Abstract
In multicellular organisms, apoptosis is a powerful method of host defense against viral infection. Apoptosis is mediated by a cascade of caspase-family proteases that commit infected cells to a form of programmed cell death. Therefore, to replicate within host cells, viruses have developed various strategies to inhibit caspase activation. In the mitochondrial cell-death pathway, release of cytochrome c from mitochondria into the cytosol triggers assembly of the oligomeric apoptosome, resulting in dimerization and activation of the apical caspase-9 (C9), and in turn its downstream effector caspases, leading to apoptosis. We previously showed that the vaccinia virus-encoded Bcl-2-like protein, F1L, which suppresses cytochrome c release by binding Bcl-2 family proteins, is also a C9 inhibitor. Here, we identify a novel motif within the flexible N-terminal region of F1L that is necessary and sufficient for interaction with and inhibition of C9. Based on functional studies and mutagenesis, we developed an atomic model of the complex in which F1L inhibits C9 by engaging the active site in the reverse orientation with respect to substrate peptides, in a manner analogous to that of XIAP-mediated inhibition of caspases-3 and -7. These studies offer new insights into the mechanism of apoptosome inhibition by F1L as well as novel probes to understand the molecular bases of apoptosome regulation and turnover. They also suggest how the two distinct functionalities of F1L (inhibition of C9 and suppression of pro-apoptotic Bcl-2 family proteins) may operate in a cellular setting.
Keywords: Animal Viruses, Apoptosis, Caspase, Cell Death, Immunosupressor, Innate Immunity, Peptide Interactions, Pox Viruses, Protease Inhibitor, Viral Protein
Introduction
During the process of host-pathogen co-evolution, the host developed a variety of defense mechanisms to cope with intracellular pathogens that the pathogen has sought to counter. One of these, apoptosis (a form of programmed cell death), provides an important defense mechanism that inhibits viral replication in infected cells (1–3). Two principal pathways leading to apoptosis are the mitochondrial/intrinsic pathway and the death receptor-dependent/extrinsic pathway (1, 4).
In the mitochondrial pathway, pro-apoptotic signals converge on mitochondria, resulting in permeabilization of the outer membrane. This process is mediated by Bcl-2 family proteins. The “BH3-only” members of this family, such as Bid and Bim, induce oligomerization of the pro-apoptotic family members, Bax and Bak, the outer mitochondrial membrane, leading to the release of cytochrome c into the cytosol, whereas anti-apoptotic family proteins, such as Bcl-2 and Bcl-XL, repress this event (4–6).
Cytosolic cytochrome c triggers the formation of the apoptosome, an oligomeric complex that includes apoptotic protease activating factor 1 (Apaf-1)4 and C9, the apical caspase in the mitochondrial cell-death pathway (7). In the currently accepted model, procaspase-9 (proC9) exists in unstimulated cells as a cytosolic pool that is monomeric and uncleaved. Apical caspases, in contrast to their downstream effector caspases, are activated primarily by dimerization rather than interdomain cleavage.
After assembly of the apoptosome, proC9 monomers bind via their N-terminal CARD domains to one of seven “acceptor” CARD domains on the apoptosome, which are flexibly tethered to the main body of the apoptosome and cluster around the seven-fold axis (8). Binding of multiple proC9 monomers to the CARD cluster creates a high local concentration that is sufficient to drive proC9 dimerization, the primary activation event (9, 10). Although bound to the apoptosome, C9 cleaves and activates its downstream effectors, caspase-3 and -7, in a proteolytic cascade that ultimately leads to apoptosis if left unchecked (11–15).
C9 is only active when bound to the apoptosome (47), and recent studies have shown that apoptosome-bound dimeric proC9 undergoes rapid cleavage (or “nicking”) within the catalytic domain to generate dimers of C9-p35/p12. Both cleaved and uncleaved C9 dimers are fully active, and the purpose of cleavage appears to be to initiate a molecular timer (16). Thus, cleaved C9 dimers have a reduced affinity/faster off-rate from the apoptosome; once released, cleaved C9 reverts to a monomeric (inactive) state and is replaced by unprocessed C9 from the cytosolic pool. This dimerization/cleavage/release cycle repeats until the cytosolic pool of unprocessed C9 is exhausted. In this scenario it is the size of the pool and apoptosome retention time of cleaved C9 that determine the duration of the apoptotic signal. However, the molecular basis for the reduced affinity of cleaved C9 for the apoptosome is unknown.
The large repertoire of anti-apoptotic proteins encoded by viruses reflects the importance of apoptosis in limiting viral replication in host cells. Many DNA viruses encode structural and functional homologs of anti-apoptotic Bcl-2 proteins (vBcl-2). Some examples are adenovirus E1B-19k, African swine fever virus A179L, cytomegalovirus vMIA, Epstein-Barr virus BHRF1, Fowlpox virus FPV039, Kaposi sarcoma-associated herpesvirus KSBcl-2, myxoma virus M11L, Orf virus ORFV125, and vaccinia virus proteins F1L and N1L (2, 3, 17).
Structural analyses have revealed strong conservation of secondary and tertiary structure between vBcl-2 proteins and their cellular counterparts. This similarity extends to the Bcl-2 homology (BH) domains, BH1-BH4, despite low (or undetectable) sequence similarity in most cases (18–24). This structural homology often correlates with function. Thus, several vBcl-2 proteins mimic their host counterparts by inhibiting apoptosis via binding to (and inactivating) pro-apoptotic Bcl-2 family proteins such as Bax, Bak, and Bim.
Vaccinia virus (and the closely related variola virus, the causative agent of smallpox) belong to the Orthopoxvirus family of the Poxviridae, whose virions package large DNA genomes (130–300 kb) encoding >200 genes. After cell entry and virion uncoating, vaccinia virus expresses a number of proteins that subvert the host machinery to enable viral replication (1, 17, 25). The vaccinia vBcl-2 protein, F1L (like its cellular counterparts), localizes to the mitochondrial outer membrane by inserting a C-terminal transmembrane helix (26, 27). F1L inhibits apoptosis in part by binding to and inhibiting the pro-apoptotic proteins, Bak and Bim (28–31). However, we recently reported that F1L utilizes a distinct yet complementary mechanism of apoptosis regulation; that is, the selective inhibition of C9 (32).
Here we define the molecular and structural determinants of this function. Specifically, we demonstrate that a conserved N-terminal 15-residue motif is necessary for binding and that synthetic peptides derived from this motif inhibit C9 activity in vitro and apoptosis in cells. The motif resembles a caspase-3-inhibitory segment of the host XIAP protein, and we show that the inhibitory mechanisms have many similarities. Based on structural comparisons and mutagenesis, we built a three-dimensional model of the F1L·C9 complex. Our analysis suggests that inhibition at the apoptosome is achieved by engagement of the N-terminal motif with the C9 active site in a reverse orientation compared with substrate peptides and offers new insights into both pathogen and host mechanisms of apoptosome regulation. Finally, experiments with uncleavable variants of C9 raise the possibility of additional or complementary mechanisms of C9 inhibition as well as suggest how F1L coordinates its dual anti-apoptotic functions during infection.
