDS) was from Cocalico Biologicals, Inc. Nitrocellulose membranes, SDS-PAGE reagents, and pre-stained protein molecular mass markers were purchased from Bio-Rad. PE-SIL G plastic-backed flexible plates used for thin layer chromatography analysis were from Whatman. Purified recombinant human LAL (rhLAL) was from Shire Human Genetic Therapies (Lexington, MA).
Preparation of Lipoproteins and Apolipoprotein A-1
LDL was isolated from pooled plasma from healthy fasting donors by density gradient ultracentrifugation (14). Radiolabeling of LDL with [1,2,6,7-3H]cholesteryl linoleate was performed as described previously (15) to a specific activity of 16–44 cpm/ng of LDL protein. ApoA-I was purified from human plasma using Q-Sepharose Fast Flow chromatography as described previously (8).
Cell Culture
Normal human skin fibroblasts were purchased from the American Type Culture Collection (Manassas, VA). CESD human skin fibroblasts were obtained via skin biopsy from an adult patient in the Cardiovascular Risk Reduction Clinic, University of Alberta Hospital, Edmonton, Alberta, Canada, following informed consent (CESD1). Genetic sequencing of CESD1 cells was kindly performed by the laboratory of Dr. Robert Hegele (Robarts Research Institute, London, Ontario, Canada) indicating a heterozygous splice mutation E8SJM (Δ254–277, 934G→A or c.894G→A), which leads to a G-to-A transition at the −1-position of exon 8. Although no previously described LAL mutations could be identified for the second allele, a second CESD mutation was assumed as the patient exhibits a classic histological phenotype of CESD based on prior liver biopsy. The second CESD human skin fibroblast cell line from an adult patient (CESD2) was generously provided by Dr. John Parks (Wake Forest University School of Medicine). This patient was a compound heterozygote for the common E8SJM mutation (c.894G→A), which leads to excision of exon 8, and a CT deletion in exon 4, causing a frameshift and termination signal four codons downstream at codon 137 (c.397–398delCT; FS 137X) (16). Low LAL enzyme activity was confirmed in both cell lines (see under “Results”).
All cells were grown in monolayers and were used between the 5th and 25th passage. Cell lines were maintained in a humidified incubator at 37 °C and 5% CO2 in DMEM containing 10% fetal bovine serum supplemented with 1% penicillin/streptomycin (Invitrogen) (growth medium). Cells were plated at 40,000/35-mm well or 120,000 cells/60-mm dish and grown to ∼60% confluence in growth medium. Cells were subsequently grown to 100% confluence in DMEM containing 10% LPDS. Where indicated, cells were then incubated with DMEM containing 50 μg/ml LDL protein for 24 h.
Conditioned Medium Experiments
For conditioned medium experiments, cells grown to confluence as above were incubated an additional 24 h in DMEM containing 5% LPDS. Conditioned medium was then removed from cells, centrifuged to pellet any cells, and added back to the indicated cell monolayers for a further 24 h prior to incubation with 50 μg/ml LDL for 24 h, as described previously (17).
Recombinant Human LAL Treatment
During optimization experiments, a stock solution of 2.9 mg/ml recombinant human LAL in 20 mm citrate, pH 5.3, was diluted to final concentrations of 0.15, 0.3, 0.6, 1.2, 2.4, or 6.0 μg/ml in DMEM and added to CESD1 (CD1) and CESD2 (CD2) cell monolayers for 24 h prior to LDL loading. Further experiments were performed using a final concentration of 1.2 μg/ml rhLAL.
Chloroquine Treatment
Where indicated, cells were pretreated for 1 h prior to LDL loading with 50 μm chloroquine diphosphate by addition of 5 μl/ml of a 10 mm stock solution, made up in DMEM with pH adjusted to 7, directly to the medium, and the same concentration of chloroquine was then added in the presence or absence of 50 μg/ml LDL for an additional 24 h as described previously (18).
Cholesterol and Phospholipid Efflux
Radiolabeling of LDL-derived cellular cholesterol pools was achieved by incubating cells for 24 h with 50 μg/ml [3H]cholesteryl linoleate-labeled LDL. Cell choline-containing phospholipids were labeled in the presence of 50 μg/ml LDL by addition of 5 μCi/ml [3H]choline chloride for 24 h (8, 19). Cells were washed two times with PBS and then incubated in DMEM containing 10 μg/ml apoA-I for 24 h or the indicated time points. Efflux media were collected and centrifuged at 3,000 rpm for 10 min at 4 °C to remove cell debris. [3H]Cholesterol in the medium was then measured directly for cells labeled with [3H]cholesteryl linoleate-labeled LDL by liquid scintillation counting. For phospholipid efflux, lipid extraction of medium was performed prior to separation of lipids by thin layer chromatography (20). Cell monolayers were kept on ice and rinsed on ice two times with cold PBS containing 1 mg/ml BSA and two times with cold PBS and stored at −20 °C until lipid extraction. Cellular lipids were extracted and separated by thin layer chromatography and assayed for radioactivity as described previously (21). For LDL-derived cholesterol efflux assays, the radioactivity of cholesteryl esters and unesterified cholesterol spots was measured using unlabeled carrier lipids to identify spots. For phospholipid assays, radiolabeled sphingomyelin and phosphatidylcholine spots were measured. Protein content of cell monolayers was determined by standard Lowry assay using BSA as standard (22).
Cholesterol Mass Assay
Cells were grown in 6-well plates and loaded with LDL as described above. Following LDL incubation, cells were washed twice with PBS, 1 mg/ml fatty acid-free albumin and incubated 24 h in the presence of 10 μg/ml apoA-I. The media fraction was collected, and cells were scraped in PBS, collected, and homogenized by sonication. Phospholipids from media or cell homogenates were digested using phospholipase C to remove the polar head groups, and samples were vortexed for 2 h at 30 °C. Total lipids were extracted by organic phase extraction with tridecanoin as the internal standard. Samples were derivatized with Sylon BFT (Supelco), and sterols were separated, analyzed by gas chromatography (Agilent Technologies, 6890 Series equipped with a Zebron capillary column (ZB-5, 15 m × 0.32 mm × 0.25 μm), and connected to a flame ionization detector (Zebron, Palo Alto, CA) (23). The separation of cholesterol and cholesteryl esters was identified, and their mass was calculated using the retention times and mass of the internal standard (8).
Quantitative Real Time PCR Analysis of ABCA1 and ABCG1 mRNA
Total RNA was isolated from fibroblast monolayers using TRIzol reagent extraction (Invitrogen), and cDNA synthesis was performed as described previously (7). ABCA1 and ABCG1 DNA amplification was performed by initial denaturation at 95 °C for 3 min. Thereafter, denaturing was at 95 °C for 20 s, annealing at 58 °C for ABCA1 or 60.9 °C for ABCG1 for 20 s, and extension at 72 °C for 40 s for a total of 40 cycles (8). SYBR Green (Quanta Biosciences) was used to detect PCR products in real time using a Realplex2 Mastercycler thermocycler (Eppendorf). The human housekeeping gene cyclophilin cDNA was amplified using the same conditions. ABCA1 mRNA levels were calculated using the comparative CT method relative to cyclophilin. The following primers were used: human ABCA1, 5′-GAC ATC CTG AAG CCA ATC CTG (forward) and 5′-CCT TGT GGC TGG AGT GTC AGG T (reverse); human ABCG1, 5′-CAC CTC GCA CAT TGG GAT CG (forward) and 5′-GCC AGG TAG TAG GCC TTC AG (reverse); human cyclophilin, 5′-ACC CAA AGG GAA CTG CAG CGA GAG C (forward) and 5′-CCG CGT CTC CTT TGA GCT GTT TGC AG (reverse).
