Abstract
The mammalian target of rapamycin (mTOR) plays a central role in the regulation of a number of cellular processes including growth, metabolism, and ion transport. mTOR is found in two multiprotein complexes, mTORC1 and mTORC2, which phosphorylate distinct substrates and regulate distinct cellular processes. SGK1 is an mTORC2 substrate, which is a key regulator of epithelial Na+ transport mediated by the epithelial sodium channel. Although it is known that SGK1 physically interacts with mTORC2, it is unknown which mTORC2 component mediates this interaction or whether this interaction plays a physiologically relevant role in specific activation of SGK1. Here we identify mSIN1 as the mTORC2 component that mediates interaction with SGK1 and demonstrate that this interaction is required for SGK1 phosphorylation and epithelial sodium channel activation. We used the yeast two-hybrid system coupled with random mutagenesis to identify a mutant mSIN1 (mSIN1/Q68H), which does not interact with SGK1. Expression of this mutant does not restore SGK1 phosphorylation to wild-type levels in mSIN1-deficient murine embryo fibroblasts. Furthermore, in kidney epithelial cells, mSIN1/Q68H has a dominant-negative effect on SGK1 phosphorylation and on SGK1-dependent Na+ transport. Interestingly, this interaction appears to be specific in that another mTORC2 substrate, Akt, does not interact with mSIN1, and its phosphorylation and activity are unaffected by the Q68H mutation. These data support the conclusion that mTORC2 uses distinct strategies to phosphorylate different substrates and suggest a mechanism for mTORC2 specificity in the regulation of diverse cellular processes.
Keywords: Epithelial Cell, Kidney, Phosphorylation Enzymes, Signal Transduction, Sodium Transport
Introduction
The mammalian target of rapamycin (mTOR)2 is a highly conserved serine/threonine kinase that plays a central role in the regulation of a number of cellular processes (1–6). It is assembled into at least two distinct multiprotein complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), which act as sensors for upstream inputs from multiple intracellular and extracellular signals that are then transduced to a wide spectrum of downstream effectors. mTORC1 has been well documented to regulate translation initiation, ribosome biogenesis, cell growth, and proliferation (7–9), whereas mTORC2 regulates actin cytoskeleton organization, ion transport, protein translation, and metabolism (10–14), among other processes. In keeping with their largely non-overlapping functional effects, mTORC1 and mTORC2 have distinct substrates; mTORC1 phosphorylates p70 S6 kinase and the eukaryotic initiation factor 4E-binding protein (4E-BP1), whereas mTORC2 phosphorylates Akt, SGK, and PKCα at a conserved site termed the hydrophobic motif (HM) (15). mTORC1 substrate preference is at least in part determined by specific interaction of one of its core components, Raptor, with a specific motif found in both S6 kinase and 4E-BP1, termed the TOR signaling motif (16). This motif is not found in mTORC2 substrates, and thus specific interaction of Raptor with the TOR signaling motif provides one mechanism for mTORC1 specificity. Previous data also have demonstrated interaction of both Akt (10) and SGK1 (13) with mTORC2 but not mTORC1 (13), suggesting that a parallel mechanism for specificity might be operating for mTORC2-regulated substrates. However, the specific component of mTORC2 mediating this interaction remains unknown, and a TOR signaling-like motif has yet to be identified in mTORC2 substrates.
SGK1 and Akt are highly homologous in their catalytic domains and regulatory motifs and, in addition to HM phosphorylation by mTORC2, both are phosphorylated in a region termed the activation loop by the PI3-kinase-dependent kinase, PDK1. Despite these similarities, these kinases have distinct functional effects and distinct targets (17). SGK1 is a key regulator of ion and solute transport processes in mammalian epithelia (18–22), whereas Akt isoforms regulate cell size and survival as well as glucose metabolism, among other functions. For both SGK1 and Akt, phosphorylation at both the HM and the activation loop is required for full kinase activity (23, 24). Activated SGK1 then regulates Na+ transport by inhibiting inhibitors of the epithelial sodium channel, ENaC (25–27), most notably, the ubiquitin ligase Nedd4-2 (21, 25, 28). In contrast, the Akt isoform Akt2 regulates glucose metabolism through a variety of mechanisms, notably stimulating cell surface expression of the glucose transporter, Glut4 (29, 30).
Because physical interaction with mTORC2 appeared to be essential for SGK1 HM phosphorylation and ENaC stimulation (13), we set out to identify the specific component(s) within mTORC2 that mediated specific interaction with SGK1. We identified the mTORC2-specific component, mSIN1 (mammalian stress-activated protein kinase-interacting protein), as an SGK1-interacting factor and then used random mutagenesis to identify an mSIN1 mutant (Q68H) that assembled appropriately into mTORC2 but failed to bind SGK1. The regulatory characteristics of wild-type and mutant mSIN1 were consistent with the hypothesized role of mSIN1 in mediating mTORC2 regulation of SGK1. However, in further experiments directed at comparing regulation of SGK1 and Akt, our data, surprisingly, did not support a parallel role of mSIN1 in Akt regulation. Taken together, our findings strongly support the idea that mSIN1 recruits SGK1 but not Akt into the mTORC2 complex to undergo HM phosphorylation. Although mTORC2 mediates Akt HM phosphorylation, it appears to use a distinct strategy independent of mSIN1, thus suggesting a novel mechanism for differential regulation of these two important signaling kinases.
