Abstract
Smoothened (Smo) is a seven-transmembrane (7-TM) receptor that is essential to most actions of the Hedgehog family of morphogens. We found previously that Smo couples to members of the Gi family of heterotrimeric G proteins, which in some cases are integral although alone insufficient in the activation of Gli transcription factors through Hedgehog signaling. In response to a report that the G12/13 family is relevant to Hedgehog signaling as well, we re-evaluated the coupling of Smo to one member of this family, G13, and investigated the capacity of this and other G proteins to activate one or more of forms of Gli. We found no evidence that Smo couples directly to G13. We found nonetheless that Gα13 and to some extent Gαq and Gα12 are able to effect activation of Gli(s). This capacity is realized in some cells, e.g. C3H10T1/2, MC3T3, and pancreatic cancer cells, but not all cells. The mechanism employed is distinct from that achieved through canonical Hedgehog signaling, as the activation does not involve autocrine signaling or in any other way require active Smo and does not necessarily involve enhanced transcription of Gli1. The activation by Gα13 can be replicated through a Gq/G12/13-coupled receptor, CCKA, and is attenuated by inhibitors of p38 mitogen-activated protein kinase and Tec tyrosine kinases. We posit that G proteins, and perhaps G13 in particular, provide access to Gli that is independent of Smo and that they thus establish a basis for control of at least some forms of Gli-mediated transcription apart from Hedgehogs.
Keywords: 7-Helix Receptor, G Protein-coupled Receptors (GPCR), G Proteins, Heterotrimeric G Proteins, Transcription Regulation, Gli, Smoothened
Introduction
The Hedgehog family of secreted proteins is essential to cell proliferation and differentiation in an array of developmental phenomena. Among the most actively studied of these in vertebrates are induction of ventral cell fates in the central nervous system and patterning of the anterior-posterior axis of the developing limb (1–3). The Hedgehog family also assumes homeostatic roles in postembryonic tissues, for example in the maintenance of certain stem cell populations (4–7). Deficits in one or more components of signaling translate into developmental syndromes and malformations (8, 9), whereas unrepressed signaling underlies several forms of cancer (10–12).
Hedgehogs in mammals exert their actions primarily through modulation of the Gli family of zinc finger transcription factors (13, 14). Here, the seven-transmembrane (7-TM)2 protein Smoothened (Smo) occupies a central position. Hedgehogs activate Smo through binding to Ptch1 (Patched 1), a 12-transmembrane protein at the cell surface that in some fashion normally holds Smo in a repressed conformation. Hedgehogs remove the inhibitory constraints of Ptch1, a process coupled with recruitment of Smo to the primary cilium. Activated Smo stabilizes Gli2 and Gli3, causing derepression of some genes and frank activation of others. Among the latter is that encoding Gli1, the third member of the Gli family.
Most efforts to understand forms of transduction employed by Smo have focused on transport and scaffolding (14). We contend that the interaction of Smo with heterotrimeric G proteins in relation to or apart from modulation of the Gli transcription factors is relevant as well (15–17). We demonstrated in studies with [35S]GTPγS binding in heterologous expression systems that Smo activates members of the Gi family and that one or more forms of Gi are required in the course of Shh (Sonic hedgehog) signaling to Gli in NIH3T3 cells (15). The data regarding coupling of Smo to Gi are in accord with effects of a pertussis toxin on Hedgehog-induced pigment aggregation (18), capillary morphogenesis (19), and (in zebrafish) selected aspects of eye, brain, and somite patterning (20). They are also consistent with the effects of dsRNA-mediated knockdown of Gαi on levels of cAMP and activation of Cubitus interuptus in Drosophila (21).
The coupling of Smo to G proteins evaluated through [35S]GTPγS binding using membranes of Sf9 (a clonal cell line derived from Spodoptera frugiperda) cells expressing these proteins was specific for members of the Gi family. Smo did not, in these studies, affect activation of Gs, Gq, G12, or G13. We were interested therefore in a report by Kasai et al. (22) that the activation of Gli in HEK293 cells by Shh is inhibited by the RGS (regulator of G protein signaling) domain of p115 RhoGEF, a domain that can inhibit G12 and G13 signaling, and by inhibitors of Rho, a monomeric G protein downstream of G12 and G13. G12 and G13 have received considerable attention in a number of phenomena relevant to developmental and oncogenic events (23).
The relevance of the G12/13 family to modulation of Gli activity by and apart from Smo was explored in this study. We find no evidence for the coupling of Smo to G12 or G13 in HEK293 cells in affirmation of our previous work with insect cells. We find, nevertheless, that the α subunit of G13, and to some extent those of Gq and G12, are capable of activating one or more forms of Gli. The activation does not involve autocrine signaling, occurs in some but not all cells, and can be recapitulated by a 7-TM receptor coupled to endogenous Gq, G12, and G13. It is attenuated by inhibitors of p38 mitogen-activated protein kinase and Tec tyrosine kinases. We posit that G13 and to some extent Gq and G12 provide an access to Gli that is independent of Smo and thus a basis for control of at least some forms of Gli transcription apart from Hedgehogs.
EXPERIMENTAL PROCEDURES
Materials
Cyclopamine, purmorphamine, KN-92, KN-93, SP600125, Y27632, diltiazam, nifedipine, and verapamil were obtained from EMD Biosciences (San Diego, CA). 8-Hydroxy-2-(dipropylamino)tetralin hydrobromide (DPAT), pertussis toxin, and a rabbit antibody specific for actin was obtained from Sigma-Aldrich. Transforming growth factor-β and a goat antibody specific for Gli1 were obtained from R&D Systems (Minneapolis, MN). SB202190 and LFM-A13 were obtained from Tocris Bioscience (Ellisville, MO). 5-Fluoro-2-indolyl des-chlorohalopemide, 9,11-dideoxy-9α,11α-methanoepoxy-prosta-5Z,13E-dien-1-oic acid (U46619), and halopemide were obtained from Cayman Chemicals (Ann Arbor, MI). Cholecystokinin-8 (CCK-8) was obtained from Peninsula Laboratories (Belmont, CA). [35S]GTPγS was purchased from PerkinElmer Life Sciences. Rabbit antisera specific for Gα subunits were described previously (24).