Western Blot Analysis of ABCA1 and LAL
Cells in 60-mm dishes were harvested in 300 μl of lysis buffer containing 20 mm Tris, 5 mm EDTA, 5 mm EGTA, 0.5% maltoside, and 1× Complete Mini Protease Inhibitor (Roche Applied Science) and homogenized using a glass mortar and Teflon pestle. Samples were then centrifuged at 2500 rpm for 10 min at 4 °C to pellet nucleic acids. The sample protein concentration was quantified using reagent from Bio-Rad. Samples to be used for human LAL (hLAL) analysis were boiled for 5 min at 100 °C. Samples used for ABCA1 analysis were not boiled, according to the antibody manufacturer's guidelines (Calbiochem). Fifteen to 30 μg of proteins were separated by a 5–15% gradient SDS-PAGE under reducing conditions and transferred to nitrocellulose membrane. Immunoblotting was performed according to standard protocols using polyclonal rabbit anti-human ABCA1 antibody (1:1000 dilution) (Calbiochem), a polyclonal rabbit anti-hLAL antibody (1:2000 dilution, Seven Hills Bioreagents), and a goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (1:10,000 dilution, Sigma). Chemiluminescence was detected using Super Signal West Femto maximum sensitivity substrate (Pierce Protein Research Products) and the Chemigenius BioImaging System (Syngene).
Two-dimensional Gel Electrophoresis of HDL Particles
To characterize apoA-I-containing particles generated by normal and CESD fibroblasts, cell monolayers in 35-mm wells were treated as described above for conditioned medium experiments with the exception of the last hour of incubation with apoA-I, when 1 mg/ml fatty acid-free albumin was added to the medium to stabilize HDL particles. Fibroblast-conditioned media were then centrifuged at 2000 × g for 5 min at 4 °C to pellet cells, and the supernatant was concentrated 10-fold by ultracentrifugation (Amicon Ultra-4, MWCO 10,000, Millipore). The concentrated media were stored at −80 °C. HDL particles in each of the concentrated apoA-I-conditioned media were separated according to our previously described methods (8), with some modifications. Briefly, 25 μl of each sample was separated in the first dimension by 0.75% agarose gel in 50 mm barbital buffer, pH 8.6, at 200 V for 5 h at 6 °C. Second dimension electrophoresis was performed with a 5–23% polyacrylamide concave gradient gel at 125 V for 24 h at 6 °C in 0.025 m Tris, 0.192 m glycine buffer, pH 8.3. High molecular weight protein standards (7.1–17.0 nm, Amersham Biosciences) were run on each gel. After electrophoresis, samples were transferred (35 V, 24 h, 4 °C) onto nitrocellulose membranes (Trans-blot, Bio-Rad), stained with Ponceau S to locate the standard proteins, and blocked for 1 h in Tris-buffered saline containing 1% Tween 20 (TBS-T) and 10% skim milk at room temperature. ApoA-I-containing particles were detected by immunoblotting with rabbit polyclonal anti-human apoA-I antibody (Calbiochem) and goat anti-rabbit horseradish peroxidase-conjugated polyclonal secondary antibody (Sigma). Chemiluminescence was detected using chemiluminescent substrate (Pierce).
Oxysterol Mass Determination
Lipid extraction of cell monolayers and solid phase purification were performed as described (24, 25). Lipid extracts were derivatized with Sigma Sil-A for 1 h at 60 °C, and the mass of 27-hydroxycholesterol was quantified by gas chromatography/mass spectrometry according to Frolov et al. (13), using d5-27-hydroxycholesterol as internal standard.
Statistics
Results were analyzed using GraphPad Prism version 5.0 and are presented as the mean ± S.D. Significant differences between experimental groups were determined using the Student's t test, with the exception of Figs. 1C and 5, C–E, where two-way analysis of variance was performed, and differences between experimental groups were determined using a Bonferroni post hoc comparison.
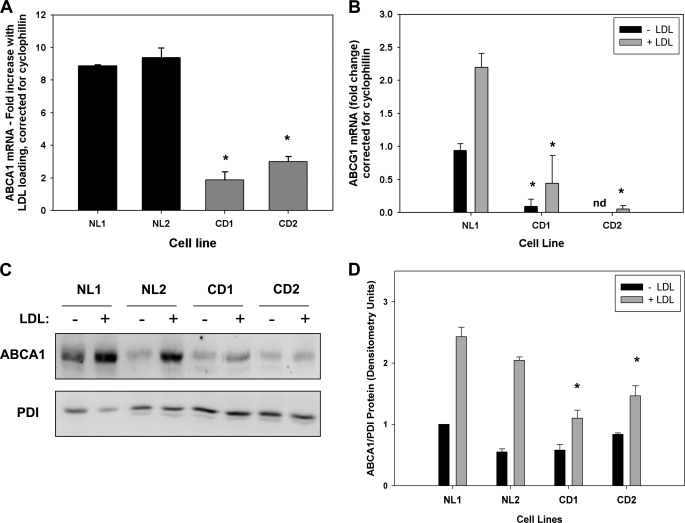
FIGURE 1.
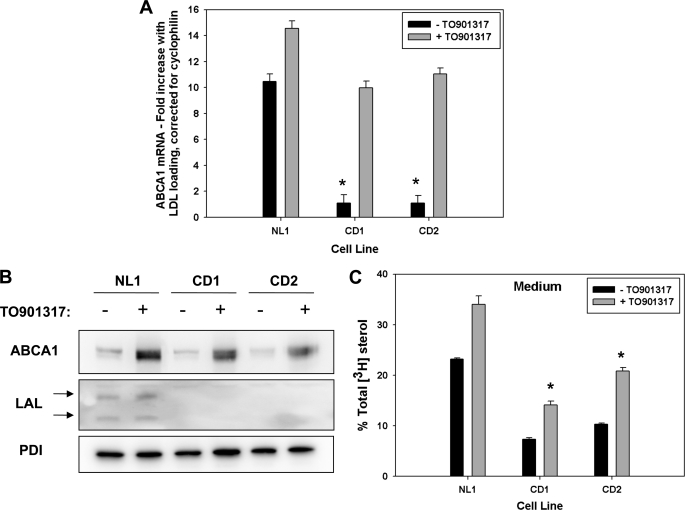
Reduced expression of ABCA1 and ABCG1 in response to LDL loading in CESD fibroblasts. Normal (NL1 and NL2) and CESD (CD1 and CD2) fibroblasts were grown to confluence in DMEM containing 5% LPDS and then incubated in the presence (+) or absence (−) of DMEM containing 50 μg/ml LDL for 24 h. A, RNA extracts were analyzed by quantitative real time PCR using primers targeting human ABCA1 (A), ABCG1 (B), and m-cyclophilin. Results are the mean fold increase in ABCA1 mRNA with LDL loading corrected for cyclophilin expression (A) or the fold increase in ABCG1 mRNA relative to NL1-LDL control, corrected for cyclophilin expression ± S.D. of three replicates, and are representative of three experiments with similar results (B). C, cells lysates were analyzed by SDS-PAGE and Western blot using polyclonal antibodies for human ABCA1 and protein-disulfide isomerase (PDI) loading control. D, Western blots were analyzed by densitometry and results expressed as the mean of ABCA1:protein-disulfide isomerase ratio ± S.D. for four experiments with NL1-LDL sample ratio set as 1. *, values less than NL1 or NL2, p < 0.01.
