Abstract
Peroxisome proliferator-activated receptor γ (PPARγ) activation induces adipogenesis and also enhances lipogenesis, mitochondrial activity, and insulin sensitivity in adipocytes. Whereas some studies implicate PPARγ coactivator 1α (PGC-1α) in the mitochondrial effect, the mechanisms involved in PPARγ regulation of adipocyte mitochondrial function are not resolved. PPARγ-activating ligands (thiazolidinediones (TZDs)) are important insulin sensitizers and were recently shown to indirectly induce PGC-1β transcription in osteoclasts. Here, we asked whether similar effects occur in adipocytes and show that TZDs also strongly induce PGC-1β in cultured 3T3-L1 cells. This effect, however, differs from the indirect effect proposed for bone and is rapid and direct and involves PPARγ interactions with an intronic PPARγ response element cluster in the PGC-1β locus. TZD treatment of cultured adipocytes results in up-regulation of mitochondrial marker genes, and increased mitochondrial activity and use of short interfering RNA confirms that these effects require PGC-1β. PGC-1β did not participate in PPARγ effects on adipogenesis or lipogenesis, and PGC-1β knockdown did not alter insulin-responsive glucose uptake into 3T3-L1 cells. Similar effects on PGC-1β and mitochondrial gene expression are seen in vivo; fractionation of obese mouse adipose tissue reveals that PPARγ and PGC-1β, but not PGC-1α, are coordinately up-regulated in adipocytes relative to preadipocytes and that TZD treatment induces PGC-1β and mitochondrial marker genes in adipose tissue of obese mice. We propose that PPARγ directly induces PGC-1β expression in adipocytes and that this effect regulates adipocyte mitochondrial activity.
Keywords: Adipocyte, Gene Regulation, Mitochondria, Nuclear Receptors, Transcription Coactivators, PGC-1, PPAR gamma, Thiazolidinedione
Introduction
Mechanisms that regulate adipogenesis and adipocyte function have come under intense scrutiny as investigators seek ways to prevent rising obesity rates (1, 2). The nuclear receptor (NR)2 peroxisome proliferator-activated receptor γ (PPARγ) is a master regulator of transcriptional cascades that are involved in commitment of preadipocyte to adipocyte differentiation and full elaboration of the adipocyte phenotype (3). Accordingly, thiazolidinediones (TZDs), synthetic PPARγ-activating ligands, induce adipocyte differentiation, lipid storage, and lipogenesis, effects that contribute to the unwanted side effect of weight gain (4–6). In addition, TZDs induce mitochondrial genes in the adipocyte, leading to mitochondrial morphological alterations and enhanced rates of oxygen (O2) consumption and fatty acid oxidation (7). Further, changes in adipocyte mitochondrial function have been linked to adipocyte insulin sensitivity (7, 8), which is also enhanced by TZDs (9–11). Thus, a critical issue is to elucidate mechanisms mediating adipogenesis and lipogenesis versus enhanced mitochondrial function and insulin sensitivity in order to prevent weight gain but retain the positive metabolic effects of PPARγ activation.
PPARγ, like other NRs, modulates gene expression by binding to PPARγ response elements (PPREs) as heterodimers with retinoid X receptors (RXRs) (3). PPREs are degenerate direct repeats of the consensus AGGTCA spaced by a single nucleotide (DR-1 element (12)) that are commonly found in the proximal promoter of PPARγ-regulated target genes (3, 12–14). However, recent genome-wide studies defining locations of PPARγ binding sites have revealed that PPREs are often located at alternate positions, including upstream enhancers, coding regions, introns, and downstream sequences (15, 16). These findings recapitulate results obtained in genome-wide analysis of binding site location for other NRs (3, 17, 18).
PPARγ coactivators, PGC-1α and PGC-1β, belong to a small family of NR coregulators that coordinate responses to metabolic stimuli and stressors (19, 20). PGCs interact with many NRs, including PPARγ, and other transcription factors and initiate assembly of larger coregulator complexes with diverse roles in gene expression, including modulation of local chromatin structure/modification state, RNA polymerase recruitment and processing, and coactivator complex turnover. Unlike many other NR and PPAR coregulators, however, PGC expression is tightly regulated at the transcriptional level, and the PGCs are induced by external stimuli. For example, cold induces PGC-1α in brown adipose tissue, where it mediates PPARγ-dependent transcriptional responses involved in mitochondrial biogenesis and uncoupling (6). In liver, fasting induces PGC1-α where it modulates gluconeogenesis and other aspects of the fasting response (20, 21). PPARγ acts through PGC-1α to coordinate responses involved in mitochondrial biogenesis (20), and PGC-1α is induced by TZDs in brown and white adipocyte cells in culture (14) and white adipose tissue (WAT) depots of ob/ob mice (7). Thus, documented TZD effects on mitochondrial activity in WAT have been attributed to TZD-dependent induction of PGC-1α (7).
PGC-1β exhibits different regulation patterns from PGC-1α. It is induced by saturated fat in liver (22), where it regulates genes involved in fat synthesis and very low density lipoprotein (VLDL) particle assembly, and by interferon γ (23) or interleukin 4 (IL-4) (24) in macrophages, where it cooperates with PPARs and STAT6 to stabilize anti-inflammatory alternative M2 macrophage polarization (25). PGC-1β is also implicated in mitochondrial activity because PGC-1β, as well as PGC-1α, knock-out mice exhibit a global reduction in oxidative phosphorylation and electron transport chain gene expression, and both PGCs play specific roles in mitochondrial oxidative activity and fatty acid oxidation in brown adipose tissue (19, 26). Interestingly, PGC-1β expression is increased along with PGC-1α in WAT of adipose-specific insulin receptor knock-out mice, and this is associated with enhanced expression of genes involved in mitochondrial activity and with longevity in the mice (27). However, little more is known about specific roles of PGC-1β in WAT.
A recent study revealed that TZDs induce PGC-1β in osteoclasts and that this establishes a transcriptional feed-forward loop required for optimal TZD induction of PPARγ target genes and genes that are regulated by other factors that bind PGC-1β (28). Therefore, we examined whether PGC-1β may also be a PPARγ target gene in the adipocyte and whether it is involved in PPARγ actions in this cell type. We show that PPARγ directly induces PGC-1β in adipocytes via interactions with an intronic PPRE cluster and that the primary role of PGC-1β in WAT is to mediate PPARγ-dependent regulation of mitochondrial genes and activity, with little or no effect on adipogenesis, lipogenesis, or insulin-mediated glucose uptake. Better understanding of PPARγ/PGC-1β actions on mitochondrial activity in adipocytes could reveal new strategies to modulate adipocyte phenotype in obesity.