Plasmid Constructs
Expression constructs for constitutively active Gα subunits (in which leucine is substituted for glutamine in the DVGGQ motif) were obtained from the Missouri cDNA Resource Center (Rolla, MO) and Dr. Silvio Gutkind (National Institute of Dental and Craniofacial Research, National Institutes of Health, Bethesda, MD). Vectors for SmoWT, SmoM2, and Shh were provided by Dr. Philip Beachy (Stanford University, Palo Alto, CA). Constructs for mouse Gli2 and both normal and mutated 8xGli luciferase reporters were obtained from Dr. Hiroshi Sasaki (RIKEN Center for Developmental Biology, Kobe, Japan) and, for experiments with pancreatic cells, Dr. Chi-chung Hui (Research Institute, Toronto, Ontario, Canada). The Bcl-2 promoter luciferase reporter was provided by Dr. Linda Boxer (Stanford University). TPα-Gα13 was constructed as described previously (25). Smo-Gα13 was constructed in a similar fashion, i.e. by PCR-directed mutagenesis, using mouse Smo cDNA; the sequence was confirmed by automated sequencing. The sequence of the fusion immediately distal to the 7-TM domain of Smo was 550RRTWCRLTGHSDDEPKR566/2ADFLPSRSVL11 (Smo/Gα13). shRNA was designed as described previously (26). The following shGli1-targeted sequences were used: CCGTCCTGCTCCAGCTAGAttcaagagaTCTAGCTGGAGCAGGACGG (shGli1, sense), CCGTCCTGCTCCAGCTAGAtctcttgaaTCTAGCTGGAGCAGGACGG (shGli1, antisense), CCTCGCCATTCTGCACCATttcaagagaATGGTGCAGAATGGCGAGG (scrambled, sense), CCTCGCCATTCTGCACCATtctcttgaaATGGTGCAGAATGGCGAGG (scrambled, antisense); capital letters represent the target sequences of the shRNA.
Cell Culture and Transfection
C3H10T1/2 cells (CCL-266), MC3T3 cells (CRL-2593), and HEK293 (CRL-1573) were obtained from the American Type Culture Collection (Manassas, VA). C3H10T1/2 cells were maintained in basal medium Eagle (Sigma) supplemented with 10% heat-inactivated fetal bovine serum. MC3T3 were maintained in minimal essential medium α supplemented with l-glutamine, ribonucleosides, and deoxyribonucleosides, without ascorbic acid (Invitrogen). HEK293 cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum added. All cell lines were grown at 37 °C with 5% CO2. For transfection, C3H10T1/2 and MC3T3 cells were seeded at 1.5 × 104 cells/well in 24-well plates and transfected with 0.1 μg firefly reporter, 0.01 μg TK Renilla reporter, and 0.13 μg construct DNA per well using FuGENE 6 transfection reagent (Roche Applied Science) according to the manufacturer's suggested protocol. HEK293 cells were seeded at 3.5 × 104 cells/well and transfected with 0.17 μg 8xGli reporter, 0.017 μg TK Renilla reporter, and 0.2 μg of construct DNA per well using Lipofectamine 2000 (Invitrogen). Serum was usually decreased to 0.5% upon attainment of confluence, and production of luciferases was assayed 24–36 h thereafter. Where noted, pertussis toxin (100 ng/ml) was added at the time serum was decreased; enzyme and channel inhibitors were added as noted 24 h prior to assay. Luciferase activities were determined using the Dual-Luciferase Reporter Assay (Promega, Madison, WI). Firefly luciferase activity was normalized to Renilla luciferase activity.
The human pancreatic cancer cell lines PANC1 (CRL-1469) and AsPC1 (CRL-1682) and the rat pancreatic acinar cell line AR42J (CRL-1492) were obtained from the American Type Culture Collection. The human cancer cell line L3.6 was kindly provided by Dr. Isaiah Fidler (University of Texas M.D. Anderson Cancer Center, Houston, TX). PANC1, AsPC1, and AR42J cells were maintained in Dulbecco's modified Eagle's medium with 10% fetal bovine serum and 100 units/ml penicillin/100 units/ml streptomycin. L3.6 cells were grown in Dulbecco's modified Eagle's medium, supplemented with 10% fetal bovine serum sodium pyruvate, nonessential amino acids, l-glutamine, and a 2-fold vitamin solution (LifeTechnologies, Rockville, MD). All pancreatic cells but PANC1 cells were transfected by electroporation at 350 V using a 10-ms pulse; PANC1 cells were electroporated at 260 V using two 10-ms pulses (BTX, Harvard Apparatus, Holliston, MA); 4 × 106 cells were placed in each cuvette together with 2 μg of firefly luciferase reporter. Where noted, 5 μg hCCKA or Gα13QL were additionally included. After electroporation, 2.5 × 105 cells were seeded into each well of a six-well plate for luciferase assays, whereas the remaining cells were plated on a 10-cm plate for expression controls. Cells were harvested and prepared for luciferase assays. To control for intersample variations in transfection efficiency, total protein for samples on each plate was quantitated using Bio-Rad protein assay (Bio-Rad), and luciferase readouts were normalized to protein content. For knockdown of expression, 18 μg of shGli1 or scrambled shRNA were used.
[35S]GTPγS Binding
The assay for receptor-promoted binding of [35S]GTPγS to Gα subunits was performed as described previously (27). Membranes (20 μg protein) were incubated with vehicle or agonist for 10 min at 30 °C then additionally with 5 nm [35S]GTPγS for another 10 min prior to solubilization and immunoprecipitation with Gα subunit-directed antibodies and scintillation spectrometry.