EXPERIMENTAL PROCEDURES
Materials
HEK-293T cells were obtained from the ATCC. Cytochrome c (from bovine heart), dATP, and Anti-FLAG M2-peroxidase antibody were purchased from Sigma. Rabbit monoclonal anti-C9 (clone E23) was purchased from Abcam Inc. (Cambridge, MA). Mouse anti-GST was purchased from BD Pharmingen. Dynabeads Protein G, anti-Bak, mouse monoclonal (clone 3E6), and rabbit polyclonal anti-GFP were obtained from Invitrogen. The caspase peptidyl substrate Ac-LEDH-AFC was purchased from BIOMOL. Glutathione-Sepharose 4B resin was obtained from GE Healthcare. All experiments utilized vaccinia virus strain Western Reserve (VACV-WR).
Plasmids
The genes encoding full-length F1L (residues 1–226) and F1LΔ34 (i.e. lacking the N-terminal 34 residues) as well as the N-terminal peptides F1L-(1–36) and F1L-(1–15) were subcloned into pEGFP-C1 (BD Biosciences Clontech) for mammalian cell expression as GFP fusion proteins. F1LΔ44-pGFP, F1LΔ59-pGFP, and F1LΔ75-pGFP plasmids were generous gifts from Dr. Michael Way (Cancer Research UK). F1LΔTM (i.e. lacking the C-terminal transmembrane motif), F1LΔTMΔ23, F1LΔTMΔ34, F1LΔTMΔ47, F1L-(1–36), and F1L-(1–47) were subcloned into pGEX-4T-1 (GE Healthcare) for bacterial expression as GST fusion proteins.
Mutants in the context of full-length F1L, L2A, S3P, M4A, F5A, C7A, C7Y, D12A, V14A, and D15A, and in the context of F1L-(1–15) or F1L-(1–36), C7A, C7Y, and C7A/D12A. were made using the QuikChange site-directed mutagenesis kit (Stratagene). Plasmids encoding viral N1L (a negative control), wild-type proC9, an active site point mutant that forms dimers but cannot be autolytically cleaved (proC9-C287A), a mutant that is activatable but cannot be cleaved (proC9-5A), a mutant that is constitutively dimeric and cleaved (C9-Dimer), and human full-length Apaf-1, have been described (10, 33–36). C9-Dimer-pET21b was a gift from Dr. Yigong Shi (Tsinghua University, Beijing, China) and was used to generate C9-Dimer-pFLAG-CMV2.
Bacterial Expression and Purification
ProC9 and its mutants were expressed in bacteria as His6-tag proteins and purified using nickel-nitrilotriacetic acid resin (Qiagen). GST-tagged F1LΔTM, F1LΔTMΔ23, F1LΔTMΔ34, F1LΔTMΔ47, F1L-(1–47), and F1L-(1–36) were expressed in bacteria and affinity-purified using glutathione-Sepharose (GE Healthcare). Further purification involved on-column thrombin cleavage of the GST tag followed by gel filtration chromatography (F1LΔTM and F1LΔTMΔ34) or Amicon ultracentrifugation with 30-kDa cut-off filters (F1L-(1–47) and F1L-(1–36)). Full-length His-tagged Apaf-1 protein was expressed in Sf9 insect cells at 27 °C for 60 h and purified as described (33).
In Vitro Protein Binding Assays
1 μg of recombinant GST, GST-F1LΔTM, or its truncated versions were incubated with 1 μg of activated C9 at 25 °C for 15 min in PBS. 10 μl of glutathione-Sepharose resin (GE Healthcare) was then added to the mixture. The protein-bound resin was washed 3 times using PBS buffer plus 20 mm imidazole and 0.1% Triton X-100, and all the associated proteins were boiled and analyzed by SDS-PAGE/immunoblotting using anti-C9 or anti-GST antibodies.
Coimmunoprecipitation (Co-IP) Assays
12 × 106 HEK293T cells were transfected using Lipofectamine 2000 (Invitrogen) with plasmids containing GFP, GFP-F1L, or its mutants together with FLAG-proC9 or its mutants as indicated. After 17 h, cells were harvested and lysed in 20 mm HEPES, pH 7.5, 150 mm NaCl, 5 mm MgCl2, 2 mm EDTA, 0.2% Nonidet P-40, 2 mm DTT, and protease inhibitors (Roche Applied Science). Cell lysates were co-immunoprecipitated with Dynabeads Protein G (Invitrogen) using mouse monoclonal anti-GFP, 3E6 (Invitrogen). Dynabeads were then washed three times with lysis buffer. Immunocomplex IPs and cell lysates were analyzed by SDS-PAGE/immunoblotting with anti-GFP or anti-FLAG antibodies.
Cell Culture, Transfection, and Apoptosis Assays
HEK293T cells were maintained in Dulbecco's modified Eagle's medium (Irvine Scientific; Irvine, CA) supplemented with 10% fetal bovine serum, 1 mm l-glutamine, and antibiotics. For transient transfection apoptosis assays, cells (5 × 105) in 6-well plates were co-transfected using Lipofectamine 2000 (Invitrogen) with 0.5 μg each of pEGFP-C1 (Clontech; Mountain View, CA) or pcDNA3-Flag-C9 together with various amounts of GFP-F1L-(1–15) plasmids or the C7A mutant.
For viral infection studies, VACV Western Reserve strain lacking the F1L coding sequence (ΔF1L) was grown and titered as previously described (31). HEK293T cells were infected with ΔF1L at a multiplicity of infection of 1 plaque-forming unit per cell; after 24 h, cells were transfected with wild-type and mutant GFP-F1L-(1–15) plasmids.
Apoptosis was assessed by DAPI-staining of cells. Briefly, 20 h after transfection both floating and adherent cells were collected, fixed with PBS containing 3.7% formaldehyde, and stained with 0.1 mg/ml DAPI in PBS. The percentages of apoptotic cells were determined by UV microscopy, counting GFP-positive cells having nuclear fragmentation and/or chromatin condensation. All assays were performed in triplicate.
In Vitro C9 Activity Assays
F1LΔTM, F1L N-terminal peptides, or N1L protein (negative control) were mixed with proC9 (200 nm) in 20 mm HEPES, pH 7.0, followed by the addition of apoptosome components (cytochrome c (1 μm), dATP (40 μm), and recombinant full-length Apaf-1 (1 μm)), and incubated at 37 °C for 10 min. The reaction was performed in 100 μl of standard caspase assay buffer (20 mm HEPES, pH 7.5, 2 mm MgCl2, 10 mm KCl, and 5 mm DTT) for 30 min at 37 °C. C9 activity was determined by hydrolysis of the fluorogenic tetrapeptide substrate, Ac-LEHD-AFC (BIOMOL), at 100 μm.
Peptide Synthesis and Purification
F1L and Bcl-2 peptides were synthesized and purified as described previously (37), and peptide mass was confirmed by MALDI-TOF spectrometry.