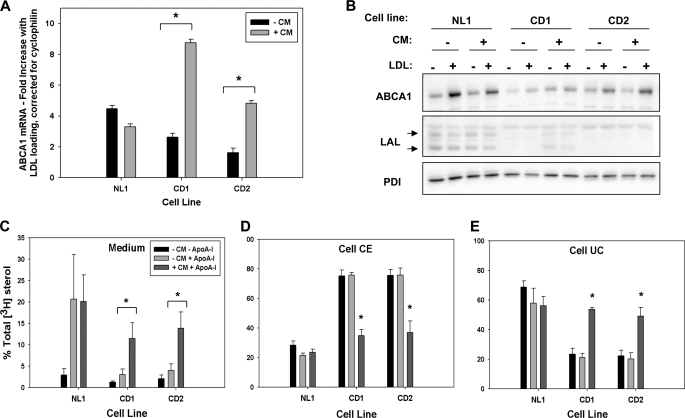
FIGURE 5.
Normal conditioned medium rescues ABCA1 expression and apoA-I-mediated cholesterol efflux in CESD fibroblasts. Normal (NL1) and CESD (CD1 and CD2) fibroblasts were grown to confluence in DMEM containing 5% LPDS. Fresh medium was applied for 24 h, and the resulting conditioned medium from normal cells or nonconditioned medium was applied as indicated for 24 h. Cell monolayers were then incubated in DMEM with (+) or without (−) 50 μg/ml LDL (A and B) or 50 μg/ml [3H]cholesteryl-linoleate LDL (C–E) for 24 h. ABCA1 mRNA expression was quantified (A), and cell lysates were analyzed by Western blot (B) as described in Fig. 1. Cells were incubated with 10 μg/ml apoA-I for 24 h and [3H]cholesterol in the medium, and cell lipid extracts were determined as in Fig. 2. Results indicate the percent of total [3H]cholesterol in the medium (C), cell cholesteryl ester (CE) (D), or cell unesterified cholesterol (UC) (E), and means ± S.D. are of triplicates and are representative of three experiments with similar results. *, values significantly greater (A, C, and E) or lower (D) than nonconditioned medium-treated CESD cells, p < 0.05. PDI, protein-disulfide isomerase.
RESULTS
Reduced LDL-induced Expression of ABCA1 and ABCG1 in CESD Fibroblasts
We previously reported impaired regulation of ABCA1 in NPC1-deficient human fibroblasts (7), where the rate of release of unesterified cholesterol out of the late endosome/lysosome compartment is slowed (26, 27), and oxysterol generation is reduced (13). The slowed hydrolysis of LDL and other lipoprotein cholesteryl esters in CESD, resulting from mutations in LAL, also results in reduced cholesterol release from this compartment and impaired cholesterol regulatory responses (11). We therefore hypothesized this would lead to impaired regulation of ABCA1 expression and activity in CESD cells. Impaired LAL activity was confirmed in skin fibroblasts obtained from two unrelated human CESD patients (CD1 and CD2) using an in vitro 4-methylumbelliferyl oleate fluorescence assay (28, 29), with CD1 showing 3.86 ± 0.86% and CD2 2.17 ± 0.85% the LAL activity of normal (NL1) fibroblasts. To determine ABCA1 expression, normal and CESD fibroblasts were grown the last 40% to confluence in LPDS and then incubated in the absence or presence of 50 μg/ml LDL for 24 h. Quantitative real time PCR analysis of cellular RNA extracts revealed the fold increase in ABCA1 mRNA expression of CESD fibroblasts in response to LDL loading was reduced to approximately ¼ that in two normal fibroblast cell lines (Fig. 1A). Basal and LDL-stimulated expressions of ABCG1 mRNA were also significantly reduced in both CESD cell lines compared with normal fibroblasts (Fig. 1B). In the absence of LDL loading, levels were consistently so low as to be undetectable from CESD2 fibroblasts; LDL loading failed to up-regulate ABCG1 mRNA expression to normal levels in either cell line (Fig. 1B). The increase in ABCA1 protein levels following LDL loading was also blunted in CESD compared with normal fibroblasts (Fig. 1, C and D). These results suggest that, as previously seen in human NPC1 disease fibroblasts (7, 8), reduced flux of cholesterol out of the late endosome/lysosome compartment in CESD cells also results in impaired transcriptional regulation of ABCA1 and ABCG1.
Reduced Phospholipid and LDL-derived Cholesterol Efflux to ApoA-I from CESD Fibroblasts
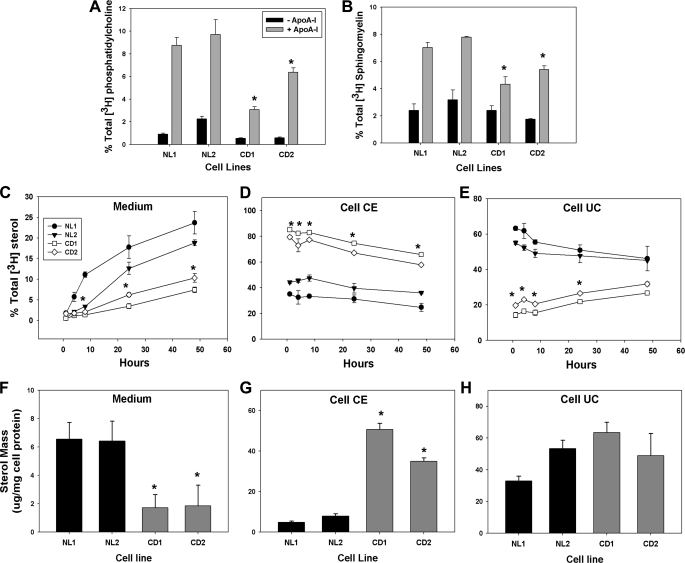
Efflux of cellular phospholipids and cholesterol to HDL apolipoproteins to form viable HDL particles is critically dependent on ABCA1 activity and is not substituted by any other known cellular mechanism or transporter (2). To determine the functional activity of ABCA1 in CESD fibroblasts, the efflux of radiolabeled phospholipids and LDL-derived cholesterol as well as total cholesterol mass to apoA-I-containing medium was measured. Efflux to apoA-I of cell phosphatidylcholine and sphingomyelin radiolabeled by addition of [3H]choline during the LDL-loading phase was significantly reduced in both CESD fibroblast lines when compared with two normal fibroblast lines (Fig. 2, A and B). To specifically measure efflux of cholesterol derived from LDL, cells were loaded with LDL containing [3H]cholesteryl linoleate prior to incubation with apoA-I. Efflux of LDL-derived cholesterol as a percentage of total medium plus cell radiolabel to 10 μg/ml apoA-I over a 48-h period was significantly lower from the two CESD cell lines (7.4 ± 0.67 and 10.3 ± 1.04% from CESD1 and CESD2 cell lines, respectively) when compared with the two normal cells lines (23.6 ± 2.70 and 18.8 ± 0.69% from NL1 and NL2, respectively) (Fig. 2C). The percentage of total medium plus cellular radiolabel in cell cholesteryl esters was persistently higher in CESD cells, consistent with reduced LAL activity and reduced hydrolysis of lysosomal cholesteryl esters over the 48-h time course of the experiment (Fig. 2D). Radiolabeled unesterified cholesterol was much lower in CESD cells at the start of the apoA-I incubation but, in contrast to normal cells, rose during the apoA-I incubation as cholesteryl esters were gradually hydrolyzed (Fig. 2E). The increase in unesterified cholesterol counts is consistent with reduced ABCA1 activity in these cells and reduced efflux to apoA-I compared with normal cells. Differences in cholesterol mass in the medium and cell cholesteryl ester pools after 24 h of incubation with 10 μg/ml apoA-I mirrored changes seen in the radiolabeled experiments, with much lower efflux of cholesterol mass to apoA-I-containing medium and much higher cholesteryl ester levels in CESD compared with normal cells (Fig. 2, F and G). Normal and CESD cells had unesterified cholesterol mass ranging between 33.0 ± 3.03 μg/mg (NL1) and 63.4 ± 6.62 μg/mg (CD1) cell protein; taken together, no significant difference could be observed between normal and CESD cells in this parameter (Fig. 2H). This result was not unexpected because, as opposed to radiolabeling experiments where the fate of [3H]LDL-cholesteryl esters was measured specifically, the total mass of unesterified cholesterol also includes that from de novo cholesterol synthesis by HMG-CoA reductase and hydrolysis of cytosolic cholesteryl esters (30).
