Abstract
5-Amino-4-imidazolecarboxamide ribonucleotide 5′-phosphate (AICAR) is a monophosphate metabolic intermediate of the de novo purine synthesis pathway that has highly promising metabolic and antiproliferative properties. Yeast mutants unable to metabolize AICAR are auxotroph for histidine. A screening for suppressors of this phenotype identified recessive and dominant mutants that result in lowering the intracellular AICAR concentration. The recessive mutants affect the adenosine kinase, which is shown here to catalyze the phosphorylation of AICAR riboside in yeast. The dominant mutants strongly enhance the capacity of the alkaline phosphatase Pho13 to dephosphorylate 5-amino-4-imidazole N-succinocarboxamide ribonucleotide 5′-phosphate(SAICAR) into its non-toxic riboside form. By combining these mutants with transcriptomics and metabolomics analyses, we establish that in yeast responses to AICAR and SAICAR are clearly linked to the concentration of the monophosphate forms, whereas the derived nucleoside moieties have no effect even at high intracellular concentration. Finally, we show that AICAR/SAICAR concentrations vary under physiological conditions known to modulate transcription of the purine and phosphate pathway genes.
Keywords: Metabolic Regulation, Purine, Transcription Regulation, Yeast Genetics, Yeast Metabolism
Introduction
AICAR3 is a monophosphate intermediate in the purine de novo synthesis pathway (see Fig. 1). This small molecule has drawn major attention recently because it has very promising properties. In sedentary mice, 5-amino-4-imidazole carboxamide ribonucleoside (AICAr; see Fig. 1B) treatment mimics exercise (1). In mammalian cells, AICAr specifically promotes apoptosis of aneuploid cells and is a promising antitumor agent (2). Indeed, AICAr inhibits proliferation of various tumor cell lines (2, 3). AICAr is metabolized to AICAR, which is an agonist of the AMP-dependent kinase (4, 5), a major regulator of metabolism. However, there are multiple examples where AICAr effects were partially or fully AMP-dependent kinase-independent (6–8). The other effectors of AICAR are not yet known, and it is not totally clear whether all the cellular effects of AICAr can be attributed to AICAR. In yeast, besides its metabolic role, AICAR has important regulatory effects. In particular, AICAR co-regulates purine synthesis and phosphate utilization by promoting interaction of the transcription factor Pho2 with either Bas1 or Pho4 (9–11). It is thus likely that AICAR will also play physiological roles in other eukaryotes.
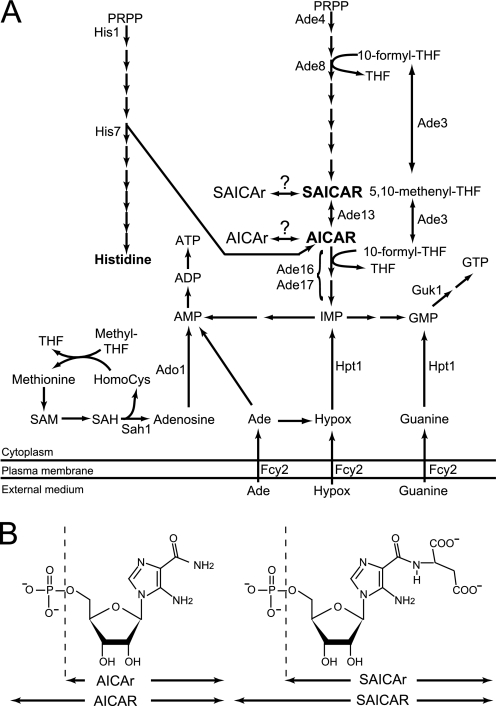
FIGURE 1.
Schematic representation of de novo purine and histidine pathways in yeast and their connection to one-carbon unit metabolism and methyl cycle. In A, only the enzymes mentioned in the text are listed. Question marks correspond to enzymatic activities catalyzed by unidentified proteins. HomoCys, homocysteine; Hypox, hypoxanthine; IMP, inosine 5′-monophosphate; PRPP, 5-phosphoribosyl-1-pyrophosphate; SAH, S-adenosylhomocysteine; SAM, S-adenosylmethionine; THF, tetrahydrofolic acid. B, chemical structure of AICAR and SAICAR derivatives.
In human, severe symptoms are associated with AICAR transformylase inosine monophosphate cyclohydrolase deficiency, a defect leading to accumulation of AICAR and derivatives (12). In yeast, AICAR is accumulated in an ade16 ade17 mutant lacking AICAR transformylase inosine monophosphate cyclohydrolase and in an ade3 mutant that cannot synthesize 10-formyltetrahydrofolate, a co-substrate for AICAR transformylase inosine monophosphate cyclohydrolase (see Fig. 1A) (9). Constitutive activation of the first step of the purine pathway is highly detrimental for cell growth when combined with ade16 ade17 (11). However, when the purine pathway is not hyperactivated, the ade16 ade17 and ade3 mutants are viable but display an auxotrophy for histidine (13). This auxotrophy is not dependent on the Pho2 and Bas1 transcription factors that regulate the purine and histidine pathways and can be suppressed by overexpression of the putative phosphomutase Pmu1 (11). In this study, we combined genetic and metabolomics approaches to get insight into AICAR metabolism and its effects on yeast cell physiology.