Protein Docking
In the first approach the crystal structure of wild-type C9 (PDB code 1JXQ) (9) was used as a model for docking of the F1L-(1–12) peptide initially in an extended conformation. GRAMM-X and ZDOCK protein-protein docking servers were used for calculations (38, 39). Both servers generated similar results, with the top hits clustered with peptide bound to the active site in the reverse orientation with respect to substrate in the active site. In the second approach, the initial model was derived by overlay of the crystallographically defined caspase-3·XIAP BIR2 complex (PDB code 1I30) (40) with C9 (PDB code 1JXQ) and energy-minimized using FIREDOCK (41). The F1L-(1–15) peptide was initially positioned based on a superposition of the caspase-3·BIR2 complex onto the crystal structure of C9 (see Fig. 3).
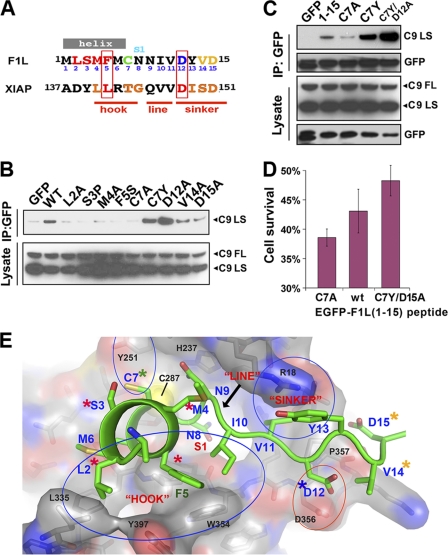
FIGURE 3.
A model for the F1L-(1–15)·C9 interaction. A, shown is sequence alignment of F1L-(1–15) with the caspase-3 inhibitory segment of XIAP. Mutated F1L residues (see below) are color-coded according to their effects on C9 binding/inhibition (residues not tested are in black). XIAP residues critical for caspase-3 inhibition are colored red and boxed; other residues involved in significant protein-protein interactions are in orange. hook, line, and sinker are defined under “Results.” B, shown is mutational analysis of full-length F1L binding to C9 in HEK239T cell lysates as judged by co-IP. C, shown is mutational analysis of the binding of GFP-F1L-(1–15) peptide fusions in the same system as B. D, shown is inhibition of VACV-ΔF1L virus-induced apoptosis of HEK293T cells by F1L peptides delivered in trans. EGFP-F1L-(1–15) peptide constructs were transfected into cells 24 h post-infection. E, shown is a hypothetical three-dimensional atomic model of the F1L-(1–15)·C9 inhibitory complex. The F1L peptide docks into the C9 active site in a reverse orientation compared with substrate binding. C9 is shown as a semitransparent surface (colored by charge potential) with a stick model evident beneath (carbon atoms are in gray; other atoms are colored by type as for the F1L peptide; the catalytic cysteine, Cys-287, is shown as a yellow ball), whereas the F1L peptide is shown as a schematic and side-chain model (carbon and the schematic are in green; non-carbon side chains are colored by atom type: oxygen = red; nitrogen = blue; sulfur = yellow). Selected C9 residues are labeled in black, and FlL is in blue. Key interactions regions, including the hook and sinker are circled. Colored asterisks indicate the effects of mutations (red = significant reduction in binding; orange = small reduction; blue = increased binding; green = mixed: i.e. C7A and C7Y mutations have opposite effects) and correspond to the color scheme in A.
RESULTS
We recently reported that F1L is a direct inhibitor of C9, the apical caspase of the intrinsic pathway to apoptosis (32). Unlike mammalian Bcl-2 family proteins, F1L possesses an N-terminal extension (residues 1–54) that is not required for interaction with Bak (18, 31), and we showed that a point mutation close to the N terminus (C7A) abrogated C9 binding and inhibition without affecting binding to Bak (32). To define further the structural determinants of this interaction, we first aligned the sequences of the orthopoxviruses in light of the recently determined crystal structure of a major fragment of F1L from the vaccinia virus strain modified vaccinia Ankara (VACV-MVA) strain of vaccinia virus (18), which is closely related to the VACV-WR strain employed here.
The crystal structure construct encompasses residues 22- 190 (VACV-WR numbering) (Fig. 1A and supplemental Fig. 1). The Bcl-2-like domain comprises residues 60–190 and forms a strong dimer both in the crystals and in solution. This region is >95% identical across the Orthopoxvirus family. Each monomer comprises a bundle of seven helices, although helix α3, which is implicated in Bak/Bim binding, is poorly ordered. The C-terminal ∼30 residues (197–226) were excluded from the construct and are predicted to form a transmembrane helix that anchors F1L into the mitochondrial membrane. In the crystals, an additional helix is observed, α0 (residues 43–58), which is N-terminal to the Bcl-2-like domain; it is stabilized by intermolecular contacts and is predicted to be linked flexibly to the body of the domain in solution (18). Residues 22–42 were not visible in the crystal structure and are presumed to be disordered.
FIGURE 1.
Common structural and functional features of the Orthopoxvirus family F1L proteins. A, shown is a three-dimensional rendering of the Bcl-2-like dimer of F1L, based on PDB code 2VTY (18). Helices 1–6 are colored in spectral order (blue through red) for each monomer. The N-terminal helix, α0 (in cyan), is predicted to be mobile; no density was observed before residue 38. Helix α7 (magenta) precedes the C-terminal transmembrane (TM) segments, which were not included in the crystallized construct. Helix α3, implicated in Bim/Bak binding, is poorly ordered in the crystal structure and is indicated by a dashed loop. B, alignment of the N-terminal segments of F1L proteins from selected orthopoxviruses is shown. Predicted helical segments are indicated by gray bars. Conserved regions are boxed in red, blue, and green. A large insertion in the F1L protein of cowpox virus strain Brighton Red (CPXV-BR) strain is marked by a red asterisk and shown separately. VACV-IOC F1L is a truncated variant comprising only the N-terminal peptide (1–68) (GenBankTM accession number ABP97442.1). VACV-WR, GenBankTM accession number NC_006998); VACV-MVA, GenBankTM accession number U94848; VARV-YUG72, GenBankTM accession number DQ441448) CPXV-BR, (GenBankTM accession number NC_003663). C, hybrid proteins were constructed by appropriate mutagenesis of the N-terminal segment of the VACV-WR F1L gene (the region highlighted in gray in B). Δ16–19 is a mimic of VACV-MVA F1L protein. MYAL-Δ16–19-S3P/Y13D is a mimic of the VARV-YUG72 F1L protein. A deletion mutant of VACV-WR F1L (Δ16–37) lacking the entire region between the N-terminal conserved element and helix α0 was also tested. Their ability to bind C9 was assessed by co-IP from HEK293T cells co-transfection with FLAG-C9 and GFP-F1L plasmids (see “Experimental Procedures”). C9 FL and C9 LS represent full-length (proC9) and the large subunit of processed C9, respectively.
