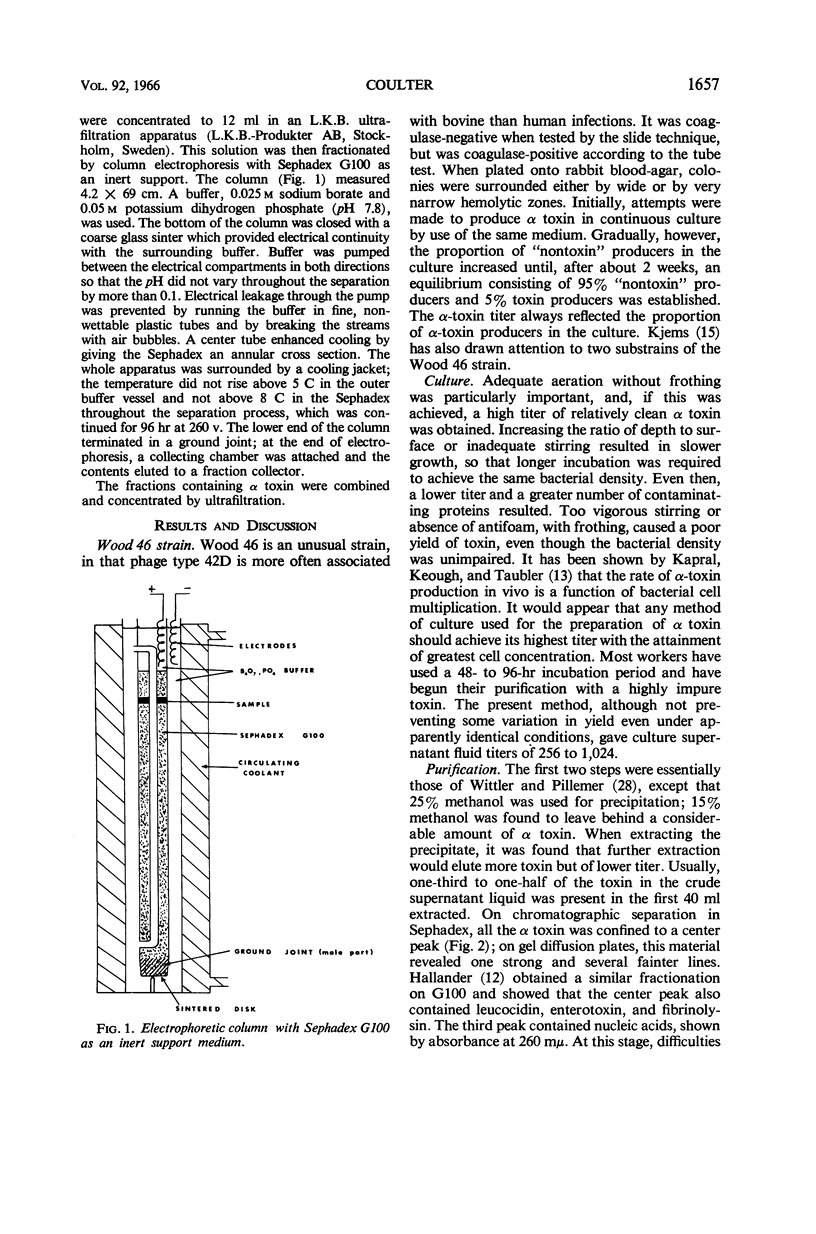
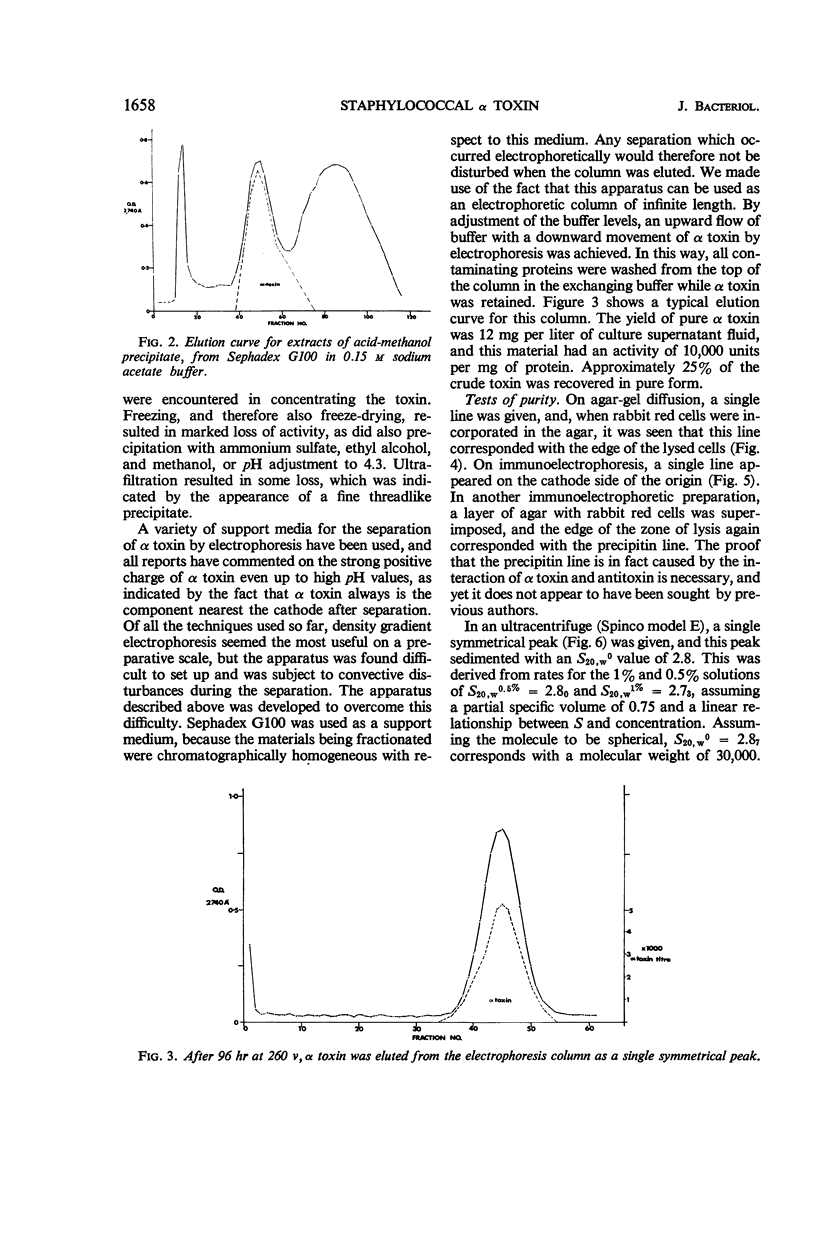
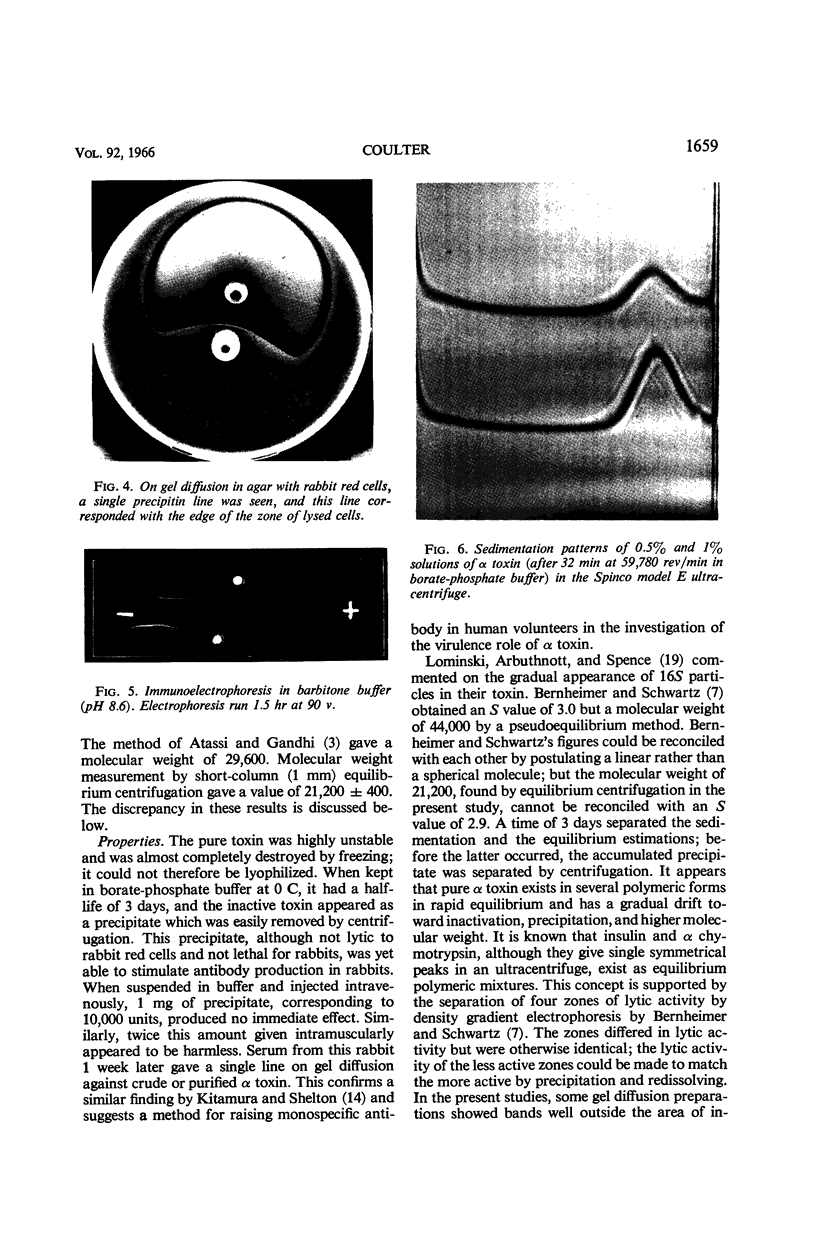
Abstract
Coulter, John R. (Institute of Medical and Veterinary Science, Adelaide, Australia). Production, purification, and composition of staphyloccocal α toxin. J. Bacteriol. 92:1655–1662. 1966—Pure staphylococcal α toxin has been prepared in quantities suitable for chemical, biological, and clinical characterization. Purification was achieved by acid-methanol precipitation, chromatography on G100 Sephadex, and electrophoresis in G100 Sephadex. We recovered 25% of the crude toxin in pure form, a yield of 12 mg/liter of crude culture supernatant fluid. The pure material gave a single line on gel diffusion and on immunoelectrophoresis and gave a single symmetrical peak in the ultracentrifuge. The α toxin was highly unstable, with a half-life of 3 days at 0 C (pH 7.8); solutions of it could not be frozen, and we found no method to stabilize it. On standing, a thready precipitate appeared; it was inactive against rabbit red cells, was not lethal to rabbits, but was able to elicit specific anti-α antibody production in the rabbit. There is evidence that α toxin is an associating molecule, with a mean sedimentation coefficient of approximately 3.0 and a molecular weight of approximately 30,000. The lowest molecular weight, found by equilibrium ultracentrifugation, was 21,200 ± 400. The amino acid composition was determined, and the high positive charge was explained by the presence of lysine, arginine, and histidine, and by amination of the aspartic and glutamic acid residues. Histidine and arginine were shown to be N-terminal amino acids, a fact which suggests the presence of two polypeptide chains. No carbohydrate was present. The ultraviolet absorption spectrum showed a maximum at 274.5 mμ, a minimum at 251.5 mμ, and a shoulder at 292 mμ. The toxin was without proteolytic or phospholipase activity, and its highly specific action on cell membranes still remains unexplained.