RESULTS
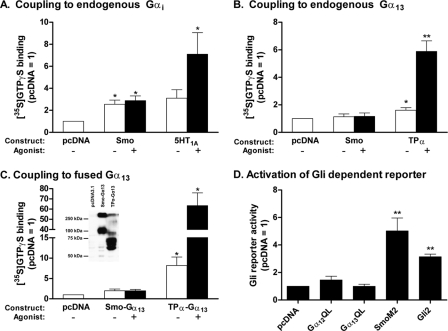
We and others (15, 18, 19, 21) demonstrated the capacity of Smo to couple to the Gi family of G proteins. Our data regarding specificity, wherein Smo was deemed unable to couple to Gs, Gq, G12, or G13, were based on a reconstitution paradigm using membranes from Sf9 cells (15). Kasai et al. (22), however, have argued the involvement of G12 and/or G13 using HEK293 cells, noting inhibition of an Shh-activated Gli reporter by the RGS domain of p115 RhoGEF. Because processing and/or targeting of Smo might differ between Sf9 and HEK293 cells, and because activation of G12 and/or G13 in the latter report was not measured directly, we evaluated the activation of the two G proteins in HEK293 cells. Smo was introduced into the cells by transfection, and activation was evaluated in subsequently isolated membranes by [35S]GTPγS binding to selected Gα subunits. As shown in Fig. 1A, Smo promoted the binding of [35S]GTPγS to the one or more forms of Gαi endogenous to HEK293 cells, as anticipated. The activity of Smo without agonist was equivalent to that with agonist (purmorphamine). The lack of agonist-promoted activity is expected for cells not expressing Ptch1 or in which levels of Smo otherwise exceed the repressive actions of Ptch1. The 5-HT1A receptor was used for comparison. In contrast to the data for Gi, no activation of G13 by Smo was evident with or without agonist (Fig. 1B) despite the activation noted for the thromboxane A2 receptor TPα used as a positive control (25). We also evaluated activation of Gα13 by means of a Smo-Gα13 fusion protein. Fusion proteins permit quantitation of relative protein expression by means of Gα-specific antibodies. Moreover, the strength of coupling between receptor and subunit is often amplified by proximity of the two proteins (25). The expression of Smo-Gα13 and TPα-Gα13 introduced into HEK293 cells was similar (Fig. 1C, inset). However, although the activation of Gα13 by TPα within the fusion protein was clearly evident (see Fig. 3C), and 5–10-fold above that noted for the independently expressed proteins, no activation of Gα13 by Smo within the fusion protein was observed. The negative data for Smo-Gα13 were not the result of a problematic conformation with truncated and fused Smo, as Gαi1 within a Smo-Gαi1 fusion protein exhibited substantial activation.3 Activation of G12 was not evident for either Smo or TPα, despite the fact TPα has an easily measured capacity to activate G12 in other settings (25). We take these data to indicate that G12 is not expressed to any appreciable level in HEK293 cells. Our data confirm the activation of Gi by Smo but rule out that of G12 and G13 in these cells.
FIGURE 1.
Smoothened does not couple to Gα13 and does not activate Gli(s) in HEK293 cells. A, HEK293 cells were transfected with vector for Smo or the 5-HT1A receptor or with empty vector (pcDNA). At 48 h, membranes were isolated and [35S]GTPγS binding to endogenous Gαi was evaluated with or without 10 μm purmorphamine (Smo) or 1 μm 8-OH-DPAT (5-HT1A receptor). [35S]GTPγS binding is normalized to levels obtained with empty vector and represents four to five individual experiments performed in triplicate. B, HEK293 cells were transfected with vector for Smo or TPα, or with empty vector, and [35S]GTPγS binding to endogenous Gα13 was evaluated as above with or without 10 μm purmorphamine (Smo) or U46619 (TPα). The data represent four individual experiments performed in triplicate. C, HEK293 cells were transfected with vector for Smo-Gα13 or TPα-Gα13, or with empty vector, and [35S]GTPγS binding was evaluated with or without 10 μm purmorphamine (Smo) or U46619 (TPα). The data represent four individual experiments performed in triplicate. Inset, Western blot showing the relative expression levels of receptor·Gα fusion proteins present in membranes using a Gα13-directed antibody. Molecular weights inferred from cDNA for Smo-Gα13 and TPα-Gα13 are 103,100 and 82,700, respectively; heterogeneity in banding is presumably due to oligomerization, varying degrees of glycosylation, and/or multiple initiation sites. D, HEK293 cells were transfected with vector for constitutively active Gα12 or Gα13 (QL mutants), for SmoM2 or Gli2, or with empty vector (pcDNA), together with 8xGli firefly and TK Renilla luciferase reporters. At 24 h, when cells reached confluence, the serum was lowered to 0.5%. Luciferase activities were assayed 24 h later. The data are means of ratios of firefly/Renilla activities normalized to those obtained for pcDNA from six independent experiments carried out in triplicate. For all panels in this figure, significance was determined using paired t tests. *, p < 0.05 and **, p < 0.01.
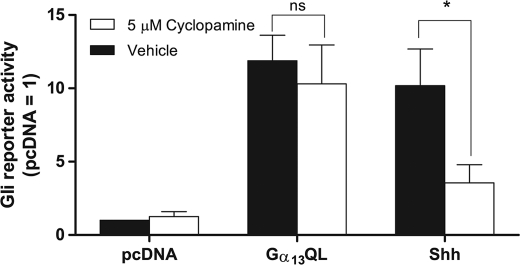
FIGURE 3.
Gα13QL activation of Gli(s) is independent of Smo. C3H10T1/2 cells were transfected with vector for constitutively active Gα13 or Shh, or empty vector, together with 8xGli firefly and TK Renilla luciferase reporters. When cells reached confluence, the serum was lowered to 0.5% and supplemented with either 5 μm cyclopamine or vehicle for 24 h. The data are means of ratios of firefly/Renilla activities normalized to those obtained for pcDNA without treatment from four independent experiments carried out in triplicate. Differences were evaluated using paired t tests. *, p < 0.05; ns, not significant.
We also examined the ability of Gα12 and Gα13 to activate one or more forms of Gli in HEK293 cells. GTPase-deficient (constitutively active) Gα subunits were introduced into the cells by means of transfection together with a reporter containing an octomeric repeat of the Gli recognition sequence (5′-GAACACCCA). Constitutively active Smo (SmoM2) and Gli2 were used as positive controls. No activation of the reporter was detected in the cells expressing either Gα12QL or Gα13QL (Fig. 1D) despite activation noted for SmoM2 and Gli2. Similar data were obtained with NIH3T3 cells (data not shown). The inability of Gα12QL and Gα13QL to activate the Gli reporter in these cells was not due to poor Gα expression as both subunits activated a serum response factor (SRF) reporter in these cells (data not shown). Although the negative data for Gli reporter activation are not necessarily inconsistent with a role for the Gα subunits in activation of Gli transcription factors, they preclude sufficiency of the subunits in these cells.