Although the entire N-terminal region (1–42) is likely to be disordered in the full-length protein (at least in the absence of a binding partner), alignment of F1L sequences reveals two strongly conserved N-terminal segments (Fig. 1B); (i) the N-terminal 15 residues (19 residues in many orthologs, which have an additional 4 residues at the N terminus) and (ii) residues 24–60, which include helix α0 (residues 41–60). Secondary structure predictions (42) point to a propensity for helix formation (similar to that for α0) at the beginning of both conserved regions. Between these conserved elements is an insert that varies greatly in length and sequence among F1L orthologs, sometimes comprising multiple tandem inserts of an aspartic acid-rich motif. Although F1L is not a substrate of C9 (32), it can be cleaved by caspase-3, -7, or -8 in vitro. We determined the cleavage sites by N-terminal protein sequencing and found that they occur predominantly in this inserted region (supplemental Fig. 2).
To explore the generality of F1L-mediated C9 inhibition among orthopoxviruses, we mutated the N-terminal segment of VACV-WR F1L to match the sequence of either its VACV-MVA (VACV-WR F1L(Δ16–19)) or variola virus strain Yugoslavia 1972 V72–164 (VARV-YUG72; VACV-WR Met-Tyr-Asn-Leu-F1L(Δ16–23)-S3P/Y13D) counterpart. Given the very high similarity between their C-terminal domains, these hybrid proteins should have nearly identical sequences to those expressed by the corresponding viruses. We found that both hybrids bound efficiently to C9, consistent with an evolutionarily conserved function (Fig. 1C).
N-terminal F1L Peptides Are Sufficient for C9 Binding and Inhibition in Vitro
We next generated a series of N-terminal F1L truncations in the context of Escherichia coli-expressed recombinant F1L lacking the C-terminal transmembrane region (F1LΔTM), a soluble construct that we previously showed to inhibit C9 (32). We found that removal of 23, 34, or 47 N-terminal residues reduced substantially (and to a similar extent) the ability of F1LΔTM to inhibit C9 in vitro (Fig. 2A).
FIGURE 2.
The N-terminal region of F1L is sufficient for C9 interaction and inhibition. A, F1LΔTM or different N-terminal truncation mutants (2 μm) lacking the first 23, 34, or 47 residues were preincubated with C9 (200 nm) that was activated using constitutively active Apaf-1ΔWD-40 (1 μm) for 10 min. C9 activity was measured by hydrolysis of Ac-LEHD-AFC (mean ± σ; n = 3). The first column shows C9 activity in the absence of F1LΔTM. B, shown are co-IP assays of F1L and truncation mutants with C9. HEK29T cells were co-transfected with plasmids expressing FLAG-C9 and either GFP, GFP-F1L, GFP-F1LΔ34, or GFP-F1LΔ44 as described under “Experimental Procedures.” IPs and cell lysates were also immunoblotted (WB) with anti-Bak antibodies as indicated. The Bak immunoblot contains a nonspecific band above the band indicated for Bak. C9 FL and C9 LS represent full-length (proC9) and the large subunit of processed C9, respectively. C, shown is N-terminal peptide containing an authentic N terminus inhibit C9. A series of F1L N-terminal peptides was mixed at different concentrations with pro-C9 (200 nm) in HEPES buffer and apoptosome components (cytochrome c, dATP, and recombinant full-length Apaf-1 (1 μm)) and incubated at 37 °C for 10 min. C9 activity was measured by hydrolysis of Ac-LEHD-AFC (mean ± S.D.; n = 3). F1L-(1–47) peptide was purified from bacterial cultures; other peptides were chemically synthesized. D, the point mutation, C7A, ablates the inhibitory activity of N-terminal peptides. Inhibition of C9 activity by F1L-(1–36) and F1L (1–36)-C7A was quantified under the same condition as in C. The value of Ki,app for F1L-(1–36), in the low μm range, was very similar that determined for F1LΔTM (see Table 1), whereas Ki,app for the mutant was estimated at >600 μm. E and F, GFP-F1L-(1–15) is sufficient to protect cells from apoptosis induced either by C9 transfection or staurosporine. E, HEK293T cells were co-transfected with FLAG-C9 plasmid (0.5 μg) and increasing amounts (0–2 μg) of GFP-F1L-(1–15) or GFP-F1L-(1–15)-C7A plasmids as indicated and incubated for 20 h. Total transfected DNA was kept constant at 3 μg by the addition of GFP plasmid. F, procedures were as in E, except that cells were treated with 0.2 μm staurosporine for 10 h rather than being transfected with FLAG-C9. In both cases, floating and adherent cells were collected, fixed, and stained with 0.1 μg/ml DAPI. The percentage of apoptotic cells was determined by counting GFP-positive cells showing nuclear fragmentation and/or chromatin condensation (mean ± σ; n = 3). Statistical pairwise comparisons were performed using the two-tailed Student's t test, where p ≤ 0.05 is considered significant, shown on E and F schematically by asterisks. For C9-induced apoptosis, p values are 0.008, 0.015, and 0.006 (wt) and 0.53, 0.1, and 0.02 (C7A). For staurosporine-induced apoptosis, p values are 0.04, 0.002, and 0.0004 (wild type) and 0.12, 0.12, and 0.03 (C7A). G and H, aliquots of the cell lysates prepared in E or F were normalized for protein content (10 μg) and incubated with the caspase-3/7 substrate Ac-DEVD-AFC. Enzyme activity was determined as in C (mean ± σ; n = 3).
We also studied binding in a eukaryotic cell setting by expressing FLAG-tagged C9 and GFP-tagged F1L mutants in HEK293T cells and found that full-length GFP-F1L co-immunoprecipitated with C9, whereas mutants that lacked the first 34 or 44 residues did not. Note that in this system both proC9 and cleaved/processed C9 (C9-p35/p12) are observed but that F1L co-IPs only with processed C9 (indicated as C9 LS for the p35 large subunit in Fig. 2B). As expected, all F1L variants retained their ability to bind Bak; this is consistent with the structural model, as the truncations (the largest of which extends to the beginning of helix α0) are predicted not to affect the structural integrity of the Bcl-2-like domain (Fig. 2B).
We next asked whether N-terminal F1L peptides (presented either as synthetic peptides or fusion proteins) were sufficient for C9 binding and inhibition. We synthesized a series of 25-residue peptides covering the N-terminal region up to and including helix α0 and found that although F1L(1–25) and F1L-(1–47) showed similar dose-dependent inhibition, peptides that lacked an authentic N terminus showed little or no inhibitory activity (Fig. 2C).
We also quantified the C9 inhibitory activity of the peptide F1L-(1–36) (Fig. 2D). We found that inhibition was saturable, with a Ki,app in the low μm range, very similar to the value for F1LΔTM (see Table 1). As a control, we tested the point mutant, C7A. Consistent with the behavior of this mutation in the context of full-length F1L (32), inhibitory activity was greatly reduced (>600-fold). We concluded that short peptides derived from the N terminus of F1L are sufficient for C9 inhibition in vitro.
TABLE 1.