FIGURE 2.
Reduced phospholipid and cholesterol efflux to apoA-I from CESD fibroblasts. Normal (NL1 and NL2) and CESD (CD1 and CD2) fibroblasts were grown to confluence in DMEM containing 5% LPDS and then in DMEM containing 5 μCi/ml [3H]choline-chloride and 50 μg/ml LDL for 24 h (A and B), 50 μg/ml [3H]cholesteryl-linoleate LDL (C–E), or 50 μg/ml LDL (F–H), followed by further incubation with DMEM in the presence (+) or absence (−) of 10 μg/ml apoA-I for 24 h or the indicated time points. The radioactivity of the medium was counted directly by liquid scintillation counting (C) or following lipid extraction (A and B). Medium and cellular lipid extracts were separated by thin layer chromatography, and radioactivity was measured in phosphatidylcholine (A) and sphingomyelin (B) spots, or cholesteryl ester (CE) (D) and unesterified cholesterol (UC) (E) spots. Total lipids were extracted from medium (F) and cells (G and H), and unesterified cholesterol (F and H) and cholesteryl ester mass (G) were determined by gas chromatography and mass spectrometry. Results are each representative of three experiments with similar results. *, values less than (A–C and E and F) or greater than (D and G) NL1 or NL2 controls, p < 0.05.
Chloroquine Blocks LDL-dependent Up-regulation of ABCA1 Expression and Activity in Normal Fibroblasts
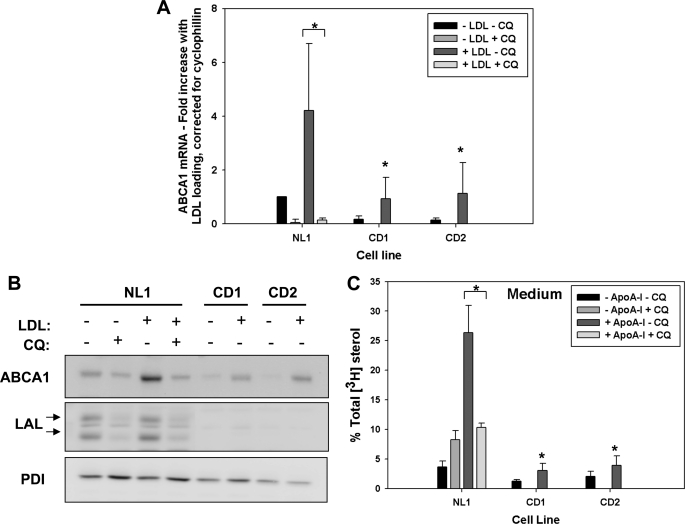
To test whether inhibition of LAL activity impairs ABCA1 expression and activity in normal fibroblasts, cells were exposed to 50 μm chloroquine for 1 h prior to and during LDL loading (11). Chloroquine treatment of normal fibroblasts reduced basal ABCA1 mRNA to nearly zero and completely blocked the increase in ABCA1 mRNA seen in response to LDL loading, which was reduced to a greater extent than seen in untreated CESD fibroblasts (Fig. 3A). The increase in ABCA1 protein in response to LDL loading was also blocked by chloroquine treatment in normal fibroblasts to a similar degree as non-chloroquine-treated CESD cells, indicating the importance of lysosomally derived cholesterol as a regulator of ABCA1 expression (Fig. 3B). Interestingly, LAL protein levels were also decreased in normal and CESD fibroblasts following chloroquine treatment (Fig. 3B), which has been attributed to enhanced secretion of newly synthesized enzyme and/or decreased re-uptake of secreted LAL (31). Therefore, chloroquine blocks LDL cholesteryl ester hydrolysis not only by inhibition of LAL activity but also by reduction of overall LAL enzyme level within the cell. Efflux of LDL-derived cholesterol to apoA-I was abolished in normal cells following chloroquine treatment before and during delivery of [3H]cholesteryl linoleate-labeled LDL to cells (Fig. 3C). Chloroquine treatment also resulted in accumulation of [3H]cholesteryl esters and reduction of cellular unesterified [3H]cholesterol in normal fibroblasts, to similar levels as those seen in non-chloroquine-treated CESD cells, confirming the inhibition of LDL-cholesteryl ester hydrolysis by chloroquine (data not shown). Therefore, the metabolic phenotype of impaired ABCA1 regulation and cellular cholesterol efflux to apoA-I observed in CESD fibroblasts could be recapitulated by pharmacological inhibition of LAL in normal fibroblasts, indicating that LAL deficiency is the reason for the impaired ABCA1 regulation observed in CESD fibroblasts.
FIGURE 3.
Chloroquine blocks up-regulation of ABCA1 in response to LDL loading and apoA-I-mediated cholesterol efflux in normal fibroblasts. Normal (NL1) and CESD (CD1 and CD2) fibroblasts were grown to confluence in LPDS. Cells were treated ± 50 μm chloroquine (CQ) for 1 h prior to and during a 24-h incubation ± 50 μg/ml LDL (A and B) or 50 μg/ml [3H]cholesteryl-linoleate LDL (C). A, cellular RNA extracts were analyzed by quantitative real time PCR as described in Fig. 1. B, cell lysates were collected and analyzed by Western blot as described in Fig. 1. C, radioactivity of the medium and cell lipid extracts was determined as in Fig. 2, and results are expressed as the percent of total medium and cell unesterified [3H]cholesterol plus cell [3H]cholesteryl ester. Values are the mean ± S.D. of triplicates and are representative of three experiments with similar results. *, values less than non-chloroquine-treated and LDL-loaded NL1 controls, p < 0.05.