EXPERIMENTAL PROCEDURES
Reagents and Plasmids
The following reagents were obtained from the indicated companies: rosiglitazone (Rosi) and GW9662 from Cayman Chemical (Ann Arbor, MI), pioglitazone from S.S.T. Corp. (Clifton, NJ), L805645 from Merck, and cycloheximide from Sigma. Pgc-1β locus DNA fragments from −48,565 to −47,014 bp, −42,077 to −41,481 bp, −38,202 to −37,663 bp, and +16,420 to +16,967 bp relative to the transcription start site, designated as Up1, Up2, Up3, and intron, respectively, were cloned by PCR from mouse genomic DNA and inserted upstream of the firefly luciferase gene in the pGL4.23 plasmid (Promega, Madison, WI). Two other DNA fragments (+16,690 and +16,516) from the intron fragment with a common downstream end at +16,967 bp were cloned and inserted into the pGL4.23 plasmid. Point mutations of putative intronic PPREs at the nucleotides +16,742 to +16,744 (TTA → CTC) and +16634 to +16736 (AGG → TTT) were generated using the QuikChange mutagenesis kit (Stratagene, La Jolla, CA). The integrity and fidelity of all constructs were verified by DNA sequencing. Renilla luciferase plasmid pRL-SV40 was purchased from Promega. PPARγ expression vector and RNAi construct for PGC-1β (pSUPER-PGC-1β) (22) were gifts from Dr. Bruce Spiegelman (Harvard University).
Cell Culture
HEK293T, CV-1, and 3T3-L1 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). HEK293T and CV-1 cells were cultured in DMEM with 10% FBS. 3T3-L1 preadipocytes were maintained in DMEM containing 10% calf serum. For differentiation, postconfluent 3T3-L1 cells were switched to DMEM containing 10% FBS, 1 μm dexamethasone, 0.5 mm isobutylmethylxanthine, and 10 μg/ml insulin for 2 days. The cells were then maintained in DMEM with FBS and insulin until harvesting. In vitro adipogenesis was detected by oil red O staining as described elsewhere (29).
Quantitative RT-PCR
Total RNA was extracted from cells and tissues by using miRNeasy mini kit (Qiagen) and was reverse-transcribed to cDNA using Taqman reverse transcription reagents (Applied Biosystems, Foster City, CA). Real-time PCR was carried out using the 7900HT fast real-time PCR system (Applied Biosystems) according to the manufacturer's instructions. The gene expression levels were normalized to 36B4. Taqman primer and probe sets were purchased from Applied Biosystems. PCR quantification for ChIP samples was performed using FastStart Universal SYBR Green PCR Master (Roche Applied Science). ChIP-qPCR data were normalized by the percentage input method (Invitrogen).
Western Blot
Whole-cell protein preparation and Western blotting were performed as described previously (30). Equal amounts of total protein (20–60 μg) were loaded. Anti-PGC-1β was obtained from Sigma. HRP-conjugated anti-actin was from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). All of the secondary antibodies were from Cell Signaling Technology (Danvers, MA).
Luciferase Assay
CV-1 cells were seeded into 24-well plates the day before transfection to give a confluence of 95% when transfection was performed. 60 ng of PPARγ expression plasmid, 320 ng of reporter plasmid, and 10 ng of pRL-SV40 were transfected per well using Lipofectamine 2000 (Invitrogen). After 24 h, culture medium was changed, and cells were incubated with or without 1 μm Rosi. After 24 h, cells were lysed and prepared for measurement of luciferase activity using a dual luciferase assay kit (Promega) and read in a microplate reader (Tecan, Männedorf, Switzerland). Renilla luciferase was used to normalize firefly luciferase activity.
Gel Shift
Plasmids bearing full-length ORFs of PPARγ and RXRα downstream of a T7 promoter were used to express these proteins using a reticulocyte lysate-based in vitro protein expression system (TNT, Promega). Binding of PPARγ to the indicated DNA elements was measured by a standard EMSA, using in vitro translated protein. Briefly, double-stranded oligonucleotides were labeled with 32P by polynucleotide kinase (New England Biolabs). For each EMSA reaction, 1 μl of translated protein was mixed with 1 pm 32P-labeled oligonucleotides (IDT) and 1 μg of poly(dI-dC) (Sigma), containing the indicated sequences in a binding buffer containing 25 mm HEPES, 50 mm KCl, 1 mm dithiothreitol, 10 mm ZnSO4, 0.1% Nonidet P-40, 5% glycerol. After a 30-min incubation at room temperature, complexes were resolved on a 5% non-denaturing gel and visualized by autoradiogram.
Chromatin Immunoprecipitation (ChIP)
Assays were performed by using the ChIP assay kit (Millipore, Billerica, MA). Cells were subjected to cross-linking with 1% formaldehyde (Fisher) for 10 min at room temperature, lysed in SDS lysis buffer, and sonicated to generate 600–1000-bp DNA fragments. Proteins were immunoprecipitated in ChIP dilution buffer using anti-PPARγ antibody (sc-7196, Santa Cruz Biotechnology, Inc.) or nonspecific rabbit IgG control (Cell Signaling Technologies). Cross-linking was reversed overnight at 65 °C, and DNA was isolated using the QIAquick PCR purification kit (Qiagen).
Retroviral Infection
Retroviruses were produced by transfection of pSUPER vector or pSUPER-PGC-1β plasmid and packing plasmid, pCL-Eco, into HEK293T cells using Fugene 6 (Roche Applied Science). Viral supernatants were supplemented with 8 μg/ml Polybrene and added to preadipocytes for infection for 2 days. Cells were selected in G418 (500 μg/ml) for 2 weeks, and pooled positive cells were used for further experiments.