EXPERIMENTAL PROCEDURES
Media, Strains, and Plasmids
SD is a synthetic minimal medium containing 5% ammonium sulfate, 0.67% yeast nitrogen base (Difco), 2% glucose. SDcasaW is SD medium supplemented with 0.2% casamino acids (Difco) and tryptophan (0.2 mm). When indicated, adenine (0.3 mm) and/or uracil (0.3 mm) were added in SDcasaW medium resulting in media named SDcasaWA (+adenine), SDcasaWU (+uracil), and SDcasaWAU (+adenine +uracil). SC medium was prepared as described by Sherman et al. (14). SC complete medium is SC medium supplemented with adenine (0.3 mm), uracil (0.3 mm), histidine (0.06 mm), leucine (0.4 mm), lysine (0.06 mm), and tryptophan (0.2 mm). Yeast strains (Table 1) belong to or are derived from a set of disrupted strains isogenic to BY4741 or BY4742 purchased from Euroscarf. Plasmids allowing expression of the PHO13 gene (either wild type or mutant) (PHO13-1) were obtained by PCR amplification with oligonucleotides 2706 (5′-CGCGGATCCAAAATGACTGCTCAACAAGGTG-3′) and 2707 (5′-CCAATGCATCTATAACTCATTATTGGTTAAGG-3′) on genomic DNA from ade3 ade16 ade17 (Y2406) and dominant mutant (Y3438) strains, respectively. PCR products were digested with BamHI and NsiI and cloned in the pCM189 vector (15) digested with BamHI and PstI, resulting in the tet-PHO13 (p4248) and tet-PHO13-1 (p4291) plasmids, respectively. The ADE1-LacZ fusion used for β-gal experiments has already been described (16).
TABLE 1.
Yeast strains
| Strain name | Genotype |
|---|---|
| FY4 | MATa |
| PLY122 | MATaleu2-3,112 lys2-Δ201 ura3–52 |
| 215 | MATa leu2-3,112 lys2-Δ201 ura3-52 ADE4-5 (=BRA11-5) |
| 220 | MATa leu2-3,112 lys2-Δ201 ura3-52 guk1-3 (=bra3-3) |
| 232 | MATa leu2-3,112 lys2-Δ201 ura3-52 fcy2-2 (=bra7-2) |
| BY4741 | MATahis3Δ1 leu2Δ0 met15Δ0 ura3Δ0 |
| BY4742 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 |
| Y508 | MATaleu2-3,112 lys2-Δ201 ura3-52 hpt1::URA3 |
| Y1095 | MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 ade16::kanMX4 ade17::kanMX4 |
| Y1162 | MATα his3Δ1 leu2Δ0 ura3Δ0 ade16::kanMX4 ade17::kanMX4 |
| Y2103 | MATaade3::kanMX4 ade16::kanMX4 ade17::kanMX4 his3Δ1::HIS3-URA3 leu2Δ0 ura3Δ0 |
| Y2406 | MATα ade3::kanMX4 ade16::kanMX4 ade17::kanMX4 his3Δ1::HIS3-LEU2 leu2Δ0 ura3Δ0 |
| Y2946 | MATα his3Δ1 leu2Δ0 ura3Δ0 |
| Y2948 | MATα his3Δ1 leu2Δ0 ura3Δ0 ade16::kanMX4 ade17::kanMX4 |
| Y2950 | MATα ade16::kanMX4 ade17::kanMX4 ade8::kanMX4 his1::kanMX4 his3Δ1 leu2Δ0 ura3Δ0 |
| Y3188 | MATα ade16::kanMX4 ade17::kanMX4 ade8::kanMX4 his1::kanMX4 ado1::LEU2 his3Δ1 leu2Δ0 ura3Δ0 |
| Y3403 | MATaade3::kanMX4 ade16::kanMX4 ade17::kanMX4 leu2Δ0 ura3Δ0 his3Δ1::HIS3-URA3 ado1-1 |
| Y3438 | MATα ade3::kanMX4 ade16::kanMX4 ade17::kanMX4 leu2Δ0 ura3Δ0 his3Δ1::HIS3-LEU2 PHO13-1 |
| Y3440 | MATα ade3::kanMX4 ade16::kanMX4 ade17::kanMX4 leu2Δ0 ura3Δ0 his3Δ1::HIS3-LEU2 ado1-3 |
| Y4090 | MATaade13-42 (m242) leu2-3,112 lys2-Δ201 ura3-52 |
| Y4143 | MATα ade3::kanMX4 his3Δ1 leu2Δ0 ura3Δ0 |
| Y4476 | MATahis3Δ1 leu2Δ0 ura3Δ0 his3Δ1::HIS3-LEU2 ado1Δ |
| Y4479 | MATα his3Δ1 leu2Δ0 ura3Δ0 his3Δ1::HIS3-LEU2 |
| Y4505 | MATaade3::kanMX4 ade16::kanMX4 ade17::kanMX4 his3Δ1::HIS3-URA3 ado1::LEU2 leu2Δ0 ura3Δ0 |
| Y4561 | MATα ade3::kanMX4 ade16::kanMX4 ade17::kanMX4 leu2Δ0 ura3Δ0 his3Δ1::HIS3-LEU2 PHO13-1 ade13::URA3 |
| Y4965 | MATa/MATα ade3::kanMX4/ade3::kanMX4 ade16::kanMX4/ade16::kanMX4 ade17::kanMX4/ade17::kanMX4 his3Δ1::HIS3-LEU2/his3Δ1::HIS3-URA3 ado1::LEU2/ado1-3 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 |
| Y4966 | MATa/MATα ade3::kanMX4/ade3::kanMX4 ade16::kanMX4/ade16::kanMX4 ade17::kanMX4/ade17::kanMX4 his3Δ1::HIS3-LEU2/his3Δ1::HIS3-URA3 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 |
| Y4972 | MATa/MATα ade3::kanMX4/ade3::kanMX4 ade16::kanMX4/ade16::kanMX4 ade17::kanMX4/ade17::kanMX4 his3Δ1::HIS3-LEU2/his3Δ1::HIS3-URA3 ADO1/ado1-3 leu2Δ0/leu2Δ0 ura3Δ0/ura3Δ0 |
| Y5969 | MATa his1::kanMX4 leu2Δ0 ura3Δ0 met15Δ0 his3Δ1::HIS3-LEU2 |
| Y6220 | MATa PHO13-1 his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 |
| Y6221 | MATα pho13::kanMX4 his3Δ1::HIS3-LEU2 leu2Δ0 lys2Δ0 ura3Δ0 |
| Y6222 | MATa pho13::kanMX4 his3Δ1::HIS3-LEU2 leu2Δ0 ura3Δ0 |
| Y6223 | MATα PHO13-1 his3Δ1 leu2Δ0 ura3Δ0 |
| Y6224 | MATα pho13::kanMX4 his3Δ1 leu2Δ0 ura3Δ0 |