LXR Agonist Corrects ABCA1 Expression but Fails to Correct ApoA-I-mediated Cholesterol Efflux in CESD Fibroblasts
We and others have previously reported that treatment with the LXR agonist TO901317 corrects ABCA1 expression and apoA-I-mediated cholesterol efflux (8, 32), and it reduces lysosomal cholesterol accumulation (8) in NPC1-deficient human fibroblasts. As found in NPC1-deficient cells, LXR agonist treatment corrected ABCA1 expression in CESD cells to similar (mRNA) or greater (protein) levels than seen in non-LXR agonist-treated normal fibroblasts (Fig. 4, A and B). Efflux of radiolabeled LDL-derived cholesterol to apoA-I, however, although increased in normal fibroblasts, was not corrected to the same level in CESD fibroblasts compared with untreated normal fibroblasts (Fig. 4C). These results suggest that although ABCA1 expression can be corrected in CESD fibroblasts, the residual deficiency of LAL activity is not overcome with this treatment, resulting in a persistent reduction of substrate unesterified cholesterol from late endosomes and lysosomes for ABCA1-mediated cholesterol efflux.
FIGURE 4.
LXR agonist up-regulates ABCA1 expression but fails to correct apoA-I-specific cholesterol efflux in CESD fibroblasts. Normal (NL1) and CESD (CD1 and) fibroblasts were grown to confluence in DMEM containing 5% LPDS and then incubated with DMEM containing 50 μg/ml LDL (A and B) or 50 μg/ml [3H]cholesteryl-linoleate LDL (C) in the presence (+) or absence (−) of 5 μm TO901317 for 24 h. ABCA1 mRNA expression was quantified (A), and cell lysates were analyzed by Western blot (B) as described in Fig. 1. C, following LDL and LXR agonist incubation, cells were further incubated with DMEM containing 10 μg/ml apoA-I for 24 h, and radioactivity of the medium and cell lipid extracts was determined as in Fig. 2. Results are expressed as the percent of total (medium and cell unesterified [3H]cholesterol plus cell lsqb]3H]cholesteryl ester); mean ± S.D. are of triplicates and are representative of three experiments with similar results. *, values less than non-LXR agonist-treated NL1 cells, p < 0.05. PDI, protein-disulfide isomerase.
Rescue of ABCA1 Expression and ApoA-I-dependent Cholesterol Efflux in CESD Fibroblasts Treated with LAL-containing Medium
Our data indicate that hydrolysis of endocytosed LDL cholesteryl esters by LAL is required for normal regulation of ABCA1 expression and activity and that the impaired ABCA1 expression and lipid efflux phenotype of CESD cells can be reproduced by inhibiting LAL activity in normal fibroblasts. Restoration of LAL activity would therefore be expected to correct the impaired ABCA1 expression and lipid efflux defects in CESD cells. Functional LAL enzyme is known to be secreted from human fibroblasts and is re-internalized via the mannose 6-phosphate receptor pathway for specific targeting to late endosomes/lysosomes (28, 33). The Goldstein and Brown laboratory has previously shown that conditioned medium from normal human fibroblasts can rescue the hydrolysis of LDL cholesteryl esters and restore normal cholesterol regulatory responses, including suppression of HMG-CoA reductase and stimulation of cholesterol re-esterification in CESD fibroblasts (17). We adapted this method to determine whether we could also rescue ABCA1 expression and lipid efflux in these cells. Normal and CESD fibroblasts were grown to confluence in LPDS, followed by an additional 24-h incubation in medium obtained from a 24-h incubation of normal fibroblasts (conditioned medium). Cells were then loaded with unlabeled or radiolabeled LDL for 24 h prior to determination of ABCA1 expression and cholesterol efflux to apoA-I. Conditioned medium from normal cells restored the LDL-induced increase in ABCA1 mRNA in CD1 and CD2 fibroblasts up to the same level seen in conditioned medium-treated normal fibroblasts (Fig. 5A). ABCA1 protein levels were also increased in both CESD cell lines in response to LDL loading following treatment with conditioned medium (Fig. 5B). No change was observed in ABCA1 mRNA or protein when CD1 or CD2 fibroblasts were treated with conditioned medium from either CESD cell line (data not shown). A similar increase in ABCA1 expression was observed upon treatment of CESD fibroblasts with purified rhLAL (supplemental Fig. 1A). Conditioned medium treatment also corrected LDL-derived cholesterol efflux to apoA-I in both CESD cell lines by ∼3.5-fold, when compared with nontreated cells, to levels similar to those seen in normal fibroblasts untreated or treated with conditioned medium (Fig. 5C). In addition, conditioned medium reduced cell cholesteryl esters and raised cell unesterified cholesterol radiolabel in CESD cells to levels similar to those found in normal cells with or without conditioned medium treatment (Fig. 5, D and E). Similarly, treatment of CESD fibroblasts with 1.2 μg/ml rhLAL also rescued cellular levels of cholesteryl esters and unesterified cholesterol to normal levels and normalized apoA-I-dependent efflux of LDL-derived [3H]cholesterol (supplemental Fig. 1, B–D). These results indicate LAL in the conditioned medium and rhLAL were internalized and targeted correctly to late endosomes/lysosomes to restore LDL cholesteryl ester hydrolysis and cholesterol homeostasis as determined by ABCA1 expression and activity.
Impaired HDL Particle Formation by CESD Fibroblasts and Correction by Treatment with Conditioned Medium from Normal Cells
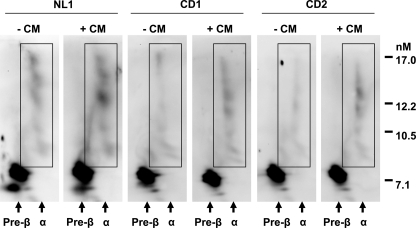
We previously reported a reduction in generation of α-migrating HDL species in apoA-I efflux medium of NPC1-deficient cells and in the plasma of a patient with NPC1 disease, using two-dimensional gel electrophoresis of HDL particles (8). We also found a correction of α-HDL formation following LXR agonist treatment in NPC1 disease cells (8). To correlate our findings of reduced ABCA1 expression and apoA-I-dependent phospholipid and cholesterol efflux from CESD fibroblasts with the reported low plasma HDL-cholesterol concentrations in CESD patients (10), we performed two-dimensional gel analysis of HDL formed in the efflux medium containing 10 μg/ml apoA-I for 24 h from normal and CESD cells. As seen in NPC1-deficient fibroblasts (8), medium from both CESD cell lines showed normal levels of pre-β-HDL but a reduction in formation of α-HDL particle species following incubation with apoA-I (Fig. 6, CD1 and CD2-CM). Incubation of CESD cells with conditioned medium from normal fibroblasts to increase LAL activity restored the level of α-HDL produced by CESD cells up to levels similar to those seen in normal cells without CM treatment (Fig. 6, CD1 and CD2 +CM). Similarly, treatment with 1.2 μg/ml rhLAL for 24 h rescued α-HDL particle formation in the medium of both CESD cell lines to normal abundance (supplemental Fig. 2).
FIGURE 6.
Reduced α-HDL particle formation by CESD fibroblasts is rescued following treatment with normal fibroblast conditioned medium. Fibroblasts were treated as in Fig. 5 with or without conditioned medium (CM) from normal fibroblasts. Efflux medium following a subsequent 24-h incubation with 10 μg/ml apoA-I was concentrated to 0.1 volume, and 20 μl of each sample was run in the first dimension on 0.75% agarose, separated in the second dimension on a 5–23% nondenaturing polyacrylamide gradient gel, and analyzed by Western blot using a rabbit polyclonal antibody to human apoA-I. Boxed areas represent α-HDL particles. Blots are representative of three experiments with similar results.