O2 Consumption Rate
Negative control and PGC-1β knockdown 3T3-L1 preadipocytes were seeded in XF 24-well cell culture microplates (Seahorse Bioscience, North Billerica, MA) at 2.5 × 104 cells/well in 500 μl of growth medium, incubated at 37 °C with 5% CO2 for 24 h, and then differentiated into adipocytes for 6 days. The basal O2 consumption was measured by an XF24 Extracellular Flux Analyzer (Seahorse Biosciences). The O2 consumption rate was calculated using the AKOS technique for determining the slope and normalized to protein amount, which was measured by a Bio-Rad protein assay.
Mitochondrial Fatty Acid Oxidation
O2 consumption in response to palmitoyl carnitine oxidation was measured to assess mitochondrial fatty acid oxidation, using Oroboros high resolution respirometry (Innsbruck, Austria). For this, 3T3-L1 cells (1 million) were resuspended in MiRO5 buffer (Oroboros) and permeabilized with 20 μg/ml digitonin, and palmitoyl carnitine (10 μm) was added as a substrate.
Glucose Uptake
3T3-L1 adipocytes were induced to differentiate for 6 days in 6-well plates and incubated in medium with or without 1 μm rosiglitazone for 3 days. After two rinses with serum-free DMEM, cells were serum-starved in DMEM with 0.5% BSA for 14 h. The cells were rinsed three times with warmed KRPH buffer (20 mm HEPES pH 7.4, 1 mm KH2PO4, 0.6 mm MgSO4, 1 mm CaCl2, 120 mm NaCl, 5 mm KCl, and 0.5% BSA) and incubated with or without 100 nm insulin in KRPH buffer at 37 °C for 20 min, followed by the addition of 50 mm 2-deoxy-d-glucose (Aldrich) and 1 mCi/well of tritiated-2-deoxy-d-glucose (MP Biomedicals, Solon, OH) for 10 min. The assay was terminated by washing the cells three times with ice-cold KRPH buffer. Cells were solubilized with 0.1% SDS, and radioactivity was determined in a liquid scintillation counter (Beckman LS6500, Fullerton, CA). Total cellular protein concentration was measured by the Bradford method (Bio-Rad).
Animal Experiments and Tissue Fractionation
10-week-old db/db mice or 9-month-old LDL receptor (LDLR)−/− male mice were treated with Rosi (1.2 g/kg chow diet) for 4 weeks. Then the animals were sacrificed, and total RNA from the epididymal fat pad was isolated for gene expression analysis. Adipocytes and SVF cells were isolated from epididymal fat pads from 9-month-old LDLR−/− male mice as described by Halleux et al. (31). SVF cells were incubated with biotin-conjugated mouse F4/80 antibody (13-4801-81, eBioscience, San Diego, CA) in isolation buffer (PBS plus 0.1% BSA and 2 mm EDTA) for 20 min at 4 °C. Cells were washed twice with isolation buffer and incubated with streptavidin-labeled magnetic FlowComp Dynabeads (Invitrogen) in 1 ml of isolation buffer for 20 min at 4 °C with mixing providing constant tilting and rotation. The tube containing cells was placed in the magnet for 1 min, and supernatant was removed. The bead-bound F4/80-positive cells (macrophage) were washed three times with isolation buffer and removed. The bead-free cells in supernatant were incubated with biotin-conjugated mouse CD-45 antibody (13-0451-81, eBioscience) and biotin-conjugated mouse CD-31 antibody (13-0311-81, eBioscience) for 20 min at 4 °C. Cells were washed twice with isolation buffer and incubated with streptavidin-labeled magnetic FlowComp Dynabeads in 1 ml of isolation buffer for 20 min at 4 °C. The tube containing the cells was placed in the magnet for 1 min, and the cells in supernatant (preadipocytes) were collected and lysed by QIAzol (Qiagen, Chatsworth, CA) for RNA isolation.
RESULTS
PPARγ Induces PGC-1β in Adipocytes
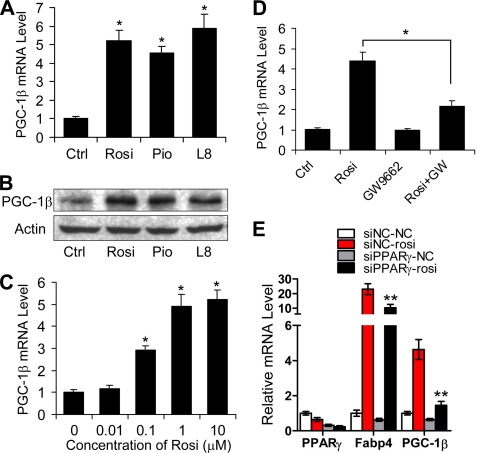
To determine whether PPARγ regulates Pgc-1β expression in adipocytes, we tested the effect of PPARγ agonists on Pgc-1β mRNA levels in 3T3-L1 adipocytes (Fig. 1A). Pgc-1β transcripts were induced 5-fold after a 2-day treatment with saturating levels of several PPARγ ligands, including TZDs, Rosi, and pioglitazone, and the non-TZD agonist L805645. PPARγ ligands also increased steady state levels of PGC-1β protein (Fig. 1B). Similar, albeit much weaker, effects were observed with Pgc-1α, but transcript levels were barely detectable in differentiated 3T3-L1 cells and very low compared with Pgc-1β (not shown). Rosi-dependent increases in Pgc-1β transcript levels exhibited characteristic dose dependence, with half-maximal response at around 0.1 μm and maximal response at 1 μm (Fig. 1C). Two lines of evidence suggested that these effects required PPARγ. First, Rosi induction of Pgc-1β was blocked by the PPARγ antagonist GW9662 (Fig. 1D). Second, transfection of a PPARγ siRNA but not a control siRNA reduced Pparγ transcript levels, Rosi induction of a well characterized PPARγ target gene that provides a functional readout of PPARγ activation (Fabp4), and Rosi induction of Pgc-1β itself (Fig. 1E). Thus, PPARγ activation results in increased Pgc-1β expression in cultured adipocytes.
FIGURE 1.