EXPERIMENTAL PROCEDURES
Yeast Two-hybrid Assays
The 1.3-kbp EcoRI-SalI cDNA fragment containing the entire coding region of the mouse SGK1 was cloned in-frame into the multiple cloning site on the yeast expression vector, pAS2-1 (Clontech, Palo Alto, CA). Yeast cells were prepared by treatment with lithium acetate. 0.1 μg of DNA was added to 100 μl of competent cells followed by the addition of 600 μl of sterile polyethylene glycol/lithium acetate solution (0.1 m lithium acetate, 10 mm Tris, pH 7.5, 1 mm EDTA). The transformation mixture was incubated at 30 °C for 30 min with shaking, treated with 10% Me2SO, heat-shocked at 42 °C for 15 min, and spread onto 100-mm plates of minimum synthetic medium lacking tryptophan. Colonies of yeast cells were allowed to grow for 4 days and harvested for subsequent transformation with mSIN1 or Rictor in the vector pACT2 (Clontech). After selection for growth on Ade−/His−/Leu−/Trp− dropout plates, positive clones were processed as described previously (31).
PCR-based Random Mutagenesis
Error-prone PCR was carried out to make single amino acid mutations (substitutions) in the candidate binding sites. The procedure relies on manganese-induced misinsertion of nucleotides by Taq polymerases as well as reduced concentration of each dNTP and an increased number of PCR cycles to decrease the fidelity of PCR amplification (32, 33). Over 50% of the mutants were found to carry single-base substitutions. A cDNA fragment corresponding to the first 160 aa at the N terminus of mSIN1 was amplified, using the forward primer 5′-CTATATGGCCATGGAGGCCATGGCCTTCTTGGACAATCCAACTATC-3′ and the reverse primer 5′-AGAAGGATCCTCAGTACTCATTAAAGGGGTTATTCAGC-3′. The amplified PCR product was purified with a QIAEX II kit (Qiagen), digested with the restriction enzymes, SfiI and BamHI (New England BioLabs) using the manufacturer's buffer solutions, purified again with a QIAEX II kit, and cloned in-frame into the pACT2 expression vector. Yeast two-hybrid assays were carried out to identify clones that failed to grow on Ade−/His−/Leu−/Trp− dropout plates. The non-binding clones were subsequently analyzed by DNA sequencing.
In Vitro Precipitation Assays
The entire coding region of the mouse mSIN1 cDNA was amplified by high fidelity PCR and cloned in-frame with the maltose-binding protein (MBP) in pMAL (New England BioLabs). Bacterial colonies were inoculated into LB medium containing ampicillin and grown for 16 h at 37 °C in a shaking incubator. The cultures were diluted 1:10 and grown for 4 h; isopropyl thiogalactoside was added to a final concentration of 0.2 mm, and incubation was continued for 1 h. Bacteria were pelleted by centrifugation at 10,000 × g for 2 min and resuspended in ice-cold PBS. Cell lysis was carried out by sonication (Sonic Dismembrator, Fisher) for 2 × 30 s. Triton X-100 was added to a final concentration of 1% to minimize aggregation of the fusion protein with bacterial proteins. Samples were centrifuged at 10,000 × g for 5 min, and the supernatants were collected, mixed with 50% slurry of amylose resin (New England BioLabs) in PBS, and incubated for 5 min at room temperature. The beads were collected by centrifugation, washed with ice-cold PBS, and stored at 4 °C in the presence of bovine serum albumin and protease inhibitors.
The pMO vector harboring the full-length SGK1 was used to produce FLAG-tagged SGK1 protein by in vitro translation using the TnT-coupled reticulocyte lysate system (Promega, Madison, WI). The tagged SGK1 protein was incubated with 2 μg of MBP-mSIN1 fusion protein bound to 30 μl of amylase resin in Nonidet P-40 buffer (150 mm NaCl, 1% Nonidet P-40, 50 mm Tris, pH 8) at 4 °C for 3 h with shaking. The beads were recovered by centrifugation, washed three times in Nonidet P-40 buffer, resuspended in Laemmli sample loading buffer (2% SDS, 10% glycerol, 100 mm dithiothreitol, 60 mm Tris, pH 6.8, and 0.001% bromphenol blue), and boiled for 3 min. After centrifugation, the supernatants were collected and subjected to Western blotting analysis using an antibody against the FLAG tag.
Generation of Recombinant Adenoviruses Harboring mSIN1
The full-length mSIN1 was cloned into the pShuttle vector (Clontech) in-frame with the FLAG tag. Positive recombinant clones were identified by restriction enzyme digestion and verified by DNA sequencing. The expression cassette harboring FLAG-tagged mSIN1 was excised using the unique restriction endonucleases PI-Sce I and I-Ceu I and subsequently cloned into the Adeno-X vector (Clontech). Recombinant adenoviral DNA harboring the expression cassette was verified by restriction enzyme digestion and PCR, cut with PacI, and transfected into HEK 293T cells. Cytopathic effect was checked periodically. When >50% of the cells detached from the culture plate, cells were harvested by centrifugation, resuspended in PBS, and lysed with three consecutive freeze-thaw cycles. To prepare high titer viruses, HEK 293T cells were infected with the low titer adenovirus harboring the FLAG-tagged mSIN1. When cytopathic effect was visible, cells were again harvested, and viral particles were isolated by the freeze-thaw method. A viral titer of 5 × 108 was routinely observed using a titration assay.