| Y6225 | MATa pho13::kanMX4 his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 |
| Y6226 | MATa PHO13-1 his3Δ1::HIS3-LEU2 leu2Δ0 lys2Δ0 ura3Δ0 |
| Y6227 | MATα PHO13-1 his3Δ1::HIS3-LEU2 leu2Δ0 ura3Δ0 |
Metabolite Extraction and Separation by Liquid Chromatography
Metabolite extractions for amino acid and nucleotide determinations were performed by the boiling water method (17) and the boiling ethanol method (18), respectively. Metabolites were separated by ionic chromatography on an ICS3000 chromatography station (Dionex, Sunnyvale, CA) and detected by integrated pulsed amperometry for amino acid detection (Amino Acids Analyzer-certified electrodes; Dionex) and by a UV diode array detector for the other metabolites (Ultimate 3000 RS, Dionex). Amino acids were separated on an aminopacPA10 column (250 × 2 mm; Dionex) with improved gradient method 3 (19). Other metabolites were usually separated on a carbopacPA1 column (250 × 2 mm; Dionex) using a sodium acetate gradient (from 300 to 800 mm) in 50 mm NaOH with a 0.25 ml/min flow as follows. Elution was started at 300 mm sodium acetate for 2 min, raised to 500 mm sodium acetate in 8 min followed by a step at 500 mm for 5 min, raised to 800 mm in 10 min, kept at 800 mm for 25 min, and returned to 300 mm in 1 min. The resin was re-equilibrated for 10 min at this sodium acetate concentration prior to any injection. For detection of physiological levels of AICAR/5-amino-4-imidazole N-succinocarboxamide ribonucleotide 5′-phosphate (SAICAR) derivatives, separation was also performed on the carbopacPA1 column with a 0.25 ml/min flow but using a modified sodium acetate gradient (from 50 to 800 mm) in 50 mm NaOH as follows. Elution was started at 50 mm sodium acetate for 2 min; raised to 75 mm in 8 min, then to 100 mm in 25 min, and finally to 350 mm in 30 min followed by a step at 350 mm for 5 min; raised to 500 mm in 10 min; kept at this sodium acetate concentration for 5 min; and finally raised to 800 mm in 10 min followed by a step at this concentration for 20 min. The resin was then equilibrated at 50 mm sodium acetate for 15 min before injection of a new sample. Peaks were identified by their retention time as well as by co-injection with standards and/or their UV spectrum signature depending on their chemical properties. Cell volume measurements used to calculate metabolite intracellular concentrations were performed using a MultisizerTM 4 Coulter Counter® (Beckman Coulter).
Pho13 Activity
A pho13Δ (Y6224) strain was transformed with plasmids allowing expression of PHO13 (either wild type (p4248) or mutant (p4291)) or by the corresponding pCM189 empty vector. For each strain, transformants were grown exponentially for 24 h in SDcasaWA medium (50 ml) and harvested by centrifugation (3000 × g, 4 min, 4 °C) when A600 nm reached 1. Cells were washed with 30 ml of extraction buffer (50 mm Tris/HCl, 100 mm NaCl, 0.5 mm DTT), resuspended in 0.4 ml of the same buffer, and then disrupted in dry ice with 300 μl of glass beads using a FastPrep (3 × 20 s; MP Biomedicals). Extracts were clarified by centrifugation (10 min, 4 °C, 21,000 × g), and the supernatant was dialyzed for 3 h at 4 °C against extraction buffer. The protein concentration for each extract was measured and diluted to 2 mg/ml. Enzyme assays were performed at 37 °C in 400 μl of extraction buffer containing 1 mm MgCl2 and SAICAR (Fig. 1B) at the indicated concentrations. Assays were started by addition of 20 μg of total proteins, and 100-μl aliquots were collected at 0, 5, 10, and 15 min, time points for which apparition of 5-amino-4-imidazole N-succinocarboxamide ribonucleoside (SAICAr; Fig. 1B) is linear over time for all SAICAR concentrations. The reaction was stopped by incubation at 95 °C in a water bath for 5 min. Concentrations of SAICAR and SAICAr were determined by HPLC using a C18 reverse phase column as described previously (20). The specific activity (μmol of SAICAr synthesized/min/mg of total proteins) was determined by linear regression of the amount of SAICAr that appeared during the time. The activity measured with protein extract from the pho13Δ strain transformed with the empty vector was considered as Pho13-independent background and was subtracted.