PPARγ-dependent induction of PGC-1β. A, 3T3-L1 preadipocytes cultured in differentiation medium were treated with 1 μm Rosi, 5 μm pioglitazone, or 1 μm L805645 (L8) for 48 h, and the levels of Pgc-1β mRNA were determined by quantitative real-time PCR. Data are presented as mean ± S.D. (error bars) from three independent experiments. *, p < 0.05 versus control (Ctrl). B, PGC-1β protein levels determined by Western blot analysis. Actin was used as loading control. C, 3T3-L1 preadipocytes cultured in differentiation medium were treated with the indicated Rosi concentrations for 24 h, and the levels of Pgc-1β mRNA were determined by real-time PCR. Data are presented as mean ± S.D. from three independent experiments. *, p < 0.05 versus 0.5 μm. D, 3T3-L1 preadipocytes cultured in differentiation medium were treated or untreated with 0.5 μm Rosi with or without 20 μm GW9662 (GW) for 48 h. Levels of Pgc-1β were determined by real-time PCR. Data are presented as mean ± S.D. from three independent experiments. *, p < 0.05. E, 3T3-L1 preadipocytes were transfected with a control siRNA or siRNA for PPARγ. After 24 h, cells were cultured in differentiation medium and either treated or untreated with 1 μm Rosi for 48 h. Total RNA was isolated, and the levels of Pparγ, Fabp4, and Pgc-1β mRNA were determined by quantitative real-time PCR. Data are presented as mean ± S.D. from three independent experiments. **, p < 0.01.
Pgc-1β Is a Direct PPARγ Target in 3T3-L1 Cells
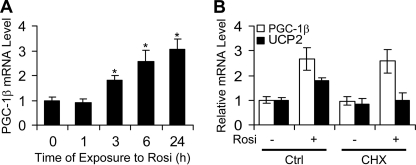
To understand how PPARγ modulates Pgc-1β expression in 3T3-L1 cells, we determined the time course of TZD induction of Pgc-1β and tested effects of the protein synthesis inhibitor cycloheximide (CHX). Rosi induction of Pgc-1β mRNA was rapid and could be detected within 3 h of ligand treatment (Fig. 2A). Further, pretreatment of 3T3-L1 cells with CHX did not alter Rosi induction of PGC-1β either at an early time point (6 h; not shown) or at 12 h of treatment (Fig. 2B). By contrast, Rosi induction of Ucp2, an indirect PPARγ target gene (32), was only detectable after 12-h ligand treatments (Fig. 2B), and this effect was blocked by CHX. Thus, Rosi induction of PGC-1β is direct and does not require new protein synthesis.
FIGURE 2.
Rosi directly induces Pgc-1β expression. A, time course of Rosi induction of Pgc-1β gene expression. B, 3T3-L1 preadipocytes cultured in differentiation medium were pretreated with 10 μg/ml CHX for 0.5 h and then co-treated with or without 1 μm Rosi for 12 h. Total RNA was isolated, and the levels of Pgc-1β and Ucp2 mRNA were determined by quantitative real-time PCR. Error bars, S.E.
The PGC-1β Locus Harbors a Potent Intronic PPRE and Far Upstream PPREs
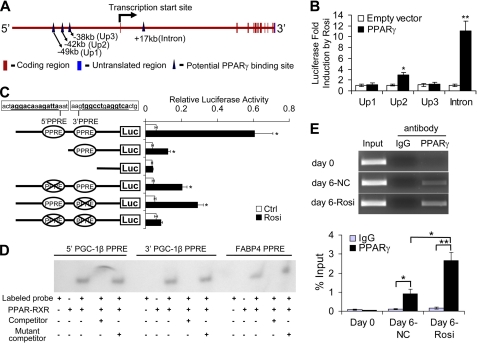
Because PPARγ directly induced Pgc-1β expression, we explored whether the Pgc-1β locus harbors PPREs. We reviewed recent genome-wide studies of PPARγ binding events during 3T3-L1 differentiation (Fig. 3A) (15, 16, 33). These studies observed PPARγ binding events at several positions far upstream of the Pgc-1β transcriptional start site (Up1, Up2, and Up3 at −49, −42, and −38 kb, respectively) and in the first Pgc-1β intron (+17 kb). Therefore, we determined whether subcloned 1-kb fragments of genomic DNA corresponding to each of these putative PPARγ binding sites would confer Rosi response upon a luciferase reporter in transfections in CV-1 cells (Fig. 3B). A reporter driven by one of the three putative upstream binding regions responded weakly to Rosi (Up2; 2.2-fold induction), but the reporter with putative intronic PPARγ binding region yielded a greater than 10-fold Rosi response.
FIGURE 3.
Identification of response elements for PPARγ in the Pgc-1β gene. A, genomic region of Pgc-1β with reported PPARγ binding sites indicated in Refs. 15 and 16. B, CV-1 cells were transfected individually with the Up1, Up2, Up3, or intron luciferase reporter plasmids, combined with or without transfection of a PPARγ expression plasmid. 24 h after transfection, cells were treated with or without 1 μm Rosi for 24 h. The luciferase activities were normalized to the internal transfection control, and the -fold induction induced by Rosi was presented. Data are presented as mean ± S.D. (error bars) from three independent experiments. *, p < 0.05; **, p < 0.01 versus 1. C, CV-1 cells were cotransfected with a luciferase reporter-containing intron fragment, truncated intron fragments, or intron fragment with mutated PPRE(s) and PPARγ expression plasmid. Luciferase activity was determined as in A. Data are presented as mean ± S.D. from three independent experiments. *, p < 0.05 versus control (Ctrl). Putative PPREs are underlined. D, autoradiographic exposure of non-denaturing gel used to separate free labeled probe from PPARγ-RXRα complex. Lanes that contain an excess of cold competitor wild type or mutant oligonucleotide are marked. E, ChIP analysis was performed using chromatin isolated from 3T3-L1 preadipocytes (day 0) and differentiated 3T3-L1 adipocytes at day 6 treated (day 6-Rosi) or untreated (day 6-NC) with 1 μm Rosi during adipogenesis. The chromatin preparations were immunoprecipitated with antibody against PPARγ and analyzed by PCR to detect the intron fragment containing putative PPREs. PCR products were visualized by 3% agarose gel electrophoresis and ethidium bromide staining (top panel). Data are shown from a representative experiment. Quantitative PCR was also performed, and the percentage of input DNA is shown in the bottom panel, presented as mean ± S.D. from three independent experiments. *, p < 0.05; **, p < 0.01.