Apparent inhibition constants (Ki,app) of F1L proteins and peptides for wild-type and uncleavable C9
| WT | ProC9-5A | |
|---|---|---|
| μm | ||
| F1LΔTM | 2.9 ± .8 | 1.8 ± .6 |
| F1LΔTMΔ34 | 41 ± 15 | 21 ± 3 |
| F1L-(1–15) | 3.5 ± 1.1 | 4.4 ± 0.9 |
| F1L-(1–36) | 2.7 ± 0.7 | |
| F1L-(1–36)-C7A | >800 | |
| F1L-(1–36)-C7A/D12A | 4.2 ± 0.9 | |
GFP-F1L-(1–15) Inhibits Apoptosis
Given the inhibitory activity of short N-terminal peptides and the strong conservation of the first 15 residues in particular, we tested the ability of the peptide fusion, GFP-F1L-(1–15) to suppress cellular apoptosis, an activity we had previously demonstrated for the full-length protein (32). In the first set of experiments, HEK293T cells were transfected with FLAG-C9 and increasing amounts (0.5–2 μg) of GFP-F1L-(1–15) plasmid. A strong concentration-dependent reduction in apoptosis was observed (Fig. 2E). In a second experiment, we found that GFP-F1L-(1–15) also suppressed (and to a similar extent) apoptosis induced by staurosporine (a more general activator of the mitochondrial cell-death pathway) (Fig. 2F). We used the C7A mutation in the same context as a control and observed only weak inhibition in both cases, as expected. To confirm these results, we used a second read-out of apoptosis (caspase activity of the same cells using a synthetic caspase-3/7 substrate, Ac-DEVD-AFC) and observed very similar F1L-(1–15) dose-dependent behavior (Fig. 2, G and H). These results demonstrate that selective inhibition of C9, mediated by GFP-F1L-(1–15), is sufficient to produce a powerful anti-apoptotic effect in transfected cells.
Toward a Model of F1L-(1–15) Inhibition of C9
We previously showed that the inhibitory mechanism of F1L is distinct from that of other viral inhibitors, such as the “suicide” inhibitors, p35 and CrmA, which bind in a substrate-like fashion and form covalent bonds to the active site cysteine (43–46)); it is also quite different from that of the cellular inhibitor, XIAP, which inhibits C9 by binding (via its BIR3 domain) to the N terminus of the monomer, thereby inhibiting dimer formation (47).
The ability of a short peptide to bind reversibly to a protease and inhibit it without being cleaved is unusual but not unprecedented. A pertinent example is the mechanism of XIAP inhibition of caspase-3 and -7 (40, 48–50). In this case XIAP utilizes a different domain, BIR2, which clamps an extended peptide segment across the active site with reversed (N to C) polarity relative to natural substrates. This arrangement sterically inhibits access to the active site by authentic substrates, and cleavage of XIAP does not occur because the catalytic machinery surrounding the nucleophilic cysteine is not arranged appropriately to drive peptide bond hydrolysis.
Comparison of the F1L-(1–15) motif with the inhibitory peptide segment of XIAP-BIR2 domain suggested a highly plausible alignment (Fig. 3A). For XIAP, inhibition requires anchor points on either side of the active site to hold the central peptide segment taut across the region containing the nucleophilic cysteine and specificity pockets (40). Thus, mutations at either anchor point (which we called the “hook” and “sinker” (40)) have been shown to ablate inhibitory activity (51). Therefore, to test our reverse binding hypothesis, we made similar mutations in the putative anchor regions of F1L-(1–15). Our choice of mutants was also guided by natural variations in F1L orthologs, notably at positions 3 (Ser/Pro), and 7 (Cys/Tyr).
We first constructed point mutants in the context of F1LΔTM and found that mutants L2A, S3P, M4A, and F5S reduced C9 binding to an extent comparable with the C7A mutant we described previously (Fig. 3B). By contrast, a D12A mutation actually enhanced binding, whereas alanine mutations at Val-14 and Asp-15 had little effect. We also mutated Cys-7 to Tyr, the residue observed in monkeypox and cowpox strains, and found that this mutation also enhanced binding, in complete contrast to the C7A mutation.
We tested a subset of these mutants for C9 binding in the context of GFP-peptide fusions and HEK293T cell lysates and obtained consistent results. Thus, although GFP-F1L-(1–15) co-immunoprecipitated efficiently with C9, the C7A mutant showed greatly diminished interaction, whereas the C7Y mutation increased interaction, which was further enhanced by the double mutation, C7ϒ/D12A (Fig. 3C).
Finally, it has been shown that deletion of the F1L gene from VACV-WR (VACV-ΔF1L) causes increased apoptosis during infection (31). We, therefore, asked whether F1L peptides could reduce apoptosis of infected cells when delivered in trans. We infected HEK293T cells with VACV-ΔF1L and 24 h later transfected them with EGFP-F1L peptide fusions. We found that both the wild-type and mutant (C7Y/D12A) peptides significantly increased cell survival compared with the C7A peptide (Fig. 3D).
An Atomic Model of the C9·F1L-(1–15) Complex
To put these mutagenesis data on a structural footing, we sought to derive an atomic model of the C9·F1L-(1–15) complex using computational approaches. We began by applying an unbiased docking procedure using an extended F1L-(1–15) peptide and the crystal structure of dimeric C9 (supplemental Fig. 3). Consistent with our reverse binding hypothesis, we found that the top solutions positioned the peptide at the active site and in the reverse orientation with respect to substrate binding. Specifically, the central peptide segment (Cys-7—Ile-11) straddled the catalytic cysteine and S1 specificity pocket.
Encouraged by this result, we built a second model based on the crystallographically defined interaction between the caspase-3 and XIAP-BIR2 (40) (Fig. 3E). The F1L-(1–15) peptide was initially positioned based on a superposition of the caspase-3·BIR2 complex (40) onto the structure of C9 (9) and assuming the sequence alignment in Fig. 3A. After replacing BIR2 side chains with those of F1L, the model was subjected to a simple energy minimization procedure (41). Remarkably, this model placed the central segment of the F1L motif, Cys-7—Ile-11, in the same location and orientation as the “unbiased” docking model (root mean square deviation on Cα < 2 Å).
The first seven N-terminal residues generally conform to the BIR2 starting model by adopting two turns of α-helix (as noted above, helical propensity is predicted for this segment) that point toward the catalytic cysteine, Cys-287. This constitutes the first anchor point (or hook), which in the model is stabilized by packing between a cluster of hydrophobic F1L residues, Leu-2, Met-4, Phe-5, Met-6, and Ile-10, and hydrophobic side chains on the protein surface (notably Leu-335, Val-352, Trp-354, and Tyr-397) as well as by intra-F1L interactions between Phe-5 and Ile-10. The model is thus consistent with the deleterious effects of the L2A, M4A, and F5S mutations, as these are predicted to disrupt the hydrophobic packing. Furthermore, the residue analogous to F1L Met-4 in XIAP (Leu-141) was one of 2 residues identified as critical for caspase-3 binding and inhibition (51).
F1L Cys-7 lies at the base of the helix; its side chain points away from the hydrophobic cluster and interacts with the protein surface. The deleterious effect of the C7A mutation may arise from loss of H-bonding interactions with the protein surface or perhaps by reducing the stability of the short α-helix (this is likely the case for the S3P mutant). The model also suggests that the C7Y mutation would allow more substantial interactions with the protein surface, providing a rationale for the stronger binding and inhibitory activity of this mutant. The additional four residues found at the N terminus of many Orthopoxvirus orthologs may extend the length and stability of this helix, and it is interesting to note that all orthologs that possess proline at position 3 have this four-residue extension, which we suggest ameliorates the helix-breaking propensity of proline.