Miscellaneous Methods
β-Gal assays were performed as described (22). Sequencing of the dominant mutants and control genomes was performed by GATC Biotech (Konstanz, Germany). DNA microarray hybridizations were performed as described (23), and microarray data were normalized without background subtraction by the global lowess method performed with Goulphar software (24).
RESULTS
Suppressors Restoring Histidine Prototrophy to ade3 ade16 ade17 Triple Mutant Strongly Decrease Intracellular AICAR Concentration
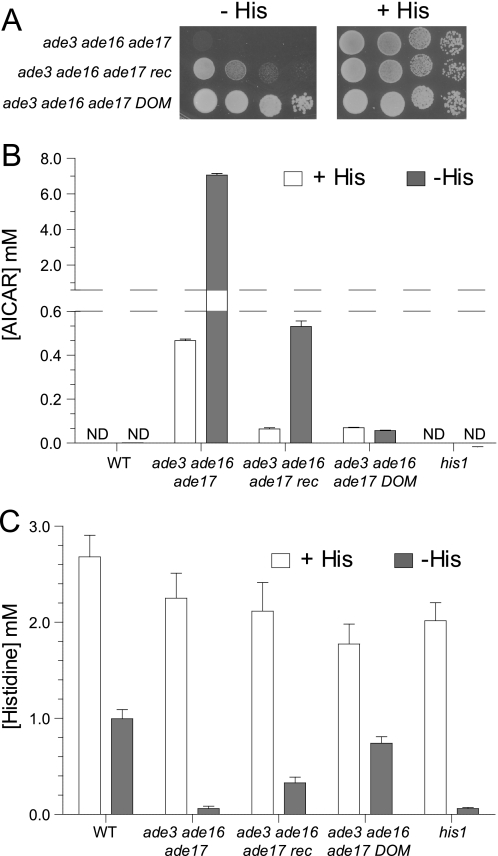
The yeast ade3 ade16 ade17 triple mutant is auxotrophic for histidine (Fig. 2A). When transferred to a growth medium lacking histidine, the mutant cells rapidly arrested in G1 with a 1N DNA content and a non-duplicated spindle pole body (data not shown). This arrest was associated with an overaccumulation of AICAR (Fig. 2B) and a strong decrease in intracellular histidine concentration down to a level comparable with that of the his1 mutant lacking the first enzyme of the histidine biosynthesis pathway (Fig. 2C). Of note, 32 h in the absence of histidine were required for the his1 mutant to fully deplete the histidine pool.
FIGURE 2.
AICAR concentration is strongly decreased in both recessive and dominant suppressors. A, cells were grown overnight, serially diluted, and spotted on SC medium containing adenine but lacking (−His; gray boxes) or containing (+His; white boxes) histidine. Plates were imaged after 3 days at 30 °C. ade3 ade16 ade17 (Y2406), ade3 ade16 ade17 recessive suppressor (rec; Y3440), and ade3 ade16 ade17 dominant suppressor (DOM; Y3438) are shown. B and C, determination of AICAR (B) and histidine (C) intracellular concentrations. Cells were grown overnight to an A600 nm of 1 in SCA medium (SC medium containing adenine) supplemented with histidine (+His; white boxes), washed twice by centrifugation in SCA medium lacking histidine and uracil, and then resuspended and incubated in the same medium for 32 h (−His; gray boxes). Metabolites were extracted, separated, and quantified as described under “Experimental Procedures.” Wild-type strain (WT; Y4479), ade3 ade16 ade17 (Y2406), ade3 ade16 ade17 rec (Y3440), ade3 ade16 ade17 DOM (Y3438), and his1 (Y5969) are shown. Results correspond to at least four independent metabolite extractions from two independent cultures, and error bars indicate variations in the mean. ND, not detectable.
A genetic screen was performed to isolate histidine prototroph suppressors from the ade3 ade16 ade17 strain mutagenized with UV light. A set of 159 suppressor mutants was further studied. Most of these suppressors (101 mutants) were recessive, whereas 58 were dominant. All the recessive mutants fell in a single complementation group, and all the tested dominant mutants (six mutants) were genetically linked. The suppression phenotype was stronger for the dominant than for the recessive suppressors (Fig. 2A). Importantly, in both dominant and recessive suppressors, AICAR accumulation was much lower than in the ade3 ade16 ade17 triple mutant (Fig. 2B), whereas intracellular histidine concentration was increased (Fig. 2C). The histidine concentration in the recessive suppressor strain was intermediate between that of the triple mutant and the wild-type strain (Fig. 2C). For the dominant suppressor, the histidine concentration was close to that of the wild-type strain (Fig. 2C). In both cases, the histidine concentration nicely followed the capacity of the suppressors to grow in the absence of histidine (Fig. 2A).