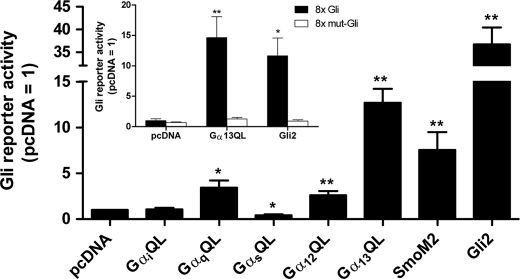
In extending experiments with the GαQL mutants to another commonly employed model of Hedgehog signaling, C3H10T1/2 cells, we found that the subunits are not always without effect on Gli activity. Substantial activation of the Gli reporter was noted for Gα13QL in these cells (Fig. 2); activation was equivalent to that of SmoM2. Activation was achieved to some degree as well by GαqQL and Gα12QL, whereas reporter activity was suppressed by GαsQL. The activation by Gα13QL was specific for the Gli recognition sequence, as no activation was achieved for a reporter in which the octomeric recognition sequences were mutated (5-GAAGTGGGA; Fig. 2, inset). Thus, in contrast to HEK293 and NIH3T3 cells, one or more forms of Gli in C3H10T1/2 cells are responsive to Gα subunits, with activation by Gα13 the most substantive.
FIGURE 2.
Gα13QL activates one or more forms of Gli in C3H10T1/2 cells. C3H10T1/2 cells were transfected with vectors for constitutively active Gα family members (QL mutants), SmoM2 or Gli2, or empty vector, together with 8xGli firefly and TK Renilla luciferase reporters. At 48 h, when cells had reached confluence, the serum was lowered to 0.5%. Luciferase activities were assayed 24 h later. The data are means of firefly/Renilla activities normalized to that obtained for pcDNA from three to 36 independent experiments carried out in triplicate. Inset, experiments were carried out in the same manner, but comparing normal with mutated 8xGli reporter plasmids. Values are means of six independent experiments carried out in triplicate. Differences from pcDNA were evaluated using paired t tests. *, p < 0.05, and **, p < 0.01.
To address the possibility that Gα13 in C3H10T1/2 cells operates through production of Shh or some other Hedgehog, i.e. that it proceeds through autocrine signaling, we employed KAAD-cyclopamine, which as an inverse agonist for Smo not only inhibits the actions of Hedgehogs but decreases Smo constitutive activity (15). We found in C3H10T1/2 cells that KAAD-cyclopamine inhibits the actions of Shh but fails to block those of Gα13QL (Fig. 3). This result demonstrates that Gα13QL acts downstream or entirely apart from pathways engaged by Smo.
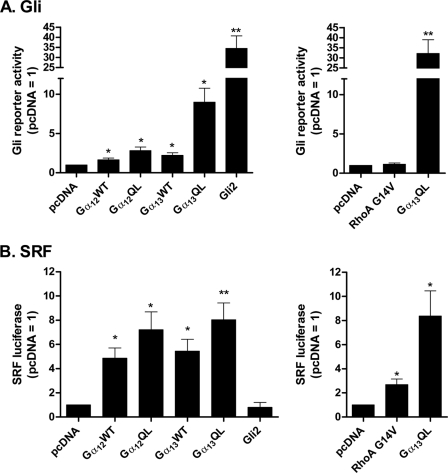
The previous experiments employed constitutively active Gα subunits. Wild type Gα12 and Gα13 were also found to promote activity of the Gli reporter (Fig. 4A), albeit the actions of these (and again Gα12QL) are considerably less than that of Gα13QL. We compared the activities of these subunits in relation to the SRF reporter gene as well (Fig. 4B). Whereas activities among the subunits toward Gli differed considerably, those toward SRF did not. These data suggest that Gli(s) is more sensitive to the nature of the subunit than SRF. Also depicted in Fig. 4 are data that show a GTPase-deficient form of RhoA (RhoA(G14V)), though activating SRF, is unable to activate Gli.
FIGURE 4.
Activation of Gli(s) is most evident for Gα13QL among variants of the G12/13 family. A, C3H10T1/2 cells were transfected with (left) empty vector or vectors for wild type or constitutively active Gα12 or Gα13, or Gli2, or (right) with empty vector or constitutively active RhoA (G14V) or Gα13QL, as well as with 8xGli firefly and TK Renilla luciferase reporters. B, C3H10T1/2 cells were transfected as in A, except substituting an SRF reporter for the 8xGli firefly reporter. The data are means of ratios of firefly/Renilla activities normalized to those obtained for pcDNA from four or five independent experiments carried out in triplicate. Differences from pcDNA were evaluated using paired t tests. *, p < 0.05; **, p < 0.01.
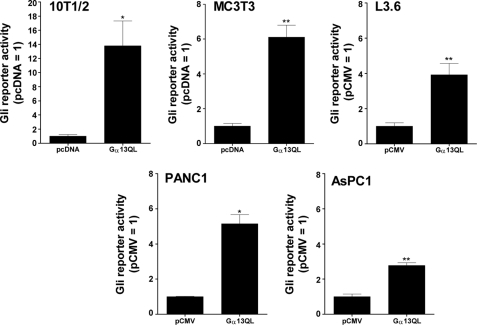
We evaluated the activation of the Gli reporter by Gα13QL in several other cells as well. C3H10T1/2 cells are equivalent to mesenchymal stem cells (28), therefore we examined MC3T3 and C2C12 cells, which reside in osteoblastic and myogenic lineages, respectively. Activation of the reporter by Gα13QL was noted in MC3T3 (Fig. 5) but not C2C12 cells (data not shown). We also examined human pancreatic cancer cell lines, for which Smo-independent forms of Gli activation have been reported (29–31). Activation of the reporter was noted in all the cells examined, i.e. L3.6, PANC1, and AsPC1 cells. These results do not constitute an extensive survey of course; however, they support the notion that the activity of Gα13QL toward Gli, although not universal, is not uncommon.