Immunoprecipitation and Immunoblotting
Murine embryonic fibroblasts (MEFs) deficient in mSIN1 were a generous gift of Dr. Bing Su (10). The cells were transfected using a high efficiency electroporation protocol (MEF1 Nucleofector kit, Amaxa Biosystems). HEK 293T cells were transfected using Lipofectamine 2000 (Invitrogen). mpkCCDc14 cells were transduced with recombinant adenoviruses. Transfected or adenovirus-transduced cells were lysed in binding buffer (50 mm Tris-HCl, pH 7.5, 10% glycerol, 1 mm EDTA, 2 mm DTT, 150 mm NaCl, and 0.3% CHAPS) for 15 min. After centrifugation, the supernatants were collected and incubated with the anti-FLAG M2 affinity beads (Sigma). The immunoprecipitates were collected by centrifugation, washed three times, and boiled for 5 min in 50 μl of cracking buffer (50 mm Tris-HCl, pH 7.0, 10% glycerol, 2% SDS, 2% β-mercaptoethanol). Immunoblotting was carried out by separating the immunoprecipitates on 10% polyacrylamide gels as described using a Bio-Rad Mini-Gel apparatus and transferring them electrophoretically to Hybond-C Extra membranes (GE Healthcare) using a Trans-Blot apparatus (Bio-Rad). The membranes were incubated to block nonspecific binding in 5% nonfat dry milk in T-PBS (1.5 mm KH2PO4, 8 mm Na2HPO4, 2.7 mm KCl, 130 mm NaCl, and 0.1% Tween 20) with gentle agitation for 1 h at room temperature and probed by Western blotting (for endogenous and transfected proteins, as described in the figure legends), using antibodies against SGK1 (Sigma), phospho-SGK1 (S422) (Santa Cruz Biotechnology), mSIN1 (Bethyl Laboratories), and mTOR, Raptor, Rictor, Akt, and PKCα (Cell Signaling). After washing with T-PBS, the membranes were incubated with peroxidase-conjugated goat anti-rabbit IgG in T-PBS for 1 h, washed three times in T-PBS, and incubated with the ECL Plus Western blotting detection system working solution (GE Healthcare) according to the manufacturer's instructions.
Glucose Uptake Assays
3T3-L1 adipocytes differentiated on 6-well plates were washed twice with PBS and incubated for 3 h in Krebs-Ringer Henseleit/BSA buffer (120 mm NaCl, 4 mm KH2PO4, 1 nm MgSO4, 0.75 nm CaCl2, 10 mm NaHCO3, and 0.1% BSA). The cells were stimulated with 10 μm insulin for 30 min. To measure glucose uptake, 3H-labeled 2-deoxy glucose was mixed with unlabeled 2-deoxy glucose and applied to the cells at a concentration of 50 μm for 30 min. After washing with cold PBS, cells were lysed in 50 mm NaOH and 1% Triton X-100. Aliquots from each sample were added with liquid scintillation fluid and counted using a Beckman LS 6000SC scintillation counter.
Measurement of ENaC-dependent Na+ Transport
For electrophysiological measurements, mpkCCDc14 cells (34) were transduced with recombinant adenoviruses and seeded on type VI collagen (Sigma) coated filters (Transwell, pore-size 0.4 μm, Corning Costar) and grown at least 24 h prior to treatment with aldosterone at a concentration of 1 μm. Transepithelial resistance and potential difference across the cell monolayer were measured using a mini-volt-ohm meter (MilliCell ERS, Millipore) at specified time points following treatment. The equivalent short-circuit current was calculated using Ohm's law.
RESULTS
Generation of an mSIN1 Mutant That Lacks Binding to SGK1
Previous immunoprecipitation experiments demonstrated physical interaction between SGK1 and mTORC2, but not mTORC1 (13). To identify a specific mTORC2 component that directly interacts with SGK1, we used the yeast two-hybrid assay, testing for interaction of SGK1 with mTORC2 components not found in mTORC1. We found no interaction of SGK1 with the original defining component of mTORC2, Rictor (data not shown); however, we found a robust interaction with a more recently identified mTORC2 component, mSIN1 (Table 1). To further map the domain in mSIN1 required for interaction with SGK1, we tested overlapping deletion mutants using the yeast two-hybrid assay and found a 160-aa domain in the N terminus of mSIN1 that was sufficient for interaction with SGK1 (Table 1).
TABLE 1.
Deletion mapping analysis of the binding domain in mSIN1 required for interaction with SGK1 using the yeast two-hybrid assay
Protein-protein interactions were examined by selection of transformed cells for growth on dropout plates. Growth (+) or no growth (−) associated with expression of the mSIN1 fragments is shown.
| mSIN1 and its deletion mutants | pAS-2 SGK1 |
|---|---|
| pACT mSIN1 (full-length) | + |
| pACT mSIN1 (aa 1–280) | + |
| pACT mSIN1 (aa 263–522) | − |
| pACT mSIN1 (aa 1–160) | + |
| pACT mSIN1 (aa 141–280) | − |
| pACT mSIN1 (aa 1–90) | − |
| pACT mSIN1 (aa 71–160) | − |
| pACT mSIN1/Q68H | − |
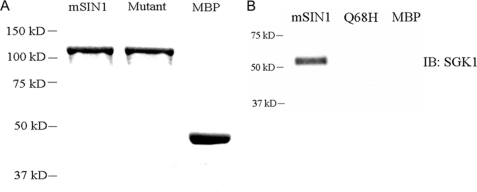
To identify specific mSIN1 residue(s) required for interaction with SGK1, we carried out random mutagenesis (by error-prone PCR) in the 160-aa fragment (aa 1–160) of mSIN1 and tested these mutants by two-hybrid assay with replica plating, as described in detail under “Experimental Procedures.” A single mSIN1 mutant, mSIN1/Q68H (Gln-to-His substitution at amino acid 68), was identified that failed to interact with SGK1 (Table 1). We next confirmed the yeast two-hybrid results by performing in vitro binding experiments. Wild-type or mutant mSIN1 was expressed in bacteria as a maltose-binding protein fusion, purified using amylose resin, and incubated with SGK1 expressed in reticulocyte lysate. The in vitro binding assay confirmed robust binding of wild-type mSIN1 to SGK1, whereas under the same conditions, no binding of mutant mSIN1 to SGK1 could be detected (Fig. 1).
FIGURE 1.
In vitro interaction between mSIN1 and SGK1. A, mSIN1 and the mSIN1/Q68H mutant were expressed as MBP fusions in Escherichia coli and purified on amylose resin. The purified mSIN1 protein, the mutant protein, and the native MBP protein are shown after Coomassie Blue staining. B, wild-type or Q68H mutant mSIN1 fusion protein or native MBP was bound to amylose resin and incubated with in vitro translated SGK1 protein. Bound proteins were recovered by boiling and analyzed by immunoblot (IB) for SGK1. Shown is a representative blot of two independent experiments.