Recessive Suppressors Are Allelic to Adenosine Kinase Gene ADO1 Required for Synthesis of AICAR from Its Riboside Form
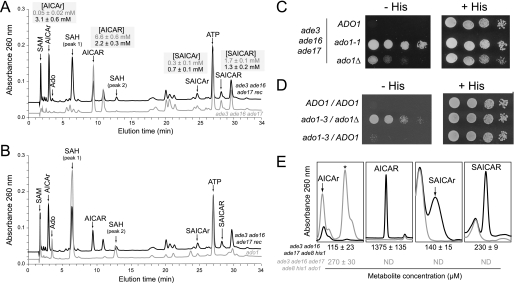
Several recessive suppressors were analyzed by ionic chromatography, and similar metabolic profiles including major peaks undetectable in the ade3 ade16 ade17 triple mutant strain were revealed (Fig. 3A). Based on their retention time, their UV spectra, and co-injection experiments, the accumulated compounds were identified as AICAr, adenosine, S-adenosylmethionine, and S-adenosylhomocysteine (Fig. 3A). Two yeast mutants potentially accumulate S-adenosylhomocysteine: sah1, which affects S-adenosylhomocysteine hydrolase (25), and ado1, which lacks adenosine kinase (26). We found that S-adenosylhomocysteine was accumulated in a sah1-1 temperature-sensitive mutant (25), but adenosine and S-adenosylmethionine were not accumulated (data not shown). By contrast, adenosine, S-adenosylmethionine, and S-adenosylhomocysteine were all accumulated in an ado1 knock-out mutant (Fig. 3B). Thus ADO1, encoding adenosine kinase (26), appeared to be a good candidate gene and was used for complementation studies in the recessive mutants. First, we showed that the ADO1 gene carried on a plasmid fully restored the histidine auxotrophy to the recessive mutant (data not shown). In addition, we found that a knock-out of ado1 when introduced in the triple ade3 ade16 ade17 mutant strain suppressed the histidine auxotrophy (Fig. 3C) although to a lesser extent than the suppressor mutants most probably because of the strong growth defect associated with the knock-out of ado1 (26). The resulting ade3 ade16 ade17 ado1 strain was used for a complementation assay with the recessive suppressor. The two suppressor mutations were clearly unable to complement each other (Fig. 3D) and thus most probably affect the same gene. Finally, sequencing of the ADO1 gene in six recessive mutants revealed in each mutant the presence of one of four different mutations leading to changes in conserved amino acids (N68K (ado1-3), G119S (ado1-2), A320P (ado1-4), and P329R (ado1-1)). From these data, we conclude that the recessive suppressors are alleles of the ADO1 gene.
FIGURE 3.
Recessive suppressors are allelic to adenosine kinase gene ADO1. A and B, representative UV chromatograms of metabolic extracts from ade3 ade16 ade17 (A, gray line; Y2406), ade3 ade16 ade17 rec (A and B, black line; Y3440), and ado1 (B, gray line; Y4476) strains grown overnight in SC complete medium to an A600 nm of 1 and then shifted for 2 h in SC medium lacking histidine. The intracellular concentrations of AICAR and derivatives in ade3 ade16 ade17 (gray numbers) and ade3 ade16 ade17 rec (black numbers) strains are shown in shaded boxes and correspond to three independent determinations. C and D, haploid (C) and diploid (D) cells were treated as in Fig. 2A. The haploid cells are ade3 ade16 ade17 (ADO1; Y2103), ade3 ade16 ade17 ado1-1 (Y3403), and ade3 ade16 ade17 ado1Δ (Y4505), and the diploid cells are ade3/ade3 ade16/ade16 ade17/ade17 (ADO1/ADO1; Y4966), ade3/ade3 ade16/ade16 ade17/ade17 ado1-3/ado1Δ (Y4965), and ade3/ade3 ade16/ade16 ade17/ade17 ado1-3/ADO1 (Y4972). E, the adenosine kinase Ado1 catalyzes AICAr phosphorylation. ade8 ade16 ade17 his1 (black line; Y2950) and ade8 ade16 ade17 his1 ado1 (gray line; Y3188) strains were grown overnight in SDcasaWAU medium supplemented with 1 mm AICAr, then diluted to an A600 nm of 0.1 in the same medium, and harvested when A600 nm reached 1. Metabolite extraction, separation, and detection were performed as described under “Experimental Procedures.” The asterisk shows the adenosine peak, which is only accumulated in the ade8 ade16 ade17 his1 ado1 mutant. Intracellular concentration is shown at the bottom of each panel (black, Y2950; gray, Y3188). ND, not detectable.
In the recessive suppressor strains, the AICAR concentration was decreased, and the AICAr concentration was increased (Fig. 3A). Thus, we hypothesized that the adenosine kinase Ado1 could be responsible for AICAr phosphorylation. This was confirmed by feeding an ade16 ade17 ade8 his1 mutant (unable to synthesize AICAR from the histidine and purine pathways; see Fig. 1A) with AICAr. In this mutant, AICAr was taken up and metabolized to AICAR (Fig. 3E). By contrast, when the ado1 knock-out mutation was introduced in this quadruple mutant strain, the resulting strain was still able to internalize AICAr but became totally unable to synthesize AICAR (as well as SAICAR and its riboside form) (Fig. 3E). Of note, in both strains grown in the absence of AICAr, none of the AICAR/SAICAR derivatives were detectable (data not shown). These results show that adenosine kinase is required to synthesize AICAR monophosphate from its riboside form (AICAr) and that the ado1 suppressors by altering the replenishment of the AICAR pool from AICAr result in a lower intracellular AICAR concentration.