The model predicts that the side chain of Asn-8 (invariant among the orthopoxviruses) projects into the S1 primary specificity pocket (this would be occupied by an Asp in a C9 substrate peptide). Residues 10–13 form a loop that constitutes the second anchor point (the “sinker”), with the side chain of I-10 also making hydrophobic interactions with the protein surface and Tyr-13 making both inter- and intramolecular contacts. The side chain of Asp-12 makes a close (unfavorable) contact with the protein side chain of Asp-356, rationalizing the enhanced binding of the D12A mutant. The equivalent mutation in XIAP (Asp-148) was the second residue shown to be critical for C-3 binding (51). Finally, Val-14 and Asp-15 make relatively few contacts with the protein, consistent with the minor effects of their mutation. The three-dimensional atomic model is thus highly consistent with the mutagenesis data.
F1L N-terminal Peptide Does Not Bind the Active Site Mutant, ProC9-C298A
As previously noted, apoptosome-mediated dimerization is the primary step in proC9 activation, whereas auto-cleavage serves mainly to promote turnover of active enzyme and thus limit the duration of the pro-apoptotic signal. To gain further insight into the F1L inhibitory mechanism, we compared the ability of full-length F1L, the truncation mutant, F1LΔ34, and the peptide, F1L-(1–36), to bind to two engineered variants of C9; (i) a catalytic site mutant (proC9-C287A) that can dimerize but is catalytically inactive and (ii) a mutant (proC9-5A) that forms dimers that are active but uncleavable. It has been shown that a similar non-cleavable active mutant promotes a constitutively active apoptosome, whereas proC9-C297A inhibits apoptosome turnover and catalytic activity (16).
We found that full-length GFP-F1L co-immunoprecipitated with both uncleavable C9 variants and with an efficiency similar or greater than for wild-type C9 (Fig. 4A). By contrast, the peptide fusion, GFP-F1L-(1–36), co-immunoprecipitated strongly with wild-type C9 and the active, uncleavable dimer (ProC9–5A) but not with the inactive dimer (proC9-C287A). Using our standard in vitro inhibition assay, we found that F1L-(1–15) peptide inhibited ProC9–5A with an efficiency nearly identical to that of F1LΔTM and very similar to the values observed for inhibition of the wild-type enzyme (Ki,app in the low μm range) (Fig. 4, B and C, and Table 1).
FIGURE 4.
Evidence for an auxiliary binding site for F1L on C9. A, shown are immunoblots (WB) from HEK293T cells transfected with plasmids encoding GFP (lane 1), GFP-F1L (lane 2), GFP-F1LΔ34 (lane 3), or GFP-F1L-(1–36) (lane 4) together with either wild type or one of two FLAG-C9 variants (inactive C9 (mutated in its active site, proC9-C287A) and uncleavable C9 (mutated in its interdomain linker, proC9-5A) that still form dimers at the apoptosome. B and C, shown is inhibition of WT and uncleavable (proC9-5A) by F1LΔTM, F1LΔTMΔ34, and F1L-(1–15) in vitro. C9 was activated with apoptosome components (cytochrome c, dATP, and full-length Apaf-1) and incubated at 37 °C for 10 min. C9 activity was measured by hydrolysis of Ac-LEHD-AFC. The values were normalized by assigning the activities of proC9 or proC9-5A as 0%, whereas that of apoptosome-activated proC9 was 100%. The percentage values are presented as the mean ± S.D. (n = 3). Vaccinia N1L protein was used as the negative control.
That the N-terminal peptide, F1L-(1–36), requires a functional enzyme with a preformed active site to bind and inhibit C9 is consistent with our model of the peptide binding directly to the active site. The ability of full-length F1L to bind proC9-C287A, which lacks an active site, therefore raises the possibility of a second or auxiliary binding site between C-terminal elements of F1L and C9. The behavior of the truncation mutant lends further support to this hypothesis. Thus, although F1LΔ34 does not co-immunoprecipitate with wild-type C9, we found that it co-immunoprecipitated strongly with both uncleavable C9 variants. Moreover, ProC9–5A displayed saturable inhibition of both wild-type C9 and proC9-5A, with Ki,app values only 20–30 times higher than for full-length F1L or F1L-(1–36), raising the possibility that an additional or complementary inhibitory mechanism, albeit of lower potency, operates in cells (see below).
DISCUSSION
We have shown that the N-terminal 15-residue motif of vaccinia virus F1L is sufficient for inhibiting the catalytic activity of C9 in vitro with a potency nearly identical to that of full-length F1L as well as inhibiting apoptosis in cells (triggered either by C9 transfection or staurosporine) when presented as a GFP-peptide fusion. We have defined the molecular determinants of inhibition by mutagenesis and developed an atomic model of the F1L-(1–15)·C9 complex that is consistent with our functional studies. In this model, inhibition is achieved by reverse binding of the F1L peptide into the C9 substrate binding groove, a mode similar to that which we previously characterized for the inhibition of caspase-3 by the BIR2 domain of XIAP (40). In addition, we have presented evidence for a second or auxiliary binding site for C9 contained within C-terminal elements of F1L.
How might inhibition work in cells? The “molecular timer” model of apoptosome function implies that within the cytosolic pool, only uncleaved C9 has pro-apoptotic potential. One plausible anti-apoptotic strategy for F1L would be to sequester the uncleaved pool before it can reach the apoptosome. An alternative strategy would be to target and inhibit the activity and turnover of the apoptosome, which would only require inhibition of the subset of apoptosome-bound dimers.
Although we do not yet have definitive answers to these questions, our data provide support for both mechanisms operating in cells. How do peptides work? We observe F1L peptides bound only to C9 (either cleaved or uncleaved) that is capable of forming a functional active site. This suggests that peptides function at the apoptosome, as this contains the active pool of C9, thus preventing C9-mediated proteolysis of its effectors caspases (caspase-3 and -7). The peptides do not seem to retard proC9 auto-cleavage, as we only observe cleaved C9 bound to F1L in cell lysates (Fig. 4A). However, the robust co-IP of F1L peptide with C9 raises the possibility that peptide increases the retention time of cleaved (inhibited) C-9 at the apoptosome, perhaps by stabilizing the dimeric state. In this regard, we previously showed that a small molecule inhibitor stabilized the dimeric form of C9 in vitro (9). A testable hypothesis, therefore, is that processing/cleavage of C9 reduces apoptosome binding affinity/retention time by reducing dimer stability and that this process can be modulated by the stabilizing effect of F1L.
Notwithstanding, dissociation of cleaved C9 from the apoptosome should promote its (irreversible) switch to a monomeric, inactive form and release of the F1L peptide. The peptide could then return to the apoptosome to engage in further inhibition. In this model, a reversible inhibitor can be seen as highly efficient: that is, it is not necessary to titrate out the entire pool of F1L; it is only required to have sufficient F1L to neutralize that small subset (the active pool) of C9 that is active at any one time. Interestingly, a strain of vaccinia virus has recently been identified (IOC, see Fig. 1B) that expresses a truncated F1L protein comprising only the N-terminal 67 residues, which may work in this fashion.