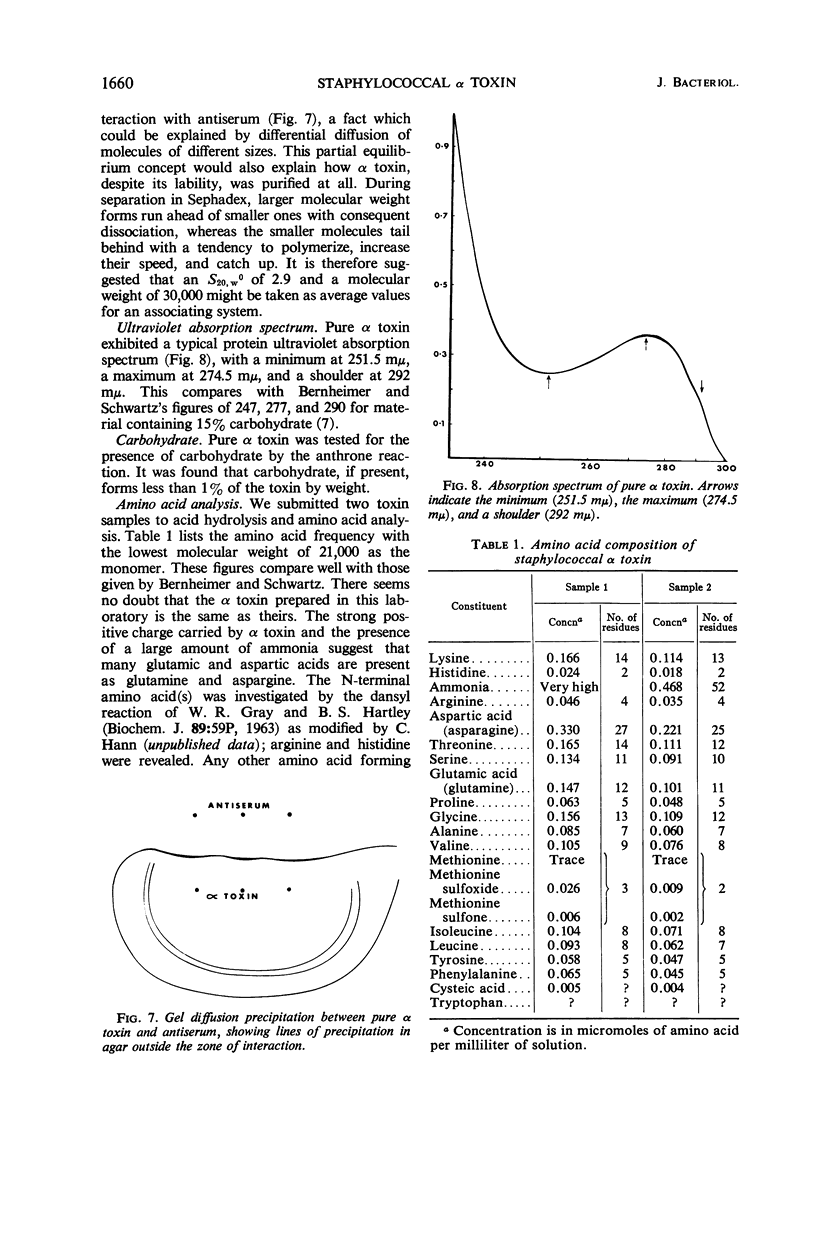
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ATASSI M. Z., GANDHI S. K. AN EMPIRICAL FORMULA FOR CALCULATING MOLECULAR WEIGHTS OF PROTEINS. Naturwissenschaften. 1965 May;52:259–259. doi: 10.1007/BF00602925. [DOI] [PubMed] [Google Scholar]
- BERNHEIMER A. W., DAVIDSON M. LYSIS OF PLEUROPNEUMONIA-LIKE ORGANISMS BY STAPHYLOCOCCAL AND STREPTOCOCCAL TOXINS. Science. 1965 May 28;148(3674):1229–1231. doi: 10.1126/science.148.3674.1229. [DOI] [PubMed] [Google Scholar]
- BERNHEIMER A. W. Resolution of mixtures of proteins by means of zone electrophoresis in sucrose density gradients. Arch Biochem Biophys. 1962 Feb;96:226–232. doi: 10.1016/0003-9861(62)90402-2. [DOI] [PubMed] [Google Scholar]
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- BUTLER L. O. Studies on the preparation and isoelectric point of staphylococcal alpha-haemolysin. Biochem J. 1959 Jan;71(1):67–73. doi: 10.1042/bj0710067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Schwartz L. L. Lysosomal disruption by bacterial toxins. J Bacteriol. 1964 May;87(5):1100–1104. doi: 10.1128/jb.87.5.1100-1104.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWSON R. M., HEMINGTON N., LINDSAY D. B. The phospholipids of the erythrocyte 'ghosts' of various species. Biochem J. 1960 Nov;77:226–230. doi: 10.1042/bj0770226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSHI K., CLUFF L. E., NORMAN P. S. Studies on the pathogenesis of staphylococcal infection. V. Purification and characterization of staphylococcal alpha hemolysin. Bull Johns Hopkins Hosp. 1963 Jan;112:15–30. [PubMed] [Google Scholar]
- HALLANDER H. O. FRACTIONATION OF STAPHYLOCOCCAL TOXINS BY GEL-FILTRATION. Acta Pathol Microbiol Scand. 1963;59:543–552. doi: 10.1111/j.1699-0463.1963.tb01258.x. [DOI] [PubMed] [Google Scholar]
- KAPRAL F. A., KEOGH A. M., TAUBLER J. H. THE NATURE OF ALPHA TOXIN PRODUCTION BY STAPHYLOCOCCUS AUREUS GROWN IN VIVO. Proc Soc Exp Biol Med. 1965 May;119:74–77. doi: 10.3181/00379727-119-30102. [DOI] [PubMed] [Google Scholar]