Inspection of the Pgc-1β intronic fragment sequence revealed two potential PPREs (5′ and 3′; Fig. 3C), which were spaced by 98 base pairs. Deletion of the 5′ element and point mutation of either or both PPREs confirmed that (i) the putative PPREs were required for optimal Rosi response and (ii) both were capable of mediating Rosi response in isolation. Gel shift analysis confirmed the association of PPARγ-RXR heterodimers with both PPREs in vitro with binding observed at equivalent levels to the well characterized fatty acid-binding protein 4 (FABP4) PPRE and competed by an excess of unlabeled native oligonucleotide but not mutant oligonucleotide (Fig. 3D). Further, chromatin immunoprecipitation (ChIP) experiments confirmed that PPARγ localized to the intronic PPRE after induction of 3T3-L1 differentiation with standard mixture and that this binding was enhanced by Rosi (Fig. 3E). Control ChIP experiments with non-PPAR-regulated gene fragment, from Gapdh, failed to reveal PPARγ binding, either with specific PPARγ antibody or nonspecific IgG (not shown). Thus, observed PPARγ binding to the Pgc-1β intron is specific, and the Pgc-1β intron harbors a potent PPRE composed of a cluster of two discrete PPAR binding sites.
PGC-1β Is Required for Rosi Induction of Mitochondrial Activity in the Adipocyte
TZD induction of mitochondrial gene expression and activity in cultured adipocytes required PGC-1β. We performed Q-PCR analysis of candidate TZD-inducible genes in 3T3-L1 cells that express short interfering RNA (siRNA) against PGC-1β or control cells with a scrambled siRNA (Fig. 4A). Rosi induced PGC-1β in control cells, and Pgc-1β mRNA was reduced about 50% by the siRNA, and this siRNA-dependent reduction in PGC-1β expression was also detected at the level of protein by Western analysis (Fig. 4A, inset). As mentioned above, TZD only induced PGC-1α weakly, and we also observed modest up-regulation of Pgc-1α levels in PGC-1β knockdown cells, consistent with previous demonstrations that Pgc-1α is up-regulated in conditions of PGC-1β knockdown (26). TZDs also up-regulated genes that encode mitochondrial proteins cytochrome c (Cycs), cytochrome c oxidase subunit IV isoform 1 (Cox4i1), and cytochrome c oxidase subunit Va (Cox5a), key components of the electron transport chain. This effect was blunted by PGC-1β siRNA. Ndusf6, a component of mitochondrial complex I and mitochondrial transcription factor A (Tfam), required for mitochondrial biogenesis, were both unaffected by TZDs or PGC-1β knockdown. This suggests that TZD effects are specific to a subset of mitochondrial components and that TZDs do not work through Tfam to induce mitochondrial biogenesis.
FIGURE 4.
PGC-1β is required for Rosi induction of mitochondrial function in 3T3-L1 adipocytes. A, Rosi induced Pgc-1β and mitochondrial genes in 3T3-L1 adipocytes. 3T3-L1 preadipocytes were infected with neomycin-selectable retroviruses expressing RNAi against PGC-1β or a scrambled RNAi and cultured under G-418 selection for 14 days and then were induced to adipogenesis for 8 days. The inset shows PGC-1β protein levels determined by Western blot analysis. Actin was used as a loading control. Gene expression was analyzed by real-time PCR. Data are presented as mean ± S.E. (error bars) from four independent experiments. *, p < 0.05; **, p < 0.01. B, O2 consumption rate (OCR) was measured by Seahorse. Data are presented as mean ± S.E. of four replicates. ns, p > 0.05; *, p < 0.05. C, O2 flux (pmol/mg) in permeabilized 3T3-L1 cells treated as shown after palmitate challenge. Mean ± S.E. from three independent experiments. *, p < 0.05; **, p < 0.01.
Several genes involved in fatty acid β-oxidation were also up-regulated by TZDs in the adipocyte. These included carnitine palmitoyl transferase 1B (Cpt1β), acetyl-CoA carboxylase 2 (Acc2), medium chain acyl-CoA dehydrogenase (Mcad), or hydroxyacyl-CoA dehydrogenase/3-ketoacyl-CoA thiolase/enoyl-CoA hydratase (Hadha) genes. Of these, however, PGC-1β siRNA only inhibited TZD induction of one of the tested genes, Cpt1β, a mitochondrial enzyme that mediates the rate-limiting step for fatty acid β-oxidation, transport of long chain fatty acids across the outer mitochondrial membrane.
Rosi effects on expression of genes that encode mitochondrial components were paralleled by increases in mitochondrial activity in 3T3-L1 cells, and these effects were also inhibited by PGC-1β knockdown. Rosi treatment increased the cellular O2 consumption rate about 3-fold, and this effect was blunted in PGC-1β siRNA-expressing cells (Fig. 4B). As expected, these effects were related to increased capacity for mitochondrial fatty acid β-oxidation (Fig. 4C); permeabilization of 3T3-L1 cells that had been pretreated with Rosi and subsequent challenge of the preparations with a long chain fatty acid palmitoyl carnitine substrate revealed that cells that had been subjected to Rosi pretreatment exhibited increased O2 consumption in response to palmitate, and these effects were blunted in PGC-1β knockdown cells.
Lack of PGC-1β Effect on Adipogenesis, Lipogenesis, or Insulin-mediated Glucose Uptake
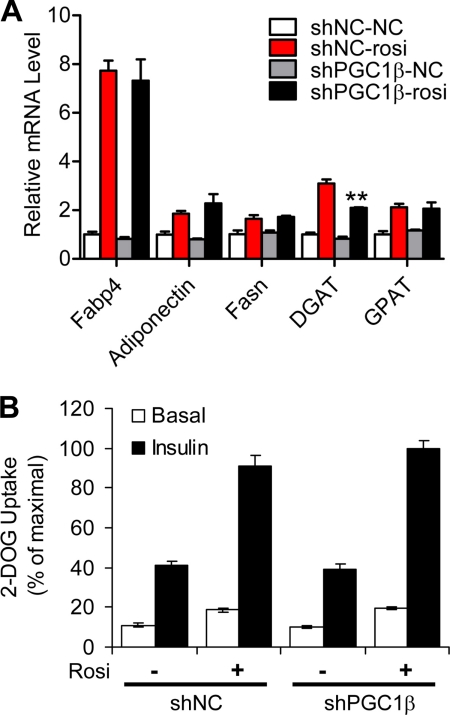
Effects of PGC-1β knockdown were specific for PPARγ target genes involved in mitochondrial activity listed above, and there was no effect on other well characterized PPARγ-regulated pathways. 3T3-L1 preadipocytes that expressed PGC-1β siRNA exhibited no obvious defect in TZD-dependent adipocyte differentiation (not shown), and accordingly, there was no effect of PGC-1β knockdown on TZD-dependent induction of adipocyte markers, including Fabp4 or adiponectin (Fig. 5A). Likewise, there was no defect in TZD induction of two lipogenic genes, fatty acid synthase (Fasn) and glycerol-3-phosphate acyltransferase-1 (Gpat), although there was a modest statistically significant decrease in diacylglycerol acyltransferase 1 (Dgat) expression in PCG-1β knock-out cells (Fig. 5A). Finally, PPARγ is known to enhance basal and insulin-stimulated glucose uptake in cultured adipocytes (9–11). Knock-out of PGC-1β in 3T3-L1 cells did not alter these effects of Rosi (Fig. 5B). Overall, these data do not support a role for PGC-1β in PPARγ-induced adipogenesis, lipogenesis, or insulin sensitization.