FIGURE 5.
Gα13QL activates Gli(s) in cells beyond C3H10T1/2 cells. C3H10T1/2 and MC3T3 cells were transfected with Gα13QL, or empty vector (pcDNA), together with reporter genes and assayed as described in the legend to Fig. 2. The data represent means from six independent experiments carried out in triplicate. L3.6, PANC1, and ASPC1 cells were transfected by electroporation. Data are means of ratios of luciferase to total cell protein normalized to that for the empty vector (pCMV) from three or four experiments carried out in triplicate. Differences from pcDNA or pCMV were evaluated using paired t tests. *, p < 0.05; **, p < 0.01.
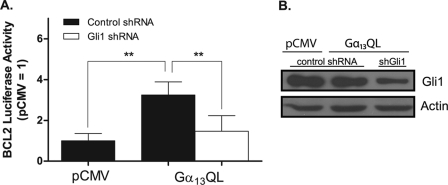
We also evaluated the actions of Gα13 in the context of the bcl-2 promoter, which contains three Gli-binding sites and mediates up-regulation of bcl-2 by Gli in settings of cell survival (32, 33). We used L3.6 cells in these experiments, as the electroporation protocol devised for them represents an efficient means of introducing DNA and monitoring sequellae for a large population of cells. Gα13QL activates the reporter containing the bcl-2 promoter (Fig. 6A, filled bars), as it does that bearing the concatameric Gli recognition sequences (see above). The activation is inhibited by Gli1-targeted shRNA (open bars). Panel B shows that Gli1 is expressed in L3.6 cells, does not increase in response to Gα13QL, and is reduced by ∼70% by the targeted shRNA. These data demonstrate that the bcl-2 promoter is activated by Gα13QL and that the activation requires Gli1 but is not attributable to an increase in this transcription factor.
FIGURE 6.
Activation of the bcl-2 promoter by Gα13QL requires Gli1. A, L3.6 cells were transfected with Gα13QL, or empty vector (pCMV), together with a luciferase reporter containing the bcl-2 promoter and either a control (scrambled) or Gli1-targeted shRNA. 36 h after transfection, cells were harvested. Data are means of luciferase to protein ratios normalized to that of vector alone for four experiments carried out in triplicate. Statistical differences were calculated using paired t tests. **, p < 0.01. B, L3.6 cells were transfected with Gα13QL, or empty vector (pCMV), together with control (scrambled) or Gli1-targeted shRNA. Levels of Gli1 were evaluated at 36 h by means of Western blotting. Shown is one of two experiments with identical results.
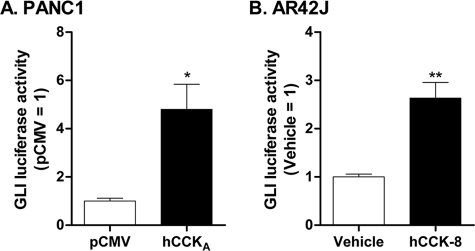
CCKA is a Gq- and G12/13-coupled receptor for cholecystokinin (34, 35). To evaluate activation of Gli in response to the activity of such a receptor, we turned to PANC1 cells in which CCKA was introduced through transfection. Activation of the Gli reporter was found to occur in this setting (Fig. 7A), ostensibly through receptor-constitutive activity. We turned as well to the pancreatic AR42J acinar cell line, in which CCKA is expressed normally. We found that treatment of AR42J cells with the agonist CCK-8 effects activation of the Gli reporter. Thus, the activation of Gli(s) occurs not only in response to introduced wild type and constitutively active Gα subunits, but to a 7-TM receptor (other than Smo) as well.
FIGURE 7.
CCKA activates Gli(s). A, PANC1 cells were transfected with CCKA, or empty vector (pCMV), together with the 8xGli reporter gene as described in the legend to Fig. 5. Data are means of luciferase to protein ratios normalized to those of pCMV for four independent experiments carried out in triplicate. The difference from pCMV was evaluated using a paired t test. *, p < 0.05. B, AR42J cells were transfected with the same reporter gene. After 36 h, medium was replaced with serum-free medium supplemented, or not (vehicle), with 100 nm CCK-8. Luciferase activity and total protein were assayed 12 h later. Data are means of ratios of luciferase/protein normalized to that for pCMV from six independent experiments carried out in triplicate. Difference from vehicle was evaluated using a paired t test. *, p < 0.05.
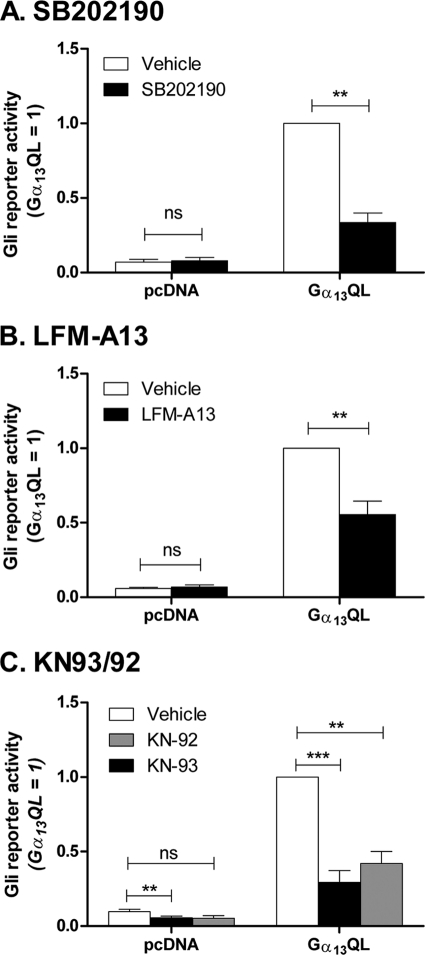
The mechanism by which G13 activates Gli remains to be determined. Studies with C3 exotoxin and Rho-targeted siRNA in C3H10T1/2 cells offer no remarkable insight, nor do inhibitors of Rho kinase (Y27632), c-Jun N-terminal kinases (SP600125), phospholipase D2 (halopemide, 5-fluoro-2-indolyl des-chlorohalopemide), and the protein tyrosine kinase PYK2 (dantrolene). We find that β-catenin, which is sometimes an effector for G13 (36, 37) and is cited to be a mediator of TGF-β activation of Gli2 (38), is not activated by Gα13 nor able to activate Gli in these cells. Inhibitors of p38(s) (SB202190) and Tec tyrosine kinases (LFM-A13) inhibit Gα13-stimulated Gli activity by 40–60% (Fig. 8); however, additional work will be required to evaluate the exact identity of the targeted proteins. KN-93, an inhibitor of Ca2+/calmodulin kinase II (39), another target for Gα13, has a substantial effect on Gli reporter activity, but so does KN-92, an analog of KN-93 having no activity toward the kinase. Both KN92 and KN93 inhibit l-type Ca2+ channels (40), among other targets (41–43); however, additional inhibitors of these channels, i.e. nifedipine, verapamil, and diltiazam, are without effect on Gα13QL-stimulated Gli reporter activity.