Effect of the Non-binding mSIN1 Mutant on mTORC2 Integrity and Interaction with SGK1
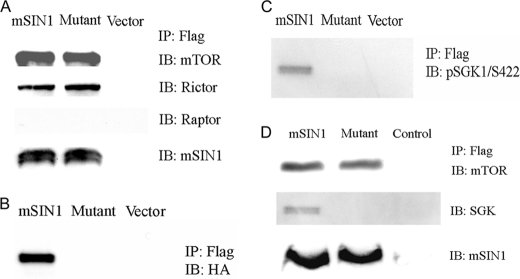
Previous reports have established that mSIN1 is essential for mTORC2 assembly; in its absence, mTORC2 does not form, as evidenced by lack of co-immunoprecipitation (co-IP) of mTOR and Rictor (10, 35). To examine the effects of the point mutant mSIN1/Q68H on mTORC2 assembly, we expressed FLAG epitope-tagged wild-type or mutant mSIN1 in HEK 293T cells, immunoprecipitated with anti-FLAG antibody, and probed Western blots with antibodies against mTOR and Rictor. No significant difference in co-IP of either mTOR or Rictor by wild-type or mutant mSIN1 was detected (Fig. 2A), indicating that the mSIN1 mutant was not impaired in its assembly into mTORC2. This is in contrast to an mSIN1 variant lacking the first 192 amino acids, which does not support formation of mTORC2 (36). These co-IP results also confirm the in vitro data that mutant mSIN1 does not physically interact with SGK1 (Fig. 2, B and C). The mSIN1/Q68H mutant was also capable of assembly into mTORC2 but did not interact with SGK1 in ENaC-expressing mpkCCD cells (Fig. 2D). Thus, mSIN1/Q68H retains its ability to assemble into mTORC2 but fails to bind SGK1.
FIGURE 2.
Effect of mSIN1/Q68H on assembly of mTORC2 and interaction of mTORC2 with SGK1. A, FLAG-tagged mSIN1 (wild-type or Q68H mutant) was co-transfected with HA-tagged SGK1 in HEK 293T cells, lysed, and subjected to immunoprecipitation with anti-FLAG antibody followed by immunoblotting (IB), as described under “Experimental Procedures.” Blots were probed with antibodies against mTOR, Rictor, and Raptor, respectively. B, blots as in A were probed with anti-HA antibody to detect SGK1. C, blots probed with anti-p-SGK1 antibody. D, mpkCCD cells were transduced with recombinant adenoviruses harboring FLAG-tagged wild-type mSIN1 or mSIN1/Q68H mutant. mSIN1 immunoprecipitation was carried out using anti-FLAG antibody, and immunoblots were probed as shown.
The Interaction between mSIN1 and SGK1 Is Necessary for SGK1 HM Phosphorylation
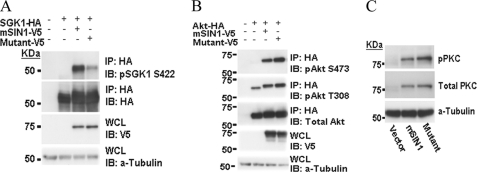
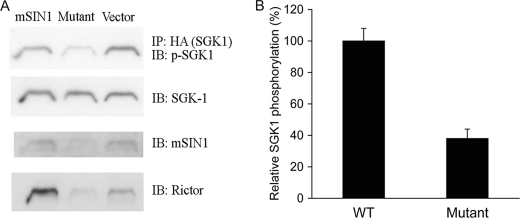
It has been well established that the kinase activity of SGK1 is tightly regulated by phosphorylation within the carboxyl-terminal HM (13, 37). To investigate the role of mSIN1-SGK1 interaction in SGK1 HM phosphorylation, we compared the effect of expressing wild-type versus the mutant mSIN1 on SGK1 phosphorylation in mSIN1-deficient MEFs. First, we examined SGK1 HM phosphorylation in unmodified mSIN1-deficient MEFs and found, as expected, that HM phosphorylation was virtually undetectable (Fig. 3A). We also confirmed previous results that Akt and PKCα HM phosphorylation is abrogated in these MEFs (Fig. 3, B and C) (10, 12, 38). Expression of wild-type mSIN1 fully restored SGK1, Akt, and PKCα HM phosphorylation to wild-type levels (Fig. 3). However, mSIN1/Q68H had a strikingly different effect; it only partially restored SGK1 HM phosphorylation (Fig. 3A), whereas fully restoring phosphorylation of Akt and PKCα. Note that our results also confirm earlier data that PKCα expression is dependent on mTORC2-dependent phosphorylation (38). Consistent with the MEF results, in HEK 293T cells (which have endogenous mSIN1), mSIN1/Q68H had a dominant-negative effect on SGK1 HM phosphorylation (Fig. 4A), causing a significant reduction in phospho-S422 (Fig. 4B) relative to wild-type mSIN1 or vector control (Fig. 4). It is also notable that the ability of SGK1 to interact with mTORC2 (as reflected by co-IP of Rictor) is also attenuated in cells expressing mSIN1/Q68H. We interpret these results (in conjunction with Fig. 2) as supporting the idea that heterologous expression of mutant mSIN1 has a dominant-negative effect on SGK1 phosphorylation by inducing the formation of mTOR complexes that cannot interact with SGK1.
FIGURE 3.