Our experiments utilizing mutants that form dimers at the apoptosome that are either inactive (proC9–287A) or active (proC9-5A) but that are uncleavable raise a number of intriguing questions concerning the behavior of full-length F1L in cells. Thus, we found that both full-length and truncated F1Ls co-immunoprecipitated with proC9-C287A and proC9-5A in transfected cells, whereas F1L peptide, as expected, bound avidly to both active mutants but failed to bind to proC9–287A. The behavior of the peptide is entirely consistent with our model of active site binding (Fig. 3D). But how can F1L bind to C9 if this binding site is not available? It seems reasonable to invoke a distinct/auxiliary binding site (an exosite) for C9 that exists within elements of F1L that lie C-terminal to the peptide region.
Evidence for an exosite is indirect, but we previously demonstrated that F1L inhibits assembly of the apoptosome and promotes the formation of F1L-C9 oligomers and that the ability of F1L to inhibit C9 in vitro is significantly reduced if its CARD domain is removed (32). A three-dimensional model that is consistent with these data is shown in Fig. 5. Although highly speculative, it does offer a working hypothesis of how the twin anti-apoptotic activities of F1L might function in the context of the mitochondrion. In this model the location of the C-terminal transmembrane helices fixes the arrangement of F1L with respect to the mitochondrial membrane. Given that F1L and C9 are both dimers, it is reasonable to align their two-fold axes. By allowing the F1L dimer to sit atop the C9 dimer, contacts with the CARD domain are feasible, and the F1L N-terminal arms (even the minimal arms defined in Fig. 1) are long enough to reach around the body of the C9 dimer and embrace each active site with an N-terminal 15-residue inhibitory motif. Note that in this model the N-terminal CARD domains (one is visible at the front, drawn schematically) would be sterically occluded from interacting with the apoptosome, so that this could provide a complementary mechanism of C9 inhibition independent of the apoptosome.
FIGURE 5.
Speculative model of the full-length F1L-C9 hetero-tetramer. The C-terminal helices transmembrane (TM) helices are shown schematically as red arrows inserted into the mitochondrial membrane. The 2-fold axes of the F1L and C9 dimers were aligned and their surfaces brought into close apposition. This arrangement allows F1L, even with minimal N-terminal arms (i.e. lacking residues 16–35 (see Fig. 1); shown in blue), to reach around the body of the C9 dimer and embrace each active site with an N-terminal 15-residue motif. Note that in this model the N-terminal CARD domains (one is visible at the front, drawn schematically) would be sterically occluded from interacting with the apoptosome, whereas the Bak/Bim binding sites are fully exposed. The location of the flexible insert containing caspase-3 cleavage sites is indicated at the elbow between F1L-(1–15) and α0.
This model still leaves the Bak/Bim binding sites fully exposed (consistent with our previous data showing that Bak does not affect the inhibitory potential of F1L in vitro) and might represent an early anti-apoptotic complex involved in preventing permeabilization of the mitochondrial membrane. We note, however, that in most F1L orthologs, there is a flexible insert at the elbow between F1L-(1–15) and α0 that contains cleavage sites for caspase-3 in vitro (Fig. 1B and supplemental Fig. 2). If such cleavage occurred in cells, it would release the C9·F1L N terminus complex from the Bcl-2-like domain of F1L (and the mitochondrion), maintaining but physically separating the two distinct antiapoptotic functions of F1L. Whether these activities of F1L are coordinated in space and time during infection in vivo remains to be seen.
The considerations above should frame the next set of experiments aimed at defining how a small viral protein exerts such a powerful function in its host as well as addressing important unanswered questions concerning the mechanism of host response. For example, the relative roles of dimerization vis à vis conformational change in apoptosome-mediated C9 activation remain an issue of keen debate (36, 52) and one that is obviously key to understanding the factors responsible for the reduced affinity/turnover of cleaved C9 for the apoptosome and, hence, the mechanism of the molecular timer (16). The peptide inhibitors identified here should provide valuable probes in this regard as well as insights (and starting points) for the development of therapies targeting selective attenuation of apoptosis either as antiviral or anti-cancer strategies.
Supplementary Material
Acknowledgments
We acknowledge Ge Wei for assistance during the early stages of this project. We are also grateful to Drs. Gombosuren and Satterthwait for peptide synthesis, Christina Pop and Mika Aoyagi for providing N1L, Yigong Shi for dimeric C9, and Drs. Postigo and Way for the ΔF1L virus.
This work was supported, in whole or in part, by National Institutes of Health Grants AI056324 and AI091967. This work was also supported by Department of Defense Grant W81XWH-10-1-0093).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–3.
- Apaf-1
- apoptotic protease activating factor 1
- IP
- immunoprecipitate
- VACV-WR
- vaccinia virus strain Western Reserve strain
- VACV-MVA
- vaccinia virus strain modified vaccinia Ankara
- VACV-ΔF1L
- VACV-WR with its F1L gene deleted
- VARV-YUG72
- variola virus strain Yugoslavia 1972 V72–164
- CPXV-BR
- cowpox virus strain Brighton Red
- TM
- transmembrane
- FL
- full-length
- LS
- large subunit
- AFC
- aminofluoromethylcoumarin.