Dominant Suppressors Are Allelic to PHO13
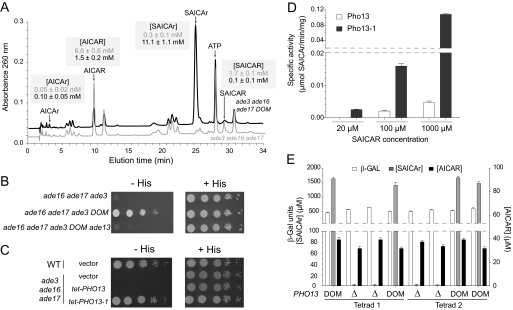
The dominant suppressors (10 mutants analyzed) massively accumulated SAICAr (Fig. 4A), the riboside derivative of SAICAR (Fig. 1B). A simple explanation would be that the dominant suppressors somehow drain AICAR to SAICAR (just upstream in the pathway) and ultimately SAICAr, thus “detoxifying” excessive AICAR. Consistently, when the gene responsible for the AICAR to SAICAR conversion (ADE13; see Fig. 1A) was disrupted in the triple ade3 ade16 ade17 mutant carrying the dominant suppressor, the resulting strain was not prototrophic for histidine anymore (Fig. 4B). This implies that Ade13 is required for the suppressor to be efficient or that the suppressor is allelic to ADE13. However, sequencing of the ADE13 locus in the ade3 ade16 ade17 mutant and several dominant suppressor strains did not reveal any differences.
FIGURE 4.
Dominant suppressors affect PHO13 gene encoding an alkaline phosphatase. A, representative metabolic profile of extracts from ade3 ade16 ade17 (gray line; Y2406) and ade3 ade16 ade17 DOM (black line; Y3438) strains grown overnight in SC complete medium to an A600 nm of 1 and then shifted for 2 h to SC medium lacking histidine. Intracellular concentrations of AICAR and derivatives of ade3 ade16 ade17 (gray numbers) and ade3 ade16 ade17 DOM (black numbers) strains are shown in shaded boxes and correspond to three independent determinations. B, ADE13 gene is required for the suppressor phenotype of the dominant mutant strain. Cells were treated as in Fig. 2A, and plates were imaged after 3 days at 30 °C. Strains shown are ade3 ade16 ade17 (Y2406), ade3 ade16 ade17 DOM (Y3438), and ade3 ade16 ade17 DOM ade13::URA3 (Y4561). C, ectopic expression of the dominant PHO13-1 allele restores histidine prototrophy in the ade3 ade16 ade17 mutant. WT (Y4479) and ade3 ade16 ade17 (Y2406) were transformed with the tet-PHO13 (p4248), tet-PHO13-1 (p4291), or the pCM189 empty vector. Transformants were isolated on SDcasaWA medium and treated as in Fig. 2A. D, in vitro dephosphorylation of SAICAR by Pho13 and Pho13-1. A pho13Δ (Y6224) mutant strain was transformed with plasmids allowing expression of PHO13 (either wild type (p4248) or mutant (p4291)). Total protein extracts were used to assay SAICAR dephosphorylation. Results correspond to four independent measurements, and error bars indicate variations in the mean. E, ADE1-LacZ expression is not affected by SAICAr accumulation. Cells (Y6220–Y6227) were transformed with an ADE1-LacZ fusion (p2281) and grown in SDcasaW medium to an A600 nm of 1. β-Gal activities were measured as described under “Experimental Procedures.” AICAR and SAICAr intracellular concentrations were determined by ionic chromatography as in Fig. 3A. Results correspond to two to four independent measurements, and error bars indicate variations in the mean.
To identify the dominant suppressor, we then used a whole genome sequencing approach and searched for polymorphisms among the genomes of three dominant suppressors and two control strains. This approach revealed among multiple single nucleotide polymorphisms a single nucleotide polymorphism (G32918A on chromosome 4) at the PHO13 (YDL236W) locus encoding an alkaline phosphatase of unknown function (27). This single nucleotide polymorphism was particularly interesting because the ionic chromatography analysis suggested that the dominant suppressor was dephosphorylating SAICAR to SAICAr (Fig. 4A). Thus, we sequenced the PHO13 locus in five dominant suppressors and confirmed the presence of the polymorphism in all cases and no other mutation in the PHO13 gene. The mutation in PHO13 affects a glycine residue that is mutated to an aspartate (G208D). It is noteworthy that Pho13 orthologs in most organisms carry a glycine at this position, although in a few cases, an aspartate residue is found in this position (28).
The dominant suppressor was then crossed to an ade3 pho13 double mutant, and we found that in the meiotic progeny (20 tetrads) none of the histidine prototroph spores carried the deletion of pho13. The suppressor and pho13 are thus tightly linked. Finally, we cloned the PHO13 coding region carrying the dominant mutation in a plasmid under the control of a heterologous promoter and found that this construct could suppress the histidine auxotrophy of an ade3 ade16 ade17 mutant (Fig. 4C). Interestingly, the wild-type PHO13 open reading frame cloned in the same vector did not allow any growth in the absence of histidine (Fig. 4C). The same plasmids were transformed in a pho13 mutant, and protein extracts from the transformants were used to measure SAICAR phosphatase activity in vitro. Consistently, the ability to dephosphorylate SAICAR to SAICAr was high in the strain carrying the tet-PHO13-1 construct and much lower in the tet-PHO13 transformants (Fig. 4D). We conclude that the mutant form of Pho13 has gained the capacity to efficiently dephosphorylate SAICAR in vivo and in vitro. Of note, we measured in vitro a very slight enzymatic activity of Pho13-1 mutant toward AICAR as a substrate (0.012 ± 0.002 μmol/min/mg with 1 mm AICAR). This activity may account for the modest accumulation of AICAr in the dominant mutant.