FIGURE 8.
Activation of the Gli reporter by Gα13QL can be inhibited by SB202190, LFM-A13, and KN compounds. C3H10T1/2 cells were transfected with Gα13QL or empty vector (pcDNA), together with 8xGli firefly or TK Renilla luciferase reporters. 24 h after transfection, the serum was lowered to 0.5% and supplemented with 10 μm SB202190 or vehicle (A); 100 μm LFM-A13 or vehicle (B); or 5 μm KN-93, 5 μm KN-92, or vehicle (C). Luciferase activities were assayed 24 h later. The data are means of ratios of firefly/Renilla activities normalized for Gα13QL without treatment from three to eight independent experiments carried out in triplicate. Significance was determined using paired t tests. **, p < 0.01; ***, p < 0.001; ns, not significant.
DISCUSSION
We demonstrate here the capacity of the α subunit of the heterotrimeric G protein G13, and of Gq and G12 as well, to activate one or more members of the Gli family of transcription factors. The mechanism employed by Gα13 is distinct from that engaged through canonical Hedgehog signaling: the activation does not require Smo, nor does it necessarily involve an increase in Gli1. We demonstrate as well that while activation of Gli(s) can be achieved through a receptor (CCKA) coupled to G13 Smo is not one such receptor. We posit that G13, and to some extent Gq and G12, serve as a means of increasing Gli transcriptional activity in a fashion altogether independent of Hedgehogs.
We were unable to corroborate the arguments of Kasai et al. (22) that the α subunit of G12 or G13 plays a significant role in signaling through Smo in HEK293 cells. Smo does not activate G12 or G13 directly in these cells, as it is unable to promote exchange of GDP for [35S]GTPγS on any Gα subunit endogenous to them except those of the Gi family. Smo is also unable to activate Gα13 to which it is fused for the purposes of amplification. Our data regarding specificity for the Gi family are in agreement with those obtained by us previously for Sf9 cells made to express Smo and individual G proteins (15). It is conceivable that G13 can be activated indirectly by Smoothened, however the specific events by which this might occur are unclear. We were also unable to demonstrate an effect of Gα13, or of Gα12, on Gli activity in these cells. Kasai et al. (22) used a reporter under control of the genomic sequence of the gli1 promoter region (−397 to +216), with an intended selectivity for Gli3 activity. Our reporter was a concatenated set of GAACACCCA sequences, which is responsive to Gli1, Gli2, and conceivably Gli3. The difference in results may therefore relate to Gli1/Gli2 versus Gli3. A concern with the genomic sequence, however, is the possibility of changes in reporter activity apart from Gli-binding elements. Our studies using mutation of the concatenated reporter and Gli1-targeted shRNA preclude this kind of error.
Most of our initial work beyond HEK293 cells focused on C3H10T1/2 cells. These cells originate from an early mouse embryo and, akin to mesenchymal stem cells with which they are equated, can differentiate into osteoblasts, chondrocytes, adipocytes, and myoblasts (28). Gα13 promoted activation of Gli in C3H10T1/2 cells to an extent comparable with that achieved by an oncogenic form of Smo, SmoM2, and greater than that achieved by Gαq and Gα12. Although relative activities among subunits await normalization of expression, we note that virtually all receptors coupled to G13 and/or G12 couple to Gq as well and that the replication or coordination of activities is not uncommon (35).
Distinctions among Gli1, Gli2, and Gli3 as the transcription factors targeted by Gα subunits, and the mechanism by which the subunits elicit activation of one or more of them, remain to be investigated. We suspect from our work with Gα13 in PANC1 cells that the activation is selective for Gli1, as activation of the bcl-2 promoter requires Gli1, and expression of Gli1 does not increase with Gα13, consistent with absence of input from Gli2 and Gli3. The latter observation is also a departure from what is observed with Hedgehog signaling (16).
Defining the path by which G13 activates Gli1 will, of course, be key to understanding the selectivity of activation among cells. RhoA is often utilized by G13; however, we find no evidence that it is employed in the context of Gli activation. β-catenin and TGF-β, too, are without effect, implying that any Gα13-initiated TGF-β autocrine loop is irrelevant. Other targets for G13 have been evaluated, with p38 and Tec tyrosine kinase offering promise; however, the list of targets to be evaluated is far from complete.
Activation of Gli transcription factors has long been thought to be the domain of Hedgehogs alone, but additional reports have challenged this notion. TGF-β controls the expression of Gli1 and Gli2 in a variety of cells independently of Hedgehogs through Smad-dependent processes (31, 38, 44). The transcriptional nature of regulation of Gli2 in the case of TGF-β, owing to SMAD and lymphoid enhancer factor/T cell factor binding elements in the promoter region of gli2 (38), departs from the mechanism by which Hedgehogs activate Gli2, which involves inhibition of Gli2 degradation (45). Oncogenic K-Ras activates Gli1 in a variety of cells independent of Hedgehogs as well. Mechanisms involve an increase in Gli1 expression (29, 46) and facilitated translocation of Gli1 to the nucleus (46). The absence of an increase in Gli1 expression with Gα13 is a clear distinction from these reports.