REFERENCES
- 1. Benedict C. A., Norris P. S., Ware C. F. (2002) Nat. Immunol. 3, 1013–1018 [DOI] [PubMed] [Google Scholar]
- 2. Cuconati A., White E. (2002) Genes Dev. 16, 2465–2478 [DOI] [PubMed] [Google Scholar]
- 3. Galluzzi L., Brenner C., Morselli E., Touat Z., Kroemer G. (2008) PLoS Pathog. 4, e1000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Creagh E. M., Conroy H., Martin S. J. (2003) Immunol. Rev. 193, 10–21 [DOI] [PubMed] [Google Scholar]
- 5. Youle R. J., Strasser A. (2008) Nat. Rev. Mol. Cell Biol. 9, 47–59 [DOI] [PubMed] [Google Scholar]
- 6. Kroemer G., Galluzzi L., Brenner C. (2007) Physiol. Rev. 87, 99–163 [DOI] [PubMed] [Google Scholar]
- 7. Bratton S. B., Walker G., Srinivasula S. M., Sun X. M., Butterworth M., Alnemri E. S., Cohen G. M. (2001) EMBO J. 20, 998–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yuan S., Yu X., Topf M., Ludtke S. J., Wang X., Akey C. W. (2010) Structure 18, 571–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Renatus M., Stennicke H. R., Scott F. L., Liddington R. C., Salvesen G. S. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 14250–14255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pop C., Timmer J., Sperandio S., Salvesen G. S. (2006) Mol. Cell 22, 269–275 [DOI] [PubMed] [Google Scholar]
- 11. Liu X., Kim C. N., Yang J., Jemmerson R., Wang X. (1996) Cell 86, 147–157 [DOI] [PubMed] [Google Scholar]
- 12. Zou H., Henzel W. J., Liu X., Lutschg A., Wang X. (1997) Cell 90, 405–413 [DOI] [PubMed] [Google Scholar]
- 13. Li P., Nijhawan D., Budihardjo I., Srinivasula S. M., Ahmad M., Alnemri E. S., Wang X. (1997) Cell 91, 479–489 [DOI] [PubMed] [Google Scholar]
- 14. Du C., Fang M., Li Y., Li L., Wang X. (2000) Cell 102, 33–42 [DOI] [PubMed] [Google Scholar]
- 15. Verhagen A. M., Ekert P. G., Pakusch M., Silke J., Connolly L. M., Reid G. E., Moritz R. L., Simpson R. J., Vaux D. L. (2000) Cell 102, 43–53 [DOI] [PubMed] [Google Scholar]
- 16. Malladi S., Challa-Malladi M., Fearnhead H. O., Bratton S. B. (2009) EMBO J. 28, 1916–1925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Postigo A., Ferrer P. E. (2009) Microbes Infect. 11, 1071–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kvansakul M., Yang H., Fairlie W. D., Czabotar P. E., Fischer S. F., Perugini M. A., Huang D. C., Colman P. M. (2008) Cell Death Differ. 15, 1564–1571 [DOI] [PubMed] [Google Scholar]
- 19. Kvansakul M., van Delft M. F., Lee E. F., Gulbis J. M., Fairlie W. D., Huang D. C., Colman P. M. (2007) Mol. Cell 25, 933–942 [DOI] [PubMed] [Google Scholar]
- 20. Aoyagi M., Zhai D., Jin C., Aleshin A. E., Stec B., Reed J. C., Liddington R. C. (2007) Protein Sci. 16, 118–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Douglas A. E., Corbett K. D., Berger J. M., McFadden G., Handel T. M. (2007) Protein Sci. 16, 695–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cooray S., Bahar M. W., Abrescia N. G., McVey C. E., Bartlett N. W., Chen R. A., Stuart D. I., Grimes J. M., Smith G. L. (2007) J. Gen. Virol. 88, 1656–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang Q., Petros A. M., Virgin H. W., Fesik S. W., Olejniczak E. T. (2003) J. Mol. Biol. 332, 1123–1130 [DOI] [PubMed] [Google Scholar]
- 24. Huang Q., Petros A. M., Virgin H. W., Fesik S. W., Olejniczak E. T. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 3428–3433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Best S. M. (2008) Annu. Rev. Microbiol. 62, 171–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wasilenko S. T., Stewart T. L., Meyers A. F., Barry M. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 14345–14350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stewart T. L., Wasilenko S. T., Barry M. (2005) J. Virol. 79, 1084–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wasilenko S. T., Banadyga L., Bond D., Barry M. (2005) J. Virol. 79, 14031–14043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Taylor J. M., Quilty D., Banadyga L., Barry M. (2006) J. Biol. Chem. 281, 39728–39739 [DOI] [PubMed] [Google Scholar]
- 30. Campbell S., Hazes B., Kvansakul M., Colman P., Barry M. (2010) J. Biol. Chem. 285, 4695–4708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Postigo A., Cross J. R., Downward J., Way M. (2006) Cell Death Differ. 13, 1651–1662 [DOI] [PubMed] [Google Scholar]
- 32. Zhai D., Yu E., Jin C., Welsh K., Shiau C. W., Chen L., Salvesen G. S., Liddington R., Reed J. C. (2010) J. Biol. Chem. 285, 5569–5580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim H. E., Du F., Fang M., Wang X. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 17545–17550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Riedl S. J., Renatus M., Snipas S. J., Salvesen G. S. (2001) Biochemistry 40, 13274–13280 [DOI] [PubMed] [Google Scholar]
- 35. Shiozaki E. N., Chai J., Rigotti D. J., Riedl S. J., Li P., Srinivasula S. M., Alnemri E. S., Fairman R., Shi Y. (2003) Mol. Cell 11, 519–527 [DOI] [PubMed] [Google Scholar]
- 36. Chao Y., Shiozaki E. N., Srinivasula S. M., Rigotti D. J., Fairman R., Shi Y. (2005) PLoS Biol. 3, e183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Faustin B., Chen Y., Zhai D., Le Negrate G., Lartigue L., Satterthwait A., Reed J. C. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 3935–3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tovchigrechko A., Vakser I. A. (2006) Nucleic Acids Res. 34, W310–W314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wiehe K., Pierce B., Tong W. W., Hwang H., Mintseris J., Weng Z. (2007) Proteins 69, 719–725 [DOI] [PubMed] [Google Scholar]
- 40. Riedl S. J., Renatus M., Schwarzenbacher R., Zhou Q., Sun C., Fesik S. W., Liddington R. C., Salvesen G. S. (2001) Cell 104, 791–800 [DOI] [PubMed] [Google Scholar]
- 41. Mashiach E., Schneidman-Duhovny D., Andrusier N., Nussinov R., Wolfson H. J. (2008) Nucleic Acids Res. 36, W229–W232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rost B. (1996) Methods Enzymol. 266, 525–539 [DOI] [PubMed] [Google Scholar]
- 43. Komiyama T., Ray C. A., Pickup D. J., Howard A. D., Thornberry N. A., Peterson E. P., Salvesen G. (1994) J. Biol. Chem. 269, 19331–19337 [PubMed] [Google Scholar]
- 44. dela Cruz W. P., Friesen P. D., Fisher A. J. (2001) J. Biol. Chem. 276, 32933–32939 [DOI] [PubMed] [Google Scholar]
- 45. Fisher A. J., Cruz W., Zoog S. J., Schneider C. L., Friesen P. D. (1999) EMBO J. 18, 2031–2039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xu G., Cirilli M., Huang Y., Rich R. L., Myszka D. G., Wu H. (2001) Nature 410, 494–497 [DOI] [PubMed] [Google Scholar]
- 47. Deveraux Q. L., Leo E., Stennicke H. R., Welsh K., Salvesen G. S., Reed J. C. (1999) EMBO J. 18, 5242–5251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Scott F. L., Denault J. B., Riedl S. J., Shin H., Renatus M., Salvesen G. S. (2005) EMBO J. 24, 645–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chai J., Shiozaki E., Srinivasula S. M., Wu Q., Datta P., Alnemri E. S., Shi Y., Dataa P. (2001) Cell 104, 769–780 [DOI] [PubMed] [Google Scholar]
- 50. Huang Y., Park Y. C., Rich R. L., Segal D., Myszka D. G., Wu H. (2001) Cell 104, 781–790 [PubMed] [Google Scholar]
- 51. Sun C., Cai M., Gunasekera A. H., Meadows R. P., Wang H., Chen J., Zhang H., Wu W., Xu N., Ng S. C., Fesik S. W. (1999) Nature 401, 818–822 [DOI] [PubMed] [Google Scholar]
- 52. Pop C., Salvesen G. S. (2009) J. Biol. Chem. 284, 21777–21781 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.