We previously reported an important regulatory role for SAICAR in transcriptional activation of the purine pathway (9, 10). The ade16 ade17 mutant strain accumulates both the monophosphate form, SAICAR (190 ± 29 μm), and the riboside form, SAICAr (11 ± 3 μm). Interestingly enough, we observed that massive accumulation (1000-fold) of SAICAr in PHO13-1 mutants (11.1 ± 0.1 mm) had no effect on the expression of an ADE1-LacZ fusion (the results for two tetrads from a PHO13-1/pho13::kanMX4 diploid are shown in Fig. 4E). Altogether, these results indicate that the monophosphate form, SAICAR, is the active form and that the riboside form, SAICAr, does not interfere with SAICAR for transcriptional up-regulation of the purine regulon.
Roles of Riboside and Monophosphate Forms of AICAR in Transcriptional Response and Toxicity
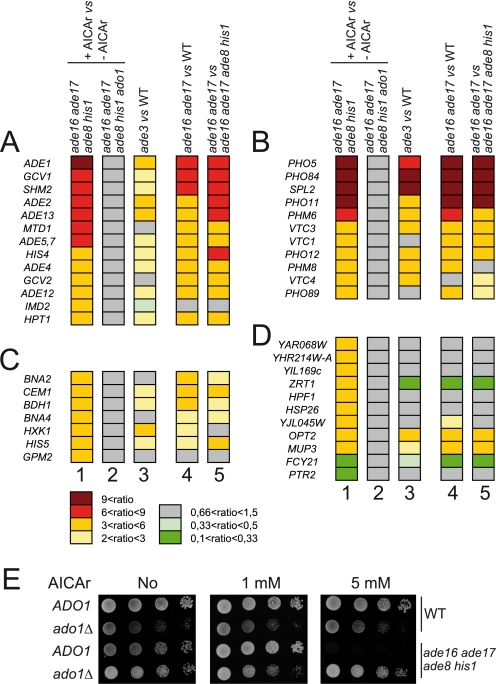
To estimate the respective regulatory roles of AICAR and its riboside derivative AICAr, we first compared the effect of addition of AICAr on the transcriptome of two isogenic strains that are capable (ADO1) or not capable (ado1) of metabolizing AICAr to AICAR (Fig. 3E). Clearly, addition of AICAr stimulated expression of the ADE regulon (Fig. 5A, column 1) and PHO regulon genes (Fig. 5B, column 1) similarly to the effect found in ade3 (Fig. 5, A and B, column 3) and ade16 ade17 mutants (Fig. 5, A and B, columns 4 and 5) that accumulate AICAR due to the block in the pathway ([AICAR] is ∼0.5 and ∼1.5 mm in ade3 and ade16 ade17 mutants, respectively). However, this AICAr-dependent effect was totally abolished by the ado1 knock-out mutation (Fig. 5, A–D, column 2) that prevents AICAR synthesis from AICAr (Fig. 3E). We conclude that the riboside form, AICAr, has no effect on transcriptional regulation of the purine and phosphate pathways and more generally that AICAr accumulation does not affect transcription in yeast. These experiments do not allow us to make conclusions on the role of SAICAR, which is also accumulated whenever AICAR is abundant, although the concentration of SAICAR is always much lower (5–10-fold) than that of AICAR under these conditions. Of note, we have attempted to use a similar approach to study the effects of SAICAr and SAICAR, but because of the low solubility of SAICAr in the culture medium and its apparent inability to be taken up by yeast cells, we could neither measure any internalized SAICAr in ade13 ade1 cells grown in the presence of this metabolite nor detect any transcriptional effect on the expression of ADE genes subsequent to SAICAr supplementation (data not shown). Finally, we could reproduce the AICAR toxicity assay (11) by feeding the ade16 ade17 ade8 his1 mutant with high concentrations of AICAr (Fig. 5E). This toxicity was abolished when ado1 was mutated (Fig. 5E), i.e. when AICAr could not be metabolized to AICAR (Fig. 3E). This result establishes that AICAR, the monophosphate form, is the toxic form of this metabolite.
FIGURE 5.
Transcriptional response and toxicity are mediated by AICAR and/or SAICAR. A–D, functional classes of genes differentially expressed (more than 3-fold in column 1) in different strains accumulating or not accumulating (S)AICAr and/or (S)AICAR. Genes were divided into functional classes: purine-responsive genes (A), phosphate-responsive genes (B), other metabolism (C), and other and unknown functions (D). Column numbers correspond to ade8 ade16 ade17 his1 (Y2950) +AICAr versus −AICAr (1), ade8 ade16 ade17 his1 ado1 (Y3188) +AICAr versus −AICAr (2), ade3 (Y4143) versus WT (Y2946) (3), ade16 ade17 (Y2948) versus WT (Y2946) (4), ade16 ade17 (Y2948) versus ade8 ade16 ade17 his1 (Y2950) (5). Raw data are available in the NCBI Gene Expression Omnibus (GEO) under accession number GSE29324. E, accumulation of AICAR and SAICAR is toxic for yeast cells. Cells were grown in SDcasaWAU medium supplemented or not with AICAr at the indicated concentration. Cells were treated as in Fig. 2A, and plates were imaged after 3 days at 30 °C. The strains shown are WT (Y4479), ado1 (Y4476), ade8 ade16 ade17 his1 (Y2950), and ade8 ade16 ade17 his1 ado1 (Y3188).