FIGURE 5.
PGC-1β induction is not required for TZD effects on adipogenesis, lipogenesis, and glucose uptake. A, PGC-1β is not required for Rosi-induced adipogenic gene expression in 3T3-L1 adipocytes. Cells were prepared as in Fig. 4A; mRNA levels were analyzed by real-time PCR. Data are presented as mean ± S.E. (error bars) from four independent experiments. *, p < 0.05; **, p < 0.01. B, results showing tritiated glucose uptake into rates into control or PGC-1β siRNA expressing 3T3-L1 adipocytes with or without Rosi and insulin treatment.
Pgc-1β Is Induced by TZDs in Mouse Adipose Tissue
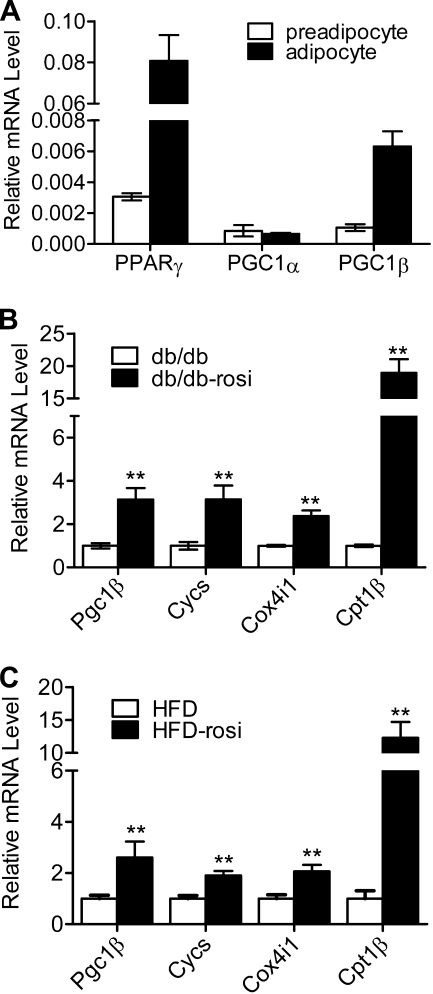
Finally, we examined expression of Pgc-1β in mouse adipose tissue. Transcript levels of Pparγ and Pgc-1β in adipocytes isolated from epididymal fat obtained from mice fed for 3 months with a high fat diet were increased relative to preadipocytes isolated from the same tissue (Fig. 6A). Although Pgc-1α was present at levels similar to those of Pgc-1β in the preadipocyte fraction, its expression was not induced in mature adipocytes. Rosi treatment of mice from several obese strains, including db/db (Fig. 6B), LDLR knock-out mice during the period of HFD (Fig. 6C), and others (not shown) resulted in increased expression of Pgc-1β along with mitochondrial genes that were proven PGC-1β targets in 3T3-L1 cells (Cycs, Cox4i1, and Cpt1β). Thus, Pparγ and Pgc-1β, but not Pgc-1α, are coordinately induced during adipocyte differentiation in vivo, and PPARγ-activating ligands induce Pgc-1β expression in WAT depots of obese mice.
FIGURE 6.
Coordinate regulation of Pparγ and Pgc-1β in adipogenesis and TZD induction of Pgc-1β and mitochondrial genes in mouse adipose tissue. A, Pparγ and Pgc-1β, but not Pgc-1α, are up-regulated in mature adipocyte fractions from WAT relative to preadipocyte fractions. Shown is a representation of real-time PCR analysis of mRNA prepared from each cell fraction. B, TZDs induce Pgc-1β and mitochondrial genes of WAT from db/db mice or db/db mice treated with Rosi (db/db-rosi) for 4 weeks. Pgc-1β, Cycs, Cox4i1, and Cpt1β mRNA levels were analyzed by real-time PCR and normalized by the relative amounts of 36B4. Data are presented as mean ± S.E. (error bars). n = 6; **, p < 0.01 versus db/db. C, as in B, except that HFD-fed LDLR−/− mice were used.
DISCUSSION
We showed that the adipogenic transcription factor PPARγ induces its own cofactor PGC-1β in adipocytes through a direct effect that is independent of new protein synthesis and is probably mediated by a combination of far upstream and intronic PPREs that are distant from the proximal promoter. We also show that this effect is closely linked to TZD-dependent changes in mitochondrial activity and capacity for fatty acid β-oxidation in the adipocyte. Thus, a PPARγ/PGC-1β autoregulatory loop participates in TZD regulation of mitochondrial activity in the adipocyte.
This is not the first study to show that TZDs act through PPARγ to enhance PGC-1β expression; a previous study demonstrated a similar effect in osteoclasts (28). In the latter case, however, TZD-dependent PGC-1β induction was attributed to indirect effects; the authors propose that TZD-dependent reductions in steady state levels of the NR coactivator protein β-catenin induce Pgc-1β transcription via derepression of c-Jun at the Pgc-1β proximal promoter. Our findings do not contradict this idea. It is clear that many NRs, including PPARγ, bind β-catenin, and this interaction results in complex effects on β-catenin protein and transcriptional outcomes at β-catenin target genes (34). Moreover, comparisons of PPARγ actions and binding locations in adipocytes and macrophages indicate that PPARγ often utilizes similar mechanisms to induce genes that are regulated similarly in both cell types (33) but that PPREs in the Pgc-1β locus are not occupied with PPARs in macrophage (Fig. 7) (15, 33, 35). Thus, it is possible that PPARγ-dependent induction of PGC-1β differs in adipocytes and osteoclasts.