Heterotrimeric G proteins can confer to agonists beyond Hedgehogs the potential to increase Gli activity. We believe this notion is exemplified by CCK. The nature and scope of signaling by such agonists will certainly differ from that by Hedgehogs. Beyond probable differences in selectivity among Gli transcription factors, agonists will certainly engage transcription factors beyond the Gli family altogether (23). We therefore anticipate that genes activated in response to agonists operating through G proteins overlap but are not identical to those activated in response to Hedgehogs.
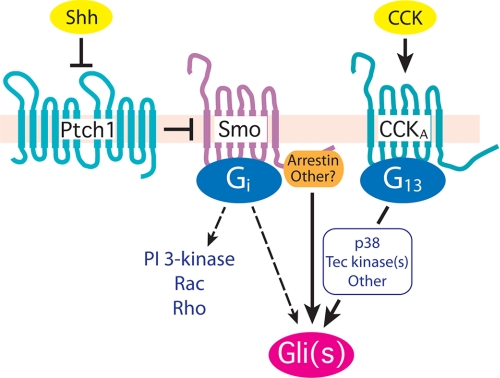
The evolving relationship of signaling by Hedgehogs and heterotrimeric G proteins is notable (Fig. 9). We have demonstrated here and previously (15) that Smo couples to members of the Gi family. The coupling to Gi is relevant in NIH3T3 cells to the activation by Hedgehogs of Gli transcription factors (15) and is germane as well, we believe, to many of the actions exerted through Smo that are presumed or known to be non-genomic in nature (17, 47–49). G13 does not serve in the same capacity, as G13 does not directly couple to Smo. Yet, G13 has an impact on Gli-mediated transcription and hence at least a subset of the events controlled through Smo.
FIGURE 9.
Activation of Gli through 7-TM receptors. The activation of Gli through Smo is initiated by the binding of Shh to Ptch1 to attenuate Ptch1 repression of Smo. Smo is translocated to the primary cilium through an arrestin-dependent event (50) where it engages one or more forms of Gli by still poorly understood events. Smo is a Gi-coupled receptor, and in some instances (dashed line) activation of Gli requires Gi (15). Gi also initiates noncanonical forms of signaling through activation of phosphatidylinositol 3-kinase, Rac, and/or Rho (17). The activation of one or more forms of Gli can also be achieved through 7-TM receptors other than Smo, represented by CCKA. CCKA couples to G13, and to Gq and G12 as well (not shown). Activated G13 appears to work in part through p38 and a Tec tyrosine family member.
Supplementary Material
Acknowledgments
We are indebted to Cherisse DiLizio and Kimberly Aranda for technical assistance.
This work was supported by National Institutes of Health Grant GM080396, Mayo Clinic Cancer Center Grant CA136526, Mayo Clinic Pancreatic SPORE Grant P50 CA102701, and Mayo Clinic Center for Cell Signaling in Gastroenterology Grant P30 DK084567 from the NIDDK.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1.
F. Shen, A. E. Douglas, and D. R. Manning, manuscript in preparation.
- TM
- transmembrane
- 5-HT
- 5-hydroxytryptamine
- CCK
- cholecystokinin
- CCK-8
- the C-terminal octapeptide of CCK
- DPAT
- 8-hydroxy-2-(dipropylamino)tetralin hydrobromide
- GTPγS
- guanosine 5′-(3-O-thio)triphosphate
- SRF
- serum response factor
- TK
- thymidine kinase
- U46619
- 9,11-dideoxy-9α,11α-methanoepoxy-prosta-5Z,13E-dien-1-oic acid.
REFERENCES
- 1. Ingham P. W., McMahon A. P. (2001) Genes Dev. 15, 3059–3087 [DOI] [PubMed] [Google Scholar]
- 2. Fuccillo M., Joyner A. L., Fishell G. (2006) Nat. Rev. Neurosci. 7, 772–783 [DOI] [PubMed] [Google Scholar]
- 3. Varjosalo M., Taipale J. (2008) Genes Dev. 22, 2454–2472 [DOI] [PubMed] [Google Scholar]
- 4. Campbell C., Risueno R. M., Salati S., Guezguez B., Bhatia M. (2008) Curr. Opin. Hematol. 15, 319–325 [DOI] [PubMed] [Google Scholar]
- 5. Palma V., Lim D. A., Dahmane N., Sánchez P., Brionne T. C., Herzberg C. D., Gitton Y., Carleton A., Alvarez-Buylla A., Ruiz i Altaba A. (2005) Development 132, 335–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ahn S., Joyner A. L. (2005) Nature 437, 894–897 [DOI] [PubMed] [Google Scholar]
- 7. Balordi F., Fishell G. (2007) J. Neurosci. 27, 5936–5947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ruiz i Altaba A. (1999) Trends Genet. 15, 418–425 [DOI] [PubMed] [Google Scholar]
- 9. Dellovade T., Romer J. T., Curran T., Rubin L. L. (2006) Annu. Rev. Neurosci. 29, 539–563 [DOI] [PubMed] [Google Scholar]
- 10. Taipale J., Beachy P. A. (2001) Nature 411, 349–354 [DOI] [PubMed] [Google Scholar]
- 11. Kasper M., Regl G., Frischauf A. M., Aberger F. (2006) Eur. J. Cancer 42, 437–445 [DOI] [PubMed] [Google Scholar]
- 12. Stecca B., Ruiz I., Altaba A. (2010) J. Mol. Cell Biol. 2, 84–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jiang J., Hui C. C. (2008) Dev. Cell 15, 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson C. W., Chuang P. T. (2010) Development 137, 2079–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riobo N. A., Saucy B., Dilizio C., Manning D. R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 12607–12612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riobo N. A., Manning D. R. (2007) Biochem. J. 403, 369–379 [DOI] [PubMed] [Google Scholar]