Attenuation of SGK1 phosphorylation in mSIN1-deficient MEFs harboring mSIN1/Q68H. A, HA-tagged SGK1 expression plasmid was transfected into mSIN1-deficient MEFs along with wild-type mSIN1 or the mSIN1/Q68H mutant. 48 h after transfection, the cells were lysed, and immunoprecipitation was carried out using agarose beads cross-linked with anti-HA antibodies. Immunoblotting (IB) was subsequently performed using antibodies as shown. WCL, whole cell lysates. B, HA-tagged Akt plasmid was transfected into mSIN1-deficient MEFs along with wild-type or mutant mSIN1, as in A, and Akt was immunoprecipitated with anti-HA antibody. Immunoblots were probed with antibodies as shown. pAkt, phosphorylated Akt. C, wild-type mSIN1 or the mSIN1/Q68H mutant was transfected into mSIN1-deficient MEFs. Immunoblotting was performed on whole cell lysates using antibodies against PKCα phospho-S657 and total PKCα. Note the dependence of PKCα phosphorylation (pPKC) and expression, as described previously (38).
FIGURE 4.
Effect of mSIN1/Q68H on SGK1 HM phosphorylation and physical interaction with mTORC2. A, HA-tagged SGK1 plasmid was transfected into HEK 293T cells along with wild-type mSIN1 or mSIN1/Q68H. 48 h after transfection, transfected cells were lysed and immunoprecipitated with anti-HA antibody and immunoblotted (IB) for phospho-S422 SGK1, total SGK1, mSIN1, and Rictor, respectively. B, densitometric quantitation of phospho-SGK1 (three independent experiments). Values were significantly different in the presence of wild-type versus mutant mSIN1 (p < 0.01) by Student's unpaired t test. Error bars indicate S.E.
Differential Regulation of Akt and SGK1 by mTORC2
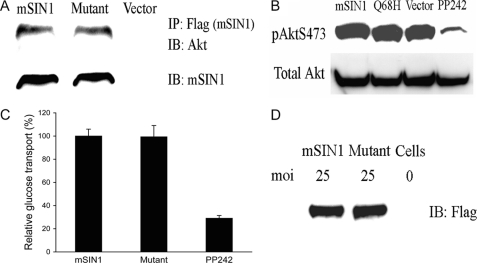
In contrast to mSIN1-SGK1 interaction, the yeast two-hybrid assay demonstrated no physical association between mSIN1 and Akt (data not shown). However, the above data demonstrated that mSIN1 is essential for both Akt and SGK1 HM phosphorylation. These data suggested that although the HMs of both Akt and SGK1 are both phosphorylated by mTORC2, the strategies might be different. To further investigate the effect of mSIN1/Q68H on Akt binding to mTORC2 and on Akt HM phosphorylation, we examined the interaction of mTORC2 with Akt when it contained wild-type versus Q68H mutant mSIN1. As shown in Fig. 5A, mTORC2 was equally capable of interacting with Akt whether it contained wild-type or mutant mSIN1, which is in marked contrast to its effect on the interaction of mTORC2 with SGK1 (Fig. 2). Furthermore, in contrast to its dominant-negative effect on SGK1 HM phosphorylation, mSIN1/Q68H had no effect on Akt HM phosphorylation (Fig. 5B). To determine whether these effects on phosphorylation were consistent with the functional role of mSIN1, we next examined the effect of mSIN1/Q68H on insulin-stimulated glucose uptake in 3T3-L1 adipocytes, a well established Akt-dependent process. Recombinant adenoviruses were used to express wild-type or mutant mSIN1, and consistent with its lack of effect on Akt HM phosphorylation, mSIN1/Q68H had no effect on Akt-mediated glucose transport (Fig. 5C).
FIGURE 5.
Effect of mSIN1/Q68H on Akt regulation by mTORC2. A, Akt physically associates with mTORC2 in the presence of either wild-type or Q68H mutant mSIN1. HEK 293T cells were transfected with FLAG-tagged wild-type or mutant mSIN1, and after 48 h, cells were lysed and immunoprecipitated with the anti-FLAG antibody. Immunoblots (IB) were probed with anti-Akt antibody. B, mSIN1/Q68H does not have dominant-negative activity on Akt S473 phosphorylation. Wild-type or mutant mSIN1 was transfected into HEK 293T cells, and after 48 h, cells were lysed and probed with anti-Akt phospho-S473 antibody. The mTOR inhibitor, PP242, was used as positive control. C, Akt-mediated glucose uptake is not inhibited by mSIN1/Q68H in 3T3-L1 cells. Differentiated 3T3-L1 adipocytes were transduced with recombinant adenoviruses harboring wild-type mSIN1 or the mSIN1/Q68H mutant. Transduced cells were stimulated with insulin, and glucose uptake was monitored by incubation with 3H-labeled 2-deoxy glucose (n = 3). There was no significant difference in glucose uptake between cells expressing wild-type and mutant mSIN1 (by Student's unpaired t test). PP242-treated cells were significantly different from cells expressing wild-type or mutant mSIN1 (p < 0.01 by unpaired t test). Error bars indicate S.E. D, transduced 3T3-L1 cells were lysed, and expression of mSIN1 and the mSIN1/Q68H mutant was determined by Western blotting. moi, multiplicity of infection.
The mSIN1-SGK1 Interaction Is Required for Na+ Transport in Kidney Epithelial Cells
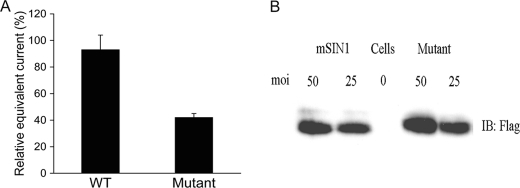
Finally, to determine whether the marked effect of the mSIN1 Q68H mutation on SGK1 HM phosphorylation was correlated with a similar effect on an SGK1-dependent physiological process, we examined its effect on ENaC-dependent Na+ transport in kidney tubule cells (18, 22). mpkCCD cells were transduced with adenoviruses harboring wild-type or mutant mSIN1 and seeded on Transwell filters for measurement of aldosterone-induced Na+ current. Expression of the mSIN1/Q68H mutant reduced Na+ current by ∼55% relative to cells transduced with wild-type mSIN1 expression (Fig. 6). These data strongly support the conclusion that interaction between mSIN1 and SGK1 is required for mTORC2-dependent phosphorylation and activation of SGK1, which is required for it to stimulate ENaC-dependent Na+ transport. Although mSIN1 is essential for mTORC2 integrity, and for this reason is also needed for Akt phosphorylation, it does not appear to interact with Akt, nor to be required to recruit Akt to the mTORC2 complex. It appears that a distinct mTORC2 component is required for this latter interaction.