- KITAMURA S., SHELTON J., THAL A. P. ISOLATION AND CHARACTERIZATION OF STAPHYLOCOCCUS ALPHA-HEMOLYSIN. Ann Surg. 1964 Nov;160:926–935. doi: 10.1097/00000658-196411000-00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KJEMS E. TWO VARIANTS OF STAPHYLOCOCCUS AUREUS WOOD 46 (NCTC 7121) DIFFERING IN RESPECT TO ALPHA TOXIN PRODUCTION. J Bacteriol. 1963 Nov;86:1127–1128. doi: 10.1128/jb.86.5.1127-1128.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORBECKI M., JELJASZESICZ J. ACTION OF STAPHYLOCOCCAL TOXINS IN CELL CULTURES. J Infect Dis. 1965 Apr;115:205–213. doi: 10.1093/infdis/115.2.205. [DOI] [PubMed] [Google Scholar]
- KUMAR S., LINDORFER R. K. The characterization of staphylococcal toxins. I. The electrophoretic migration of the alpha hemolytic, dermonecrotic, lethal, and leucocidal activities of crude toxin. J Exp Med. 1962 Jun 1;115:1095–1106. doi: 10.1084/jem.115.6.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MADOFF M. A., WEINSTEIN L. Purification of staphylococcal alpha-hemolysin. J Bacteriol. 1962 Apr;83:914–918. doi: 10.1128/jb.83.4.914-918.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macfarlane M. G., Knight B. C. The biochemistry of bacterial toxins: The lecithinase activity of Cl. welchii toxins. Biochem J. 1941 Sep;35(8-9):884–902. doi: 10.1042/bj0350884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noll H. Chain initiation and control of protein synthesis. Science. 1966 Mar 11;151(3715):1241–1245. doi: 10.1126/science.151.3715.1241. [DOI] [PubMed] [Google Scholar]
- Ogston A. Micrococcus Poisoning. J Anat Physiol. 1882 Jul;16(Pt 4):526–567. [PMC free article] [PubMed] [Google Scholar]
- ROBINSON J., THATCHER F. S., MONTFORD J. Studies with staphylococcal toxins. V. Possible identification of alpha hemolysin with a proteolytic enzyme. Can J Microbiol. 1960 Apr;6:183–194. doi: 10.1139/m60-020. [DOI] [PubMed] [Google Scholar]
- ROBINSON J., THATCHER F. S., MONTFORD J. Studies with staphylococcal toxins. VI. Some properties of a nonhemolytic dermonecrotic toxin produced by some strains of Staphylococcus aureus. Can J Microbiol. 1960 Apr;6:195–201. doi: 10.1139/m60-021. [DOI] [PubMed] [Google Scholar]
- SIEGEL I., COHEN S. ACTION OF STAPHYLOCOCCAL TOXIN ON HUMAN PLATELETS. J Infect Dis. 1964 Dec;114:488–502. doi: 10.1093/infdis/114.5.488. [DOI] [PubMed] [Google Scholar]
- SMITH E. W., GOSHI K., NORMAN P. S., CLUFF L. E. STUDIES ON THE PATHOGENESIS OF STAPHYLOCOCCAL INFECTION. VIII. THE HUMAN CUTANEOUS REACTION TO INJECTION OF ALPHA HEMOLYSIN. Bull Johns Hopkins Hosp. 1963 Nov;113:247–260. [PubMed] [Google Scholar]