Impaired LDL-induced Oxysterol Formation in CESD Cells and Correction by Addition of LAL
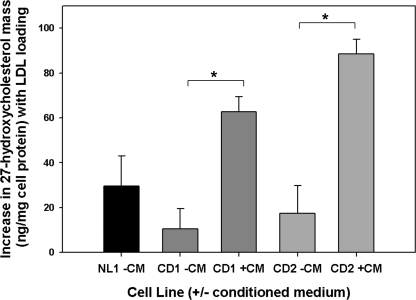
Production of endogenous oxysterols in response to LDL loading is impaired in NPC1- and NPC2-deficient human fibroblasts (13), providing a reason for the impaired regulation of ABCA1 in NPC disease (7). The reduced flux of unesterified cholesterol out of lysosomes in CESD, plus the ability of an exogenous LXR agonist to correct ABCA1 expression in these cells (Fig. 4, A and B), suggests that endogenous oxysterol generation required for LXR activation and ABCA1 expression is also impaired in CESD. To test this hypothesis, we measured the increase in 27-HC mass, the predominant enzymatically generated oxysterol in fibroblasts (34, 35) and key regulator of HMG-CoA reductase (34) and ABCA1 (35) expression in response to LDL loading of fibroblasts, following incubation of CESD cells with LDL. The increase in combined medium and cellular 27-HC mass in response to LDL loading was less in both CESD cell lines when compared with normal fibroblasts (Fig. 7). Treatment with conditioned medium from normal cells to provide LAL activity significantly increased production of 27-HC mass in both CESD cell lines when compared with nontreated cells, to levels similar to or higher than LDL-loaded normal fibroblasts (Fig. 7). Addition of rhLAL also increased 27-HC production by CESD cells (supplemental Fig. 3), suggesting the correction of ABCA1 expression and activity under this condition was due to endogenously produced rather than exogenous oxysterol. These results provide the first demonstration of reduced oxysterol production in CESD fibroblasts, and further evidence that the impaired flux of cholesterol out of lysosomes and the consequent reduction in oxysterol formation is responsible for the impaired regulation of ABCA1 and HDL formation in CESD.
FIGURE 7.
Reduced 27-hydroxycholesterol mass in CESD cells and correction following treatment with normal fibroblast conditioned medium. Normal (NL1) and CESD (CD1 and CD2) fibroblasts were treated as in Fig. 5, either with (+) or without (−) conditioned media (CM). Lipids from cells and media were extracted, and the pooled lipid fractions were separated by gas chromatography, and the mass of 27-HC quantified as described under “Experimental Procedures.” Results represent the difference between LDL loading (+LDL) and non-LDL-loaded controls; means ± S.D. are of triplicates and are representative of two experiments with similar results. *, values significantly greater than non- conditioned medium-treated cells, p < 0.05.
DISCUSSION
The studies presented here provide several additional lines of evidence that the rate of flux of cholesterol out of lysosomes is a key regulator of ABCA1 expression and activity and therefore HDL particle formation. Human CESD fibroblasts with very low residual activity of LAL and slowed rate of hydrolysis of cholesteryl esters in late endosome/lysosomes exhibited a marked decrease in LDL-stimulated expression of ABCA1 at the mRNA and protein level, resulting in impaired ABCA1-dependent phospholipid and cholesterol efflux to apoA-I, and reduced production of larger α-HDL particles. Treatment of normal fibroblasts with chloroquine to inhibit LAL activity induced the same defects in LDL-stimulated increases in ABCA1 expression and cholesterol efflux to apoA-I as seen in the CESD cells. Treatment of CESD cells with LAL-containing conditioned medium from normal cells or purified recombinant human LAL corrected cholesteryl ester hydrolysis, ABCA1 expression, cholesterol efflux to apoA-I, and formation of α-HDL particles to levels similar to those seen in normal fibroblasts. Formation of the key oxysterol formed in response to LDL loading in fibroblasts, 27-hydroxycholesterol, was reduced in CESD fibroblasts, consistent with reduced ABCG1 as well ABCA1 expression in these cells. 27-HC formation was increased upon addition of LAL-containing medium to correct cholesteryl ester hydrolysis. Together with our previous findings using cells from patients with the lysosomal cholesterol storage disorder NPC1 disease (7, 8), these results demonstrate further the critical role of the rate of flux of cholesterol out of late endosomes/lysosomes in regulating ABCA1 expression and HDL particle formation. They also provide the first likely explanation for the low plasma HDL-C seen in CESD patients, impaired regulation of ABCA1.
In both NPC1 disease and CESD, previous studies using cultured fibroblasts showed that the reduced rate of release of unesterified cholesterol from late endosomes/lysosomes leads to impaired down-regulation of HMG-CoA reductase and LDL receptor activity and therefore inappropriately high levels of de novo cholesterol synthesis and LDL uptake (11, 36). Reduced trafficking of unesterified cholesterol to the endoplasmic reticulum also results in reduced levels of cholesterol esterification by acyl-CoA:cholesterol acyltransferase in both diseases (11, 37). At the same time, our previous work (7, 8) and this study suggest the low rate of cholesterol egress from lysosomes and oxysterol generation in both these diseases results in reduced ABCA1 expression and HDL particle formation. Fibroblasts from NPC1 and CESD patients therefore fail to sense the accumulation of excess cholesterol in late endosomes/lysosomes and to up-regulate ABCA1 appropriately in response to cholesterol loading, thereby resulting in impaired HDL formation.
27-HC has been shown to be the predominant oxysterol formed and primary regulator of both HMG-CoA reductase and ABCA1 expression in response to LDL or acetylated LDL loading in human fibroblasts and other cell types (34, 35). The reduced generation of 27-HC in NPC disease (13) and in CESD cells in response to LDL loading found in this study is striking also in demonstrating the key role of lysosomally derived cholesterol in the formation of this key regulatory oxysterol required for ABCA1 expression. Despite increased HMG-CoA reductase activity and de novo cholesterol synthesis in both CESD and NPC disease cells (11, 36), newly synthesized cholesterol is apparently not contributing a significant pool of cholesterol or regulatory oxysterols affecting ABCA1 expression. Although synthesis of 24(S),25-epoxycholesterol has been shown to increase coordinately with de novo cholesterol synthesis via HMG-CoA reductase (38), synthesis of this oxysterol is apparently not enough to impact ABCA1 expression in the face of reduced flux of cholesterol out of lysosomes. Further demonstration of the specific and critical role of lysosomally derived cholesterol in regulating ABCA1 expression was seen in our experiments using chloroquine, where levels of ABCA1 mRNA were reduced to near zero in cells pretreated with lipoprotein-deficient serum or following LDL loading (Fig. 3A).
Treatment of CESD cells with the non-oxysterol LXR agonist TO-901317 increased ABCA1 mRNA and protein levels up to either normal or higher levels than those seen in untreated normal fibroblasts (Fig. 4, A and B). In contrast to our previous findings of complete correction of apoA-I-mediated cholesterol efflux and HDL particle formation in NPC1-deficient fibroblasts treated with this LXR agonist (8), CESD cells showed a persistent reduction in apoA-I-mediated cholesterol efflux (Fig. 4C). These results suggest that treatment with this agonist and increased expression of ABCA1 can bypass a deficiency in NPC1 activity to induce mobilization of lysosomal (unesterified) cholesterol, but it cannot overcome the deficiency in LAL activity and reduced LDL cholesteryl ester hydrolysis in CESD cells. These results also support the conclusion of previous studies (39, 40) that lysosomally derived cholesterol forms a significant fraction of the substrate pool of cholesterol mobilized by ABCA1 for HDL particle formation.