FIGURE 7.
PPARγ binding sites near and within the Pgc-1β loci, originally identified in 3T3-L1 adipocytes (blue) and macrophages (red), aligned to the mouse genome (mm8) with the UCSC genome browser.
Our data are among the first to define a role of PGC-1β in WAT, and results point to a role in mitochondrial activity. TZDs induce PGC-1β and downstream PGC-1β target genes in cultured adipocytes and mouse adipose tissue, and PPARγ and PGC-1β are coordinately up-regulated in mature mouse adipocytes relative to preadipocytes. Additionally, the use of short interfering RNA against PGC-1β demonstrated that this coregulator is required for TZD-dependent increases in mitochondrial gene expression, O2 consumption, and palmitate oxidation in 3T3-L1 cells. In further support of the idea that PGC-1β plays a key role in regulation of mitochondrial gene expression in WAT, our comparisons (not shown) of gene expression in obese versus lean mice confirm previous findings that obesity leads to down-regulation of PPARγ-dependent mitochondrial genes in WAT and that Rosi treatment reverses these effects (7) but also extend these findings to show that PGC-1β displays an identical regulation pattern in lean and obese animals (not shown). This is coupled with the fact that our studies indicate that requirements for PGC-1β are specific to a subset of tested PPARγ target genes involved in mitochondrial activity (Cycs, Cox4i1, Cox5a, and Cpt1β) and that PGC-1β was not needed for TZD effects on genes involved in adipogenesis, lipogenesis, or most genes in the β-oxidation pathway nor for PPARγ-dependent effects on insulin-mediated or basal glucose uptake or adipogenesis at the whole cell level. Our data strongly suggest that PGC-1β plays a key role in TZD-dependent effects on mitochondrial genes in the adipocyte.
Earlier studies indicated that TZDs induce Pgc-1α in brown adipose tissue and WAT cells in culture (14) and in adipose tissue of ob/ob mice (7, 8), suggesting that PGC-1α may mediate TZD-dependent effects on adipocyte biology and adipocyte mitochondrial activity. Our data, however, suggest that Pgc-1α transcripts are present at slightly reduced levels in mature adipocyte versus preadipocyte fractions prepared from WAT depots in mice, unlike Pgc-1β, which is induced during this transition. Additionally, Pgc-1α transcripts are barely detectable and present at much lower levels than those of Pgc-1β in differentiated 3T3-L1 cells or WAT (not shown). This suggests that Pgc-1β may play an even more important role in mitochondrial metabolism in mature WAT than Pgc-1α. Although PGC-1α and PGC-1β play similar roles in mitochondrial activity, their actions are only partially redundant (19). It will be important to understand the special role for PGC-1β in the mitochondrial function in adipocyte and WAT.
Our studies do not address physiologic relevance of PPARγ-dependent induction of PGC-1β in WAT and concomitant increases in WAT mitochondrial activity, but we envisage several possibilities. PGC-1β induction could enhance mitochondrial function and β-oxidation in WAT to prevent excess fat storage and promote energy balance in response to other actions of PPARγ. Alternatively, adipocyte function requires energy, and this need is met by local β-oxidation (7); perhaps TZDs stimulate mitochondrial activity to meet cellular energy requirements associated with differentiation, increased triglyceride synthesis and storage, or other TZD-dependent responses. Relatedly, PGC-1β-dependent increases in fatty acid oxidation will produce acetyl groups to regenerate tricarboxylic acid (TCA) cycle intermediates, and this could fulfill demands for metabolites required for fat function. Triglyceride synthesis and storage certainly result in large demands for glycerol phosphate that are met by gluconeogenesis (7, 36, 37), and it is possible that adipogenesis could also trigger requirements for other intermediates that are synthesized from TCA cycle precursors. In any of these cases, however, the reasons why PGC-1β, but not PGC-1α, is up-regulated in mature adipocytes to meet proposed needs are not obvious. Clearly, more studies will be needed to understand the role of PGC-1β and mitochondrial function in WAT in both normal individuals and disease states.
Acknowledgments
We thank Dr. Qiang Tong and Dr. Fei Wang (Baylor College of Medicine) for assistance with the glucose uptake experiment.
This work was supported, in whole or in part, by National Institutes of Health Grants 1R24DK087723-01 (to W. A. H. and J. D. B.) and U01 GM094614 (to P. W.). This work was also supported by American Diabetes Association Fellowship 7-07-CVD-12 (to T. D.) and the MacDonald Foundation (to W. A. H.).
- NR
- nuclear receptor
- PPAR
- peroxisome proliferator-activated receptor
- TZD
- thiazolidinedione
- WAT
- white adipose tissue
- PPRE
- PPARγ response element
- Rosi
- rosiglitazone
- CHX
- cycloheximide
- RXR
- retinoid X receptor
- LDLR
- low density lipoprotein receptor.