- 17. Polizio A. H., Chinchilla P., Chen X., Kim S., Manning D. R., Riobo N. A. (2011) J. Biol. Chem. 286, 19589–19596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DeCamp D. L., Thompson T. M., de Sauvage F. J., Lerner M. R. (2000) J. Biol. Chem. 275, 26322–26327 [DOI] [PubMed] [Google Scholar]
- 19. Kanda S., Mochizuki Y., Suematsu T., Miyata Y., Nomata K., Kanetake H. (2003) J. Biol. Chem. 278, 8244–8249 [DOI] [PubMed] [Google Scholar]
- 20. Hammerschmidt M., McMahon A. P. (1998) Dev. Biol. 194, 166–171 [DOI] [PubMed] [Google Scholar]
- 21. Ogden S. K., Fei D. L., Schilling N. S., Ahmed Y. F., Hwa J., Robbins D. J. (2008) Nature 456, 967–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kasai K., Takahashi M., Osumi N., Sinnarajah S., Takeo T., Ikeda H., Kehrl J. H., Itoh G., Arnheiter H. (2004) Genes Cells 9, 49–58 [DOI] [PubMed] [Google Scholar]
- 23. Kelly P., Casey P. J., Meigs T. E. (2007) Biochemistry 46, 6677–6687 [DOI] [PubMed] [Google Scholar]
- 24. Butkerait P., Zheng Y., Hallak H., Graham T. E., Miller H. A., Burris K. D., Molinoff P. B., Manning D. R. (1995) J. Biol. Chem. 270, 18691–18699 [DOI] [PubMed] [Google Scholar]
- 25. Zhang L., DiLizio C., Kim D., Smyth E. M., Manning D. R. (2006) Mol. Pharmacol. 69, 1433–1440 [DOI] [PubMed] [Google Scholar]
- 26. Fernandez-Zapico M. E., Gonzalez-Paz N. C., Weiss E., Savoy D. N., Molina J. R., Fonseca R., Smyrk T. C., Chari S. T., Urrutia R., Billadeau D. D. (2005) Cancer Cell 7, 39–49 [DOI] [PubMed] [Google Scholar]
- 27. Windh R. T., Lee M. J., Hla T., An S., Barr A. J., Manning D. R. (1999) J. Biol. Chem. 274, 27351–27358 [DOI] [PubMed] [Google Scholar]
- 28. Yamaguchi A., Komori T., Suda T. (2000) Endocr. Rev. 21, 393–411 [DOI] [PubMed] [Google Scholar]
- 29. Ji Z., Mei F. C., Xie J., Cheng X. (2007) J. Biol. Chem. 282, 14048–14055 [DOI] [PubMed] [Google Scholar]
- 30. Fernández-Zapico M. E. (2008) Pancreatology 8, 227–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nolan-Stevaux O., Lau J., Truitt M. L., Chu G. C., Hebrok M., Fernández-Zapico M. E., Hanahan D. (2009) Gene. Dev. 23, 24–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cayuso J., Ulloa F., Cox B., Briscoe J., Martí E. (2006) Development 133, 517–528 [DOI] [PubMed] [Google Scholar]
- 33. Regl G., Kasper M., Schnidar H., Eichberger T., Neill G. W., Philpott M. P., Esterbauer H., Hauser-Kronberger C., Frischauf A. M., Aberger F. (2004) Cancer Res. 64, 7724–7731 [DOI] [PubMed] [Google Scholar]
- 34. Murthy K. S., Zhou H., Grider J. R., Makhlouf G. M. (2001) Am. J. Physiol. Gastrointest. Liver Physiol. 280, G381–G388 [DOI] [PubMed] [Google Scholar]
- 35. Riobo N. A., Manning D. R. (2005) Trends Pharmacol. Sci. 26, 146–154 [DOI] [PubMed] [Google Scholar]
- 36. Meigs T. E., Fields T. A., McKee D. D., Casey P. J. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 519–524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meigs T. E., Fedor-Chaiken M., Kaplan D. D., Brackenbury R., Casey P. J. (2002) J. Biol. Chem. 277, 24594–24600 [DOI] [PubMed] [Google Scholar]
- 38. Dennler S., André J., Verrecchia F., Mauviel A. (2009) J. Biol. Chem. 284, 31523–31531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sumi M., Kiuchi K., Ishikawa T., Ishii A., Hagiwara M., Nagatsu T., Hidaka H. (1991) Biochem. Biophys. Res. Commun. 181, 968–975 [DOI] [PubMed] [Google Scholar]
- 40. Gao L., Blair L. A., Marshall J. (2006) Biochem. Biophys. Res. Commun. 345, 1606–1610 [DOI] [PubMed] [Google Scholar]
- 41. Ledoux J., Chartier D., Leblanc N. (1999) J. Pharmacol. Exp. Ther. 290, 1165–1174 [PubMed] [Google Scholar]
- 42. Rezazadeh S., Claydon T. W., Fedida D. (2006) J. Pharmacol. Exp. Ther. 317, 292–299 [DOI] [PubMed] [Google Scholar]
- 43. Itoh H., Toyohira Y., Ueno S., Saeki S., Zhang H., Furuno Y., Takahashi K., Tsutsui M., Hachisuka K., Yanagihara N. (2010) Naunyn-Schmiedebergs Arch. Pharmacol. 382, 235–243 [DOI] [PubMed] [Google Scholar]
- 44. Dennler S., André J., Alexaki I., Li A., Magnaldo T., ten Dijke P., Wang X. J., Verrecchia F., Mauviel A. (2007) Cancer Res. 67, 6981–6986 [DOI] [PubMed] [Google Scholar]
- 45. Pan Y., Bai C. B., Joyner A. L., Wang B. (2006) Mol. Cell Biol. 26, 3365–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stecca B., Mas C., Clement V., Zbinden M., Correa R., Piguet V., Beermann F., Ruiz I., Altaba A. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 5895–5900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Trousse F., Martí E., Gruss P., Torres M., Bovolenta P. (2001) Development 128, 3927–3936 [DOI] [PubMed] [Google Scholar]
- 48. Charron F., Stein E., Jeong J., McMahon A. P., Tessier-Lavigne M. (2003) Cell 113, 11–23 [DOI] [PubMed] [Google Scholar]
- 49. Yam P. T., Langlois S. D., Morin S., Charron F. (2009) Neuron 62, 349–362 [DOI] [PubMed] [Google Scholar]
- 50. Kovacs J. J., Whalen E. J., Liu R., Xiao K., Kim J., Chen M., Wang J., Chen W., Lefkowitz R. J. (2008) Science 320, 1777–1781 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.