FIGURE 6.
Inhibition of ENaC-dependent Na+ current by mSIN1/Q68H. A, mpkCCD cells were transduced with recombinant adenoviruses harboring mSIN1 or the mSIN1/Q68H mutant. Transduced cells were plated on Transwell filters, and after reaching high electrical resistance (24–72 h), amiloride-sensitive equivalent current was measured by Evometer. Values in the bar graph are significantly different (p < 0.01) by Student's unpaired t test in three independent experiments. Error bars indicate S.E. B, transduced cells were lysed, and expression of mSIN1 and the mSIN1/Q68H mutant was determined by Western blotting (IB). moi, multiplicity of infection.
DISCUSSION
SGK1 is one of the most intensively studied regulators of epithelial Na+ transport and has been particularly recognized for its importance in integrating the effects of aldosterone on the ENaC with those of other hormones, such as insulin, which regulate SGK1 through various kinase networks (23, 24, 39). Signal integration occurs in part because SGK1 is under dual control; its gene transcription is controlled by aldosterone (and other regulators), and its activity is controlled by phosphorylation (18, 23). HM phosphorylation is central to SGK1 activation, and most recent evidence shows that mTORC2 mediates this phosphorylation (13, 37) (although there is one study suggesting that in some contexts, SGK1 may be a target of mTORC1 (40)). Importantly, in ENaC-expressing kidney cells, mTORC2 is the kinase implicated in SGK1 activation. Based on prior data that mTORC2, but not mTORC1, interacts with SGK1 in co-IP experiments, we set out to identify a single component of the kinase complex that might mediate this interaction. Using the yeast two-hybrid assay, we explored the interaction between SGK1 and mTORC2 components not found in mTORC1. We failed to find direct interaction of SGK1 with Rictor but found robust interaction of SGK1 with mSIN1, another defining component of mTORC2 (Table 1). Previous work established that mSIN1 is required for mTORC2 formation (10, 35), and hence its elimination abrogates phosphorylation of all mTORC2 substrates. To identify mSIN1 amino acids specifically involved in physical interaction with SGK1, we used PCR-based random mutagenesis in conjunction with the yeast two-hybrid assay to identify mSIN1 mutants that could incorporate into mTORC2 but could not physically interact with SGK1. Using this approach, we identified mSIN1/Q68H, which assembled normally into mTORC2 but failed to bind to SGK1 in yeast two-hybrid assay, in in vitro pulldown assays, or in co-IP assays (Table 1; Figs. 1 and 2).
We next demonstrated the functional importance of SGK1-mSIN1 interaction in mSIN1-deficient MEFs. It is notable that in the absence of mSIN1, SGK1 phosphorylation in these MEFs was virtually undetectable (Fig. 3), similar to prior data for Akt (10). As expected, wild-type mSIN1 fully restored SGK1 phosphorylation. In contrast, the ability of mSIN1/Q68H to restore SGK1 phosphorylation was markedly attenuated. Interestingly, truncated mSIN1, lacking the first 192 amino acids (mSIN1/Δ192), did not restore phosphorylation of either SGK1 or Akt (data not shown). This latter observation is consistent with previously published data demonstrating that a natural splice variant of mSIN1, which lacks the N-terminal 192 amino acids, does not sustain formation of mTORC2 (36). The behavior of the point mutant Q68H is quite different from Δ192 in that it selectively restores phosphorylation of Akt but not SGK1 (Fig. 3). As addressed further below, these data suggest that mSIN1 plays distinct roles in mTORC2-dependent phosphorylation of SGK1 and Akt (Fig. 5).
The functional importance of the interaction of SGK1 with mSIN1 was also supported by the dominant-negative effect of mSIN1/Q68H on SGK1 HM phosphorylation (Fig. 4) and on ENaC-dependent Na+ transport in kidney epithelial cells (Fig. 6). Based on our previous work and that of others (13, 37, 41, 42), full activation of SGK1 requires mTORC2-dependent HM phosphorylation and PDK1-dependent activation loop phosphorylation (at Thr-256). The fully activated SGK1 then stimulates ENaC-dependent Na+ transport by phosphorylating a variety of targets including the ubiquitin ligase Nedd4-2 as well as c-Raf (19, 21, 25–27) and the channel itself (43, 44). Our present data demonstrate that overexpression of the interaction-deficient mutant disrupts SGK1 phosphorylation and inhibits ENaC-dependent Na+ current, consistent with the idea that it incorporates into mTORC2 but does not sustain recruitment and phosphorylation of SGK1.
In addition to the MEF data described above, several lines of evidence suggest that a different mechanism, not involving mSIN1, underlies mTORC2-mediated regulation of Akt. First, the non-binding mSIN1 mutant showed no effect on binding of Akt to mTORC2 (Fig. 5). Second, although the non-binding mSIN1 mutant displayed a dominant-negative effect on SGK1 HM phosphorylation and ENaC-dependent Na+ transport, no adverse effect on Akt HM phosphorylation, nor on Akt-dependent glucose uptake, was observed (Fig. 5). Finally, in the yeast two-hybrid assay, no physical association between mSIN1 and Akt was detected (data not shown).