REFERENCES
- 1. Lefterova M. I., Lazar M. A. (2009) Trends Endocrinol. Metab. 20, 107–114 [DOI] [PubMed] [Google Scholar]
- 2. White U. A., Stephens J. M. (2010) Mol. Cell. Endocrinol. 318, 10–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tontonoz P., Spiegelman B. M. (2008) Annu. Rev. Biochem. 77, 289–312 [DOI] [PubMed] [Google Scholar]
- 4. Lebovitz H. E. (2002) Diabetes Metab. Res. Rev. 18, Suppl. 2, S23–S29 [DOI] [PubMed] [Google Scholar]
- 5. Fonseca V. (2003) Am. J. Med. 115, Suppl. 8A, 42S–48S [DOI] [PubMed] [Google Scholar]
- 6. Shah P., Mudaliar S. (2010) Expert Opin. Drug Saf. 9, 347–354 [DOI] [PubMed] [Google Scholar]
- 7. Wilson-Fritch L., Nicoloro S., Chouinard M., Lazar M. A., Chui P. C., Leszyk J., Straubhaar J., Czech M. P., Corvera S. (2004) J. Clin. Invest. 114, 1281–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi X., Burkart A., Nicoloro S. M., Czech M. P., Straubhaar J., Corvera S. (2008) J. Biol. Chem. 283, 30658–30667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mukherjee R., Hoener P. A., Jow L., Bilakovics J., Klausing K., Mais D. E., Faulkner A., Croston G. E., Paterniti J. R., Jr. (2000) Mol. Endocrinol. 14, 1425–1433 [DOI] [PubMed] [Google Scholar]
- 10. Nugent C., Prins J. B., Whitehead J. P., Savage D., Wentworth J. M., Chatterjee V. K., O'Rahilly S. (2001) Mol. Endocrinol. 15, 1729–1738 [DOI] [PubMed] [Google Scholar]
- 11. Hu X., Feng Y., Liu X., Zhao X. F., Yu J. H., Yang Y. S., Sydow-Bäckman M., Hörling J., Zierath J. R., Leng Y. (2007) Diabetologia 50, 1048–1057 [DOI] [PubMed] [Google Scholar]
- 12. Heinäniemi M., Uski J. O., Degenhardt T., Carlberg C. (2007) Genome Biol. 8, R147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tontonoz P., Hu E., Graves R. A., Budavari A. I., Spiegelman B. M. (1994) Genes Dev. 8, 1224–1234 [DOI] [PubMed] [Google Scholar]
- 14. Hondares E., Mora O., Yubero P., Rodriguez de la Concepción M., Iglesias R., Giralt M., Villarroya F. (2006) Endocrinology 147, 2829–2838 [DOI] [PubMed] [Google Scholar]
- 15. Lefterova M. I., Zhang Y., Steger D. J., Schupp M., Schug J., Cristancho A., Feng D., Zhuo D., Stoeckert C. J., Jr., Liu X. S., Lazar M. A. (2008) Genes Dev. 22, 2941–2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nielsen R., Pedersen T. A., Hagenbeek D., Moulos P., Siersbaek R., Megens E., Denissov S., Børgesen M., Francoijs K. J., Mandrup S., Stunnenberg H. G. (2008) Genes Dev. 22, 2953–2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carroll J. S., Brown M. (2006) Mol. Endocrinol. 20, 1707–1714 [DOI] [PubMed] [Google Scholar]
- 18. Siersbaek R., Nielsen R., Mandrup S. (2010) FEBS Lett. 584, 3242–3249 [DOI] [PubMed] [Google Scholar]
- 19. Lin J. D. (2009) Mol. Endocrinol. 23, 2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Handschin C., Spiegelman B. M. (2006) Endocr. Rev. 27, 728–735 [DOI] [PubMed] [Google Scholar]
- 21. Rhee J., Inoue Y., Yoon J. C., Puigserver P., Fan M., Gonzalez F. J., Spiegelman B. M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 4012–4017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lin J., Yang R., Tarr P. T., Wu P. H., Handschin C., Li S., Yang W., Pei L., Uldry M., Tontonoz P., Newgard C. B., Spiegelman B. M. (2005) Cell 120, 261–273 [DOI] [PubMed] [Google Scholar]
- 23. Sonoda J., Laganière J., Mehl I. R., Barish G. D., Chong L. W., Li X., Scheffler I. E., Mock D. C., Bataille A. R., Robert F., Lee C. H., Giguère V., Evans R. M. (2007) Genes Dev. 21, 1909–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vats D., Mukundan L., Odegaard J. I., Zhang L., Smith K. L., Morel C. R., Wagner R. A., Greaves D. R., Murray P. J., Chawla A. (2006) Cell Metab. 4, 13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Odegaard J. I., Ricardo-Gonzalez R. R., Goforth M. H., Morel C. R., Subramanian V., Mukundan L., Red Eagle A., Vats D., Brombacher F., Ferrante A. W., Chawla A. (2007) Nature 447, 1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Uldry M., Yang W., St-Pierre J., Lin J., Seale P., Spiegelman B. M. (2006) Cell Metab. 3, 333–341 [DOI] [PubMed] [Google Scholar]
- 27. Katic M., Kennedy A. R., Leykin I., Norris A., McGettrick A., Gesta S., Russell S. J., Bluher M., Maratos-Flier E., Kahn C. R. (2007) Aging Cell 6, 827–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei W., Wang X., Yang M., Smith L. C., Dechow P. C., Sonoda J., Evans R. M., Wan Y. (2010) Cell Metab. 11, 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Soukas A., Socci N. D., Saatkamp B. D., Novelli S., Friedman J. M. (2001) J. Biol. Chem. 276, 34167–34174 [DOI] [PubMed] [Google Scholar]
- 30. Deng T., Shan S., Li P. P., Shen Z. F., Lu X. P., Cheng J., Ning Z. Q. (2006) Endocrinology 147, 875–884 [DOI] [PubMed] [Google Scholar]
- 31. Halleux C. M., Declerck P. J., Tran S. L., Detry R., Brichard S. M. (1999) J. Clin. Endocrinol. Metab. 84, 4097–4105 [DOI] [PubMed] [Google Scholar]
- 32. López-Solache I., Marie V., Vignault E., Camirand A., Silva J. E. (2002) Endocrine 19, 197–208 [DOI] [PubMed] [Google Scholar]
- 33. Lefterova M. I., Steger D. J., Zhuo D., Qatanani M., Mullican S. E., Tuteja G., Manduchi E., Grant G. R., Lazar M. A. (2010) Mol. Cell. Biol. 30, 2078–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mulholland D. J., Dedhar S., Coetzee G. A., Nelson C. C. (2005) Endocr. Rev. 26, 898–915 [DOI] [PubMed] [Google Scholar]
- 35. Kent W. J., Sugnet C. W., Furey T. S., Roskin K. M., Pringle T. H., Zahler A. M., Haussler D. (2002) Genome Res. 12, 996–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Olswang Y., Cohen H., Papo O., Cassuto H., Croniger C. M., Hakimi P., Tilghman S. M., Hanson R. W., Reshef L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 625–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Franckhauser S., Muñoz S., Pujol A., Casellas A., Riu E., Otaegui P., Su B., Bosch F. (2002) Diabetes 51, 624–630 [DOI] [PubMed] [Google Scholar]