Our findings that addition of LAL-containing conditioned medium from normal fibroblasts or purified LAL rescued ABCA1 expression and activity are consistent with previous results showing correction of LDL cholesteryl ester hydrolysis and new cholesteryl ester formation as well as suppression of HMG-CoA reductase by addition of normal fibroblast conditioned medium to CESD fibroblasts (17). Correction of LDL-derived radiolabeled cholesteryl ester and unesterified cholesterol levels in CESD cells to the same levels as seen in normal fibroblasts (Fig. 5, D and E, and supplemental Fig. 1, C and D) indicated LAL in the rescue medium was targeted to and active in late endosomes/lysosomes of the CESD cells. Correction of LAL activity also resulted in normalization of apoA-I-mediated cholesterol efflux (Fig. 5C and supplemental Fig. 1B), HDL particle formation (Fig. 6 and supplemental Fig. 2), and 27-HC formation (Fig. 7 and supplemental Fig. 3) in the CESD cells. Although differences in 27-HC production between normal and CESD cells in the absence or presence of purified LAL enzyme did not reach significance, the increases in 27-HC production by CESD cells treated with both conditioned medium or purified LAL were sufficient to correct ABCA1 expression and activity in both cases. These results suggest that the rate of production of 27-HC, in addition to total 27-HC mass produced over a 24-h period, is important in correcting ABCA1 expression following normalization of cholesteryl ester hydrolysis. This increase in 27-HC formation and correction of the regulatory defect in ABCA1 expression and activity by adding back LAL to CESD cells provides further evidence for reduced lysosomal cholesteryl ester hydrolysis and flux of unesterified cholesterol out of the late endosome/lysosome compartment, and therefore the reduced delivery of this cholesterol to sites of oxysterol generation, as the reason for impaired ABCA1 regulation and HDL formation in CESD.
A striking aspect of both NPC disease and CESD is the low plasma HDL-C in humans with these disorders (9, 10) but not in the mouse models of NPC1 and LAL deficiency (41, 42). Previous studies indicated hepatic ABCA1 is a critical regulator of plasma HDL-C in mice (43) and that hepatocyte ABCA1 is not reduced in NPC1−/− mice (44). The absence of low plasma HDL-C in LAL-deficient mice is consistent with these observations, because mouse hepatic ABCA1 is reported to be unresponsive to LXR stimulation (45, 46) and would not be expected to show reduced expression in the face of reduced flux of cholesterol out of lysosomes and oxysterol generation with LAL deficiency. Human hepatocyte ABCA1, conversely, is responsive to LXR activation in both primary human hepatocytes (47, 48) and HepG2 cells (49, 50). Human hepatocyte ABCA1 would therefore be expected to show reduced expression in the face of reduced LAL (and NPC1) activity and the reduced production of oxysterol agonist of LXR, which we have demonstrated here in fibroblasts. These conclusions are consistent with both a major role of hepatic ABCA1 in predicting plasma HDL-C and the approximately half-normal HDL-C levels seen in humans with CESD (10, 51). Additional studies are required to explore the role of LAL or NPC1 deficiency on human hepatocyte ABCA1 expression and HDL formation.
In summary, the results presented provide further evidence that the rate of flux of cholesterol out of late endosomes/lysosomes is a critical regulator of the expression of ABCA1 and HDL particle formation, and is not corrected by the increased de novo cholesterol synthesis seen in cells from patients with two different diseases of lysosomal cholesterol storage, cholesteryl ester storage disease and NPC1 disease. These results also provide the first plausible explanation for the hypoalphalipoproteinemia seen in CESD, impaired regulation of ABCA1. We therefore propose a model for CESD cells where hydrolysis of cholesteryl esters from endocytosed LDL in late endosomes and lysosomes is impaired, and there is a reduced rate of unesterified cholesterol release from these compartments to other intracellular sites for regulatory effects, including production of 27-HC, and therefore reduced LXR-dependent regulation of ABCA1. In addition, less unesterified cholesterol is available to join the substrate pool of ABCA1 for new HDL particle formation. Although we did not find an up-regulation of ABCA1 expression in normal cells in response to addition of conditioned medium containing LAL, further studies are required to assess the potential role of LAL as a target to increase ABCA1 expression and HDL formation at the cellular and clinical level.
Supplementary Material
This work was supported by Canadian Institutes of Health Research Grant MOP-79532 (to G. A. F.). This work was also supported by National Institutes of Health Grants HL067773 (to D. S. O.) and HL087001 (to H. D.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- LXR
- liver X receptor
- CESD
- cholesteryl ester storage disease
- 27-HC
- 27-hydroxycholesterol
- LAL
- lysosomal acid lipase
- LPDS
- lipoprotein-deficient serum
- NPC
- Niemann-Pick type C
- LPDS
- lipoprotein-deficient serum
- rh
- recombinant human.
REFERENCES
- 1. Francis G. A. (2010) Biochim. Biophys. Acta 1801, 1286–1293 [DOI] [PubMed] [Google Scholar]
- 2. Oram J. F., Heinecke J. W. (2005) Physiol. Rev. 85, 1343–1372 [DOI] [PubMed] [Google Scholar]
- 3. Costet P., Luo Y., Wang N., Tall A. R. (2000) J. Biol. Chem. 275, 28240–28245 [DOI] [PubMed] [Google Scholar]
- 4. Venkateswaran A., Laffitte B. A., Joseph S. B., Mak P. A., Wilpitz D. C., Edwards P. A., Tontonoz P. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 12097–12102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kennedy M. A., Venkateswaran A., Tarr P. T., Xenarios I., Kudoh J., Shimizu N., Edwards P. A. (2001) J. Biol. Chem. 276, 39438–39447 [DOI] [PubMed] [Google Scholar]
- 6. Wang N., Lan D., Chen W., Matsuura F., Tall A. R. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 9774–9779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi H. Y., Karten B., Chan T., Vance J. E., Greer W. L., Heidenreich R. A., Garver W. S., Francis G. A. (2003) J. Biol. Chem. 278, 32569–32577 [DOI] [PubMed] [Google Scholar]
- 8. Boadu E., Choi H. Y., Lee D. W., Waddington E. I., Chan T., Asztalos B., Vance J. E., Chan A., Castro G., Francis G. A. (2006) J. Biol. Chem. 281, 37081–37090 [DOI] [PubMed] [Google Scholar]