Taken together, these data support the hypothesis that mSIN1 plays a dual role in mTORC2 function. On the one hand, it is absolutely required for mTORC2 formation and hence kinase activity toward all substrates; on the other hand, it specifically recruits SGK1, but not Akt, to mTORC2, and this interaction is essential for normal levels of SGK1 phosphorylation. Although the 192-aa region at the N terminus of mSIN1 is implicated in both SGK1 interaction and incorporation into mTORC2, Gln-68 appears to selectively mediate interaction with SGK1. Our data strongly suggest that an mTORC2 component other than mSIN1 mediates interaction with Akt. This conclusion is in contrast to that of Jacinto et al. (10), who suggested that mSIN1 mediates Akt interaction with mTORC2. However, their conclusion was based on co-IP experiments, performed in mSIN1-deficient MEFs, in which mTORC2 formation is compromised. When wild-type mSIN1 was expressed, mTORC2 was reconstituted, and so was co-IP of mSIN1 and Akt. This does not demonstrate direct physical interaction of Akt with mSIN1, but rather supports the conclusion that Akt interacts with mTORC2 and that mSIN1 is necessary for mTORC2 formation. In contrast, our functional and biochemical data indicate that mSIN1 does not directly interact with Akt under conditions in which it demonstrates robust interaction with SGK1 (Fig. 5). It will be of considerable interest to identify the mTORC2 component that mediates physical interaction with Akt.
Interestingly, another mTORC2 component, Protor-1, has also been shown to be required for SGK1 phosphorylation (45). In Protor-1 knock-out mice, SGK1 phosphorylation in the kidney is dramatically reduced. Further work is warranted to delineate the mechanism underlying Protor-1-mediated regulation of SGK1 phosphorylation. Because no physical association between Protor-1 and SGK1 was observed (45), the regulation of SGK1 phosphorylation by Protor-1 may be indirect. It is possible that Protor-1 may impact indirectly on the interaction between mSIN1 and SGK1. In contrast to kidney cells, SGK1 activity appears normal in Protor-1 knock-out MEFs, suggesting cell context-specific regulation of SGK1 by Protor-1 (45).
In summary, our findings show that physical association between SGK1 and mSIN1 is important for mTORC2-mediated phosphorylation of the SGK1 HM and subsequent kinase activation and stimulation of ENaC. It appears that a distinct mechanism is used for HM phosphorylation of Akt. Our findings suggest the interesting possibility that mTOR can selectively associate with its substrates and thereby regulate specific cellular processes in response to various input signals. With respect to mSIN1 in particular, it is appealing to speculate that its interaction with SGK1 is modulated by covalent modification(s), thus altering recruitment to mTORC2 and HM phosphorylation. Selective modification of mSIN1 would in principle have no effect on Akt, which might be controlled by distinct signals that trigger modification of the Akt-recruiting factor. It remains to be determined which protein in mTORC2 interacts with Akt and how this impacts on HM phosphorylation. Identification of the interaction surface(s) and, importantly, the signals that modify them, will provide further insight into the mechanisms underlying the regulation of these important mTOR substrates and the physiological processes they control.
Acknowledgments
We are grateful to Dr. Bing Su for providing the mSIN1-deficient MEFs and to Drs. Kaveh Ashrafi and Kevan Shokat for comments on the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants DK056695 and DK085101 (to D. P.).
- mTOR
- mammalian target of rapamycin
- ENaC
- epithelial sodium channel
- HM
- hydrophobic motif
- MBP
- maltose-binding protein
- MEF
- mouse embryonic fibroblast
- aa
- amino acid(s)
- co-IP
- co- immunoprecipitation.
REFERENCES
- 1. Huang J., Manning B. D. (2009) Biochem. Soc. Trans. 37, 217–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Inoki K., Guan K. L. (2006) Trends Cell Biol. 16, 206–212 [DOI] [PubMed] [Google Scholar]
- 3. Laplante M., Sabatini D. M. (2009) J. Cell Sci. 122, 3589–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wullschleger S., Loewith R., Hall M. N. (2006) Cell 124, 471–484 [DOI] [PubMed] [Google Scholar]
- 5. Yang Q., Guan K. L. (2007) Cell Res. 17, 666–681 [DOI] [PubMed] [Google Scholar]
- 6. Zoncu R., Efeyan A., Sabatini D. M. (2011) Nat. Rev. Mol. Cell Biol. 12, 21–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Holz M. K., Ballif B. A., Gygi S. P., Blenis J. (2005) Cell 123, 569–580 [DOI] [PubMed] [Google Scholar]
- 8. Hara K., Maruki Y., Long X., Yoshino K., Oshiro N., Hidayat S., Tokunaga C., Avruch J., Yonezawa K. (2002) Cell 110, 177–189 [DOI] [PubMed] [Google Scholar]
- 9. Kim D. H., Sarbassov D. D., Ali S. M., King J. E., Latek R. R., Erdjument-Bromage H., Tempst P., Sabatini D. M. (2002) Cell 110, 163–175 [DOI] [PubMed] [Google Scholar]
- 10. Jacinto E., Facchinetti V., Liu D., Soto N., Wei S., Jung S. Y., Huang Q., Qin J., Su B. (2006) Cell 127, 125–137 [DOI] [PubMed] [Google Scholar]
- 11. Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Science 307, 1098–1101 [DOI] [PubMed] [Google Scholar]
- 12. Jacinto E., Loewith R., Schmidt A., Lin S., Rüegg M. A., Hall A., Hall M. N. (2004) Nat. Cell Biol. 6, 1122–1128 [DOI] [PubMed] [Google Scholar]
- 13. Lu M., Wang J., Jones K. T., Ives H. E., Feldman M. E., Yao L. J., Shokat K. M., Ashrafi K., Pearce D. (2010) J. Am. Soc. Nephrol. 21, 811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oh W. J., Wu C. C., Kim S. J., Facchinetti V., Julien L. A., Finlan M., Roux P. P., Su B., Jacinto E. (2010) EMBO J. 29, 3939–3951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vanhaesebroeck B., Alessi D. R. (2000) Biochem. J. 346, 561–576 [PMC free article] [PubMed] [Google Scholar]
- 16. Nojima H., Tokunaga C., Eguchi S., Oshiro N., Hidayat S., Yoshino K., Hara K., Tanaka N., Avruch J., Yonezawa K. (2003) J. Biol. Chem. 278, 15461–15464 [DOI] [PubMed] [Google Scholar]
- 17. Lang F., Cohen P. (2001) Sci. STKE 2001, re17. [DOI] [PubMed] [Google Scholar]
- 18. Chen S. Y., Bhargava A., Mastroberardino L., Meijer O. C., Wang J., Buse P., Firestone G. L., Verrey F., Pearce D. (1999) Proc. Natl. Acad. Sci. U.S.A. 96, 2514–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Debonneville C., Flores S. Y., Kamynina E., Plant P. J., Tauxe C., Thomas M. A., Münster C., Chraïbi A., Pratt J. H., Horisberger J. D., Pearce D., Loffing J., Staub O. (2001) EMBO J. 20, 7052–7059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lang F., Böhmer C., Palmada M., Seebohm G., Strutz-Seebohm N., Vallon V. (2006) Physiol. Rev. 86, 1151–1178 [DOI] [PubMed] [Google Scholar]
- 21. Snyder P. M., Olson D. R., Thomas B. C. (2002) J. Biol. Chem. 277, 5–8 [DOI] [PubMed] [Google Scholar]
- 22. Wulff P., Vallon V., Huang D. Y., Völkl H., Yu F., Richter K., Jansen M., Schlünz M., Klingel K., Loffing J., Kauselmann G., Bösl M. R., Lang F., Kuhl D. (2002) J. Clin. Invest. 110, 1263–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Park J., Leong M. L., Buse P., Maiyar A. C., Firestone G. L., Hemmings B. A. (1999) EMBO J. 18, 3024–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Webster M. K., Goya L., Ge Y., Maiyar A. C., Firestone G. L. (1993) Mol. Cell Biol. 13, 2031–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Soundararajan R., Melters D., Shih I. C., Wang J., Pearce D. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 7804–7809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Soundararajan R., Wang J., Melters D., Pearce D. (2007) J. Biol. Chem. 282, 36303–36313 [DOI] [PubMed] [Google Scholar]
- 27. Soundararajan R., Zhang T. T., Wang J., Vandewalle A., Pearce D. (2005) J. Biol. Chem. 280, 39970–39981 [DOI] [PubMed] [Google Scholar]
- 28. Kamynina E., Staub O. (2002) Am. J. Physiol. Renal Physiol. 283, F377–F387 [DOI] [PubMed] [Google Scholar]
- 29. Bae S. S., Cho H., Mu J., Birnbaum M. J. (2003) J. Biol. Chem. 278, 49530–49536 [DOI] [PubMed] [Google Scholar]
- 30. Cho H., Mu J., Kim J. K., Thorvaldsen J. L., Chu Q., Crenshaw E. B., 3rd, Kaestner K. H., Bartolomei M. S., Shulman G. I., Birnbaum M. J. (2001) Science 292, 1728–1731 [DOI] [PubMed] [Google Scholar]
- 31. Lu M., Holliday L. S., Zhang L., Dunn W. A., Jr., Gluck S. L. (2001) J. Biol. Chem. 276, 30407–30413 [DOI] [PubMed] [Google Scholar]
- 32. Lin-Goerke J. L., Robbins D. J., Burczak J. D. (1997) BioTechniques 23, 409–412 [DOI] [PubMed] [Google Scholar]
- 33. Lu M., Ammar D., Ives H., Albrecht F., Gluck S. L. (2007) J. Biol. Chem. 282, 24495–24503 [DOI] [PubMed] [Google Scholar]
- 34. Bens M., Vallet V., Cluzeaud F., Pascual-Letallec L., Kahn A., Rafestin-Oblin M. E., Rossier B. C., Vandewalle A. (1999) J. Am. Soc. Nephrol. 10, 923–934 [DOI] [PubMed] [Google Scholar]
- 35. Yang Q., Inoki K., Ikenoue T., Guan K. L. (2006) Genes Dev. 20, 2820–2832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frias M. A., Thoreen C. C., Jaffe J. D., Schroder W., Sculley T., Carr S. A., Sabatini D. M. (2006) Curr. Biol. 16, 1865–1870 [DOI] [PubMed] [Google Scholar]
- 37. García-Martínez J. M., Alessi D. R. (2008) Biochem. J. 416, 375–385 [DOI] [PubMed] [Google Scholar]
- 38. Facchinetti V., Ouyang W., Wei H., Soto N., Lazorchak A., Gould C., Lowry C., Newton A. C., Mao Y., Miao R. Q., Sessa W. C., Qin J., Zhang P., Su B., Jacinto E. (2008) EMBO J. 27, 1932–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wang J., Barbry P., Maiyar A. C., Rozansky D. J., Bhargava A., Leong M., Firestone G. L., Pearce D. (2001) Am. J. Physiol. Renal Physiol. 280, F303–F313 [DOI] [PubMed] [Google Scholar]
- 40. Hong F., Larrea M. D., Doughty C., Kwiatkowski D. J., Squillace R., Slingerland J. M. (2008) Mol. Cell 30, 701–711 [DOI] [PubMed] [Google Scholar]
- 41. Biondi R. M., Cheung P. C., Casamayor A., Deak M., Currie R. A., Alessi D. R. (2000) EMBO J. 19, 979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Biondi R. M., Kieloch A., Currie R. A., Deak M., Alessi D. R. (2001) EMBO J. 20, 4380–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Diakov A., Korbmacher C. (2004) J. Biol. Chem. 279, 38134–38142 [DOI] [PubMed] [Google Scholar]
- 44. Yang L. M., Rinke R., Korbmacher C. (2006) J. Biol. Chem. 281, 9859–9868 [DOI] [PubMed] [Google Scholar]
- 45. Pearce L. R., Sommer E. M., Sakamoto K., Wullschleger S., Alessi D. R. (2011) Biochem. J. 436, 169–179 [DOI] [PubMed] [Google Scholar]