- 9. Garver W. S., Jelinek D., Meaney F. J., Flynn J., Pettit K. M., Shepherd G., Heidenreich R. A., Vockley C. M., Castro G., Francis G. A. (2010) J. Lipid Res. 51, 406–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Assmann G., Seedorf U. (2001) in The Metabolic and Molecular Bases of Inherited Disease (Scriver C. R., Beaudet A. L., Sly W. S., Valle D. eds) 7th Ed., pp. 3551–3572, McGraw Hill Inc., New York [Google Scholar]
- 11. Goldstein J. L., Dana S. E., Faust J. R., Beaudet A. L., Brown M. S. (1975) J. Biol. Chem. 250, 8487–8495 [PubMed] [Google Scholar]
- 12. Du H., Sheriff S., Bezerra J., Leonova T., Grabowski G. A. (1998) Mol. Genet. Metab. 64, 126–134 [DOI] [PubMed] [Google Scholar]
- 13. Frolov A., Zielinski S. E., Crowley J. R., Dudley-Rucker N., Schaffer J. E., Ory D. S. (2003) J. Biol. Chem. 278, 25517–25525 [DOI] [PubMed] [Google Scholar]
- 14. Chung B. H., Wilkinson T., Geer J. C., Segrest J. P. (1980) J. Lipid Res. 21, 284–291 [PubMed] [Google Scholar]
- 15. Sattler W., Stocker R. (1993) Biochem. J. 294, 771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Anderson R. A., Bryson G. M., Parks J. S. (1999) Mol. Genet. Metab. 68, 333–345 [DOI] [PubMed] [Google Scholar]
- 17. Brown M. S., Sobhani M. K., Brunschede G. Y., Goldstein J. L. (1976) J. Biol. Chem. 251, 3277–3286 [PubMed] [Google Scholar]
- 18. Brown M. S., Dana S. E., Goldstein J. L. (1975) Proc. Natl. Acad. Sci. U.S.A. 72, 2925–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Waddington E. I., Boadu E., Francis G. A. (2005) Methods 36, 196–206 [DOI] [PubMed] [Google Scholar]
- 20. Folch J., Lees M., Sloane Stanley G. H. (1957) J. Biol. Chem. 226, 497–509 [PubMed] [Google Scholar]
- 21. Francis G. A., Oram J. F., Heinecke J. W., Bierman E. L. (1996) Biochemistry 35, 15188–15197 [DOI] [PubMed] [Google Scholar]
- 22. Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951) J. Biol. Chem. 193, 265–275 [PubMed] [Google Scholar]
- 23. Sahoo D., Trischuk T. C., Chan T., Drover V. A., Ho S., Chimini G., Agellon L. B., Agnihotri R., Francis G. A., Lehner R. (2004) J. Lipid Res. 45, 1122–1131 [DOI] [PubMed] [Google Scholar]
- 24. Bligh E. G., Dyer W. J. (1959) Can. J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 25. Guardiola F., Codony R., Rafecas M., Boatella J. (1995) J. Chromatogr. A 705, 289–304 [DOI] [PubMed] [Google Scholar]
- 26. Pentchev P. G., Kruth H. S., Comly M. E., Butler J. D., Vanier M. T., Wenger D. A., Patel S. (1986) J. Biol. Chem. 261, 16775–16780 [PubMed] [Google Scholar]
- 27. Kruth H. S., Comly M. E., Butler J. D., Vanier M. T., Fink J. K., Wenger D. A., Patel S., Pentchev P. G. (1986) J. Biol. Chem. 261, 16769–16774 [PubMed] [Google Scholar]
- 28. Sando G. N., Henke V. L. (1982) J. Lipid Res. 23, 114–123 [PubMed] [Google Scholar]
- 29. Sheriff S., Du H., Grabowski G. A. (1995) J. Biol. Chem. 270, 27766–27772 [DOI] [PubMed] [Google Scholar]
- 30. Brown M. S., Dana S. E., Goldstein J. L. (1974) J. Biol. Chem. 249, 789–796 [PubMed] [Google Scholar]
- 31. Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. (1980) J. Cell Biol. 85, 839–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Langmade S. J., Gale S. E., Frolov A., Mohri I., Suzuki K., Mellon S. H., Walkley S. U., Covey D. F., Schaffer J. E., Ory D. S. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 13807–13812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sando G. N., Rosenbaum L. M. (1985) J. Biol. Chem. 260, 15186–15193 [PubMed] [Google Scholar]
- 34. Axelson M., Larsson O. (1995) J. Biol. Chem. 270, 15102–15110 [DOI] [PubMed] [Google Scholar]
- 35. Fu X., Menke J. G., Chen Y., Zhou G., MacNaul K. L., Wright S. D., Sparrow C. P., Lund E. G. (2001) J. Biol. Chem. 276, 38378–38387 [DOI] [PubMed] [Google Scholar]
- 36. Pentchev P. G., Comly M. E., Kruth H. S., Tokoro T., Butler J., Sokol J., Filling-Katz M., Quirk J. M., Marshall D. C., Patel S., et al. (1987) FASEB J. 1, 40–45 [DOI] [PubMed] [Google Scholar]
- 37. Pentchev P. G., Comly M. E., Kruth H. S., Vanier M. T., Wenger D. A., Patel S., Brady R. O. (1985) Proc. Natl. Acad. Sci. U.S.A. 82, 8247–8251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wong J., Quinn C. M., Brown A. J. (2007) Lipids Health Dis. 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen W., Sun Y., Welch C., Gorelik A., Leventhal A. R., Tabas I., Tall A. R. (2001) J. Biol. Chem. 276, 43564–43569 [DOI] [PubMed] [Google Scholar]
- 40. Chen W., Wang N., Tall A. R. (2005) J. Biol. Chem. 280, 29277–29281 [DOI] [PubMed] [Google Scholar]
- 41. Xie C., Turley S. D., Dietschy J. M. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 11992–11997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Du H., Heur M., Duanmu M., Grabowski G. A., Hui D. Y., Witte D. P., Mishra J. (2001) J. Lipid Res. 42, 489–500 [PubMed] [Google Scholar]
- 43. Timmins J. M., Lee J. Y., Boudyguina E., Kluckman K. D., Brunham L. R., Mulya A., Gebre A. K., Coutinho J. M., Colvin P. L., Smith T. L., Hayden M. R., Maeda N., Parks J. S. (2005) J. Clin. Invest. 115, 1333–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang M. D., Franklin V., Sundaram M., Kiss R. S., Ho K., Gallant M., Marcel Y. L. (2007) J. Biol. Chem. 282, 22525–22533 [DOI] [PubMed] [Google Scholar]
- 45. Brunham L. R., Kruit J. K., Pape T. D., Parks J. S., Kuipers F., Hayden M. R. (2006) Circ. Res. 99, 672–674 [DOI] [PubMed] [Google Scholar]
- 46. Tang W., Ma Y., Jia L., Ioannou Y. A., Davies J. P., Yu L. (2008) Arterioscler. Thromb. Vasc. Biol. 28, 448–454 [DOI] [PubMed] [Google Scholar]
- 47. Whitney K. D., Watson M. A., Goodwin B., Galardi C. M., Maglich J. M., Wilson J. G., Willson T. M., Collins J. L., Kliewer S. A. (2001) J. Biol. Chem. 276, 43509–43515 [DOI] [PubMed] [Google Scholar]
- 48. Menke J. G., Macnaul K. L., Hayes N. S., Baffic J., Chao Y. S., Elbrecht A., Kelly L. J., Lam M. H., Schmidt A., Sahoo S., Wang J., Wright S. D., Xin P., Zhou G., Moller D. E., Sparrow C. P. (2002) Endocrinology 143, 2548–2558 [DOI] [PubMed] [Google Scholar]
- 49. Aravindhan K., Webb C. L., Jaye M., Ghosh A., Willette R. N., DiNardo N. J., Jucker B. M. (2006) J. Lipid Res. 47, 1250–1260 [DOI] [PubMed] [Google Scholar]
- 50. Zelcer N., Hong C., Boyadjian R., Tontonoz P. (2009) Science 325, 100–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ginsberg H. N., Le N. A., Short M. P., Ramakrishnan R., Desnick R. J. (1987) J. Clin. Invest. 80, 1692–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.