AICAR and SAICAR Intracellular Concentrations Vary under Physiological Conditions
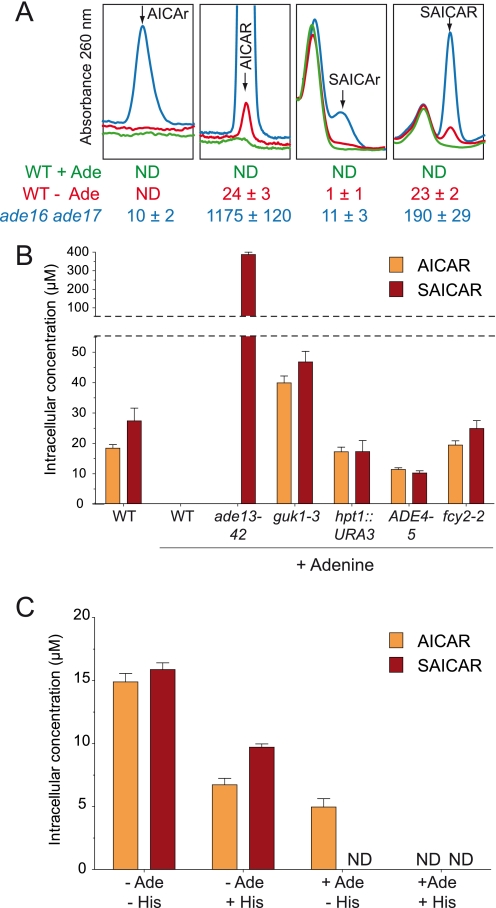
Results presented in the previous sections indicate that AICAR and SAICAR are the effective regulatory molecules. In a previous work, we showed that AICAR binds the Pho2 and Pho4 transcription factors and that up-regulation of ADE and PHO pathway genes is clearly associated with increased AICAR concentration (9–11). Together, these results indicate that AICAR and SAICAR are likely to be signal molecules under physiological conditions. Should this be true, the concentration of AICAR and/or SAICAR should vary under growth conditions known to induce activation of both the ADE and PHO pathways (16, 29), i.e. in response to external adenine. This expectation was directly addressed by ionic chromatography using a highly sensitive detection device. As shown in Fig. 6A, both AICAR and SAICAR were found in the adenine-depleted culture, whereas both compounds were undetectable in the adenine-replete growth condition (Fig. 6A). Of note, AICAr could not be detected in both growth conditions (Fig. 6A). Additionally, we found that in previously characterized adenine-unresponsive mutants (30) AICAR and/or SAICAR could be detected even in the presence of adenine (Fig. 6B). Together, these results strongly substantiate the physiological regulatory role of AICAR and SAICAR in transcriptional activation.
FIGURE 6.
Physiological concentrations of (S)AICAR derivatives. A, metabolite extracts were obtained from wild-type (BY4742) and ade16 ade17 (Y1095) strains grown in SDcasaWU medium supplemented (+Ade) or not (−Ade) with adenine and separated as in Fig. 3A. Intracellular concentrations shown at the bottom of each panel correspond to three independent analyses, and error bars indicate variations in the mean. Red, green, and blue lines correspond to WT −Ade, WT +Ade, and ade16 ade17 +Ade extracts, respectively. B, AICAR and SAICAR concentrations in adenine-non-responsive mutants. Cells were grown in SDcasaWU medium supplemented or not (left two bars) with adenine. Strains shown are WT (PLY122), ade13-42 (Y4090), guk1-3 (220), hpt1::URA3 (Y508), ADE4-5 (215), and fcy2-2 (232). C, physiological concentrations of AICAR and SAICAR in a prototrophic strain. FY4 cells were grown in SD minimal medium in the presence or absence of adenine and/or histidine. In all panels, metabolites were extracted and separated as in Fig. 3A. Results are an average of at least three independent metabolite extractions, and error bars indicate variations in the mean. ND, not detectable.
Finally, measurement of AICAR and SAICAR concentrations in a prototrophic strain grown in minimal medium containing or not containing adenine and histidine allowed us to establish that under physiological conditions both pathways contribute to AICAR synthesis, whereas only the purine pathway supplies SAICAR synthesis (Fig. 6C). In the presence of histidine and adenine (+Ade +His), both pathways are feedback-inhibited by their final product, and neither AICAR nor SAICAR can be detected (Fig. 6C). When the purine pathway is feedback-inhibited, but the histidine pathway is not (+Ade −His), only AICAR is detected (Fig. 6C). Finally, as soon as the purine pathway is not feedback-inhibited (−Ade +His), both AICAR and SAICAR are detected (Fig. 6C). The highest levels for these metabolites were found when both adenine and histidine were absent (−Ade −His; Fig. 6C).
DISCUSSION
Our genetic approach to understand why AICAR-accumulating mutants are histidine auxotrophs, although rather exhaustive, only allowed the identification of two loci. In both cases, histidine prototrophy was most probably due to decreased AICAR concentration, suggesting that no single mutation could reverse the effect of AICAR leading to histidine auxotrophy. Our work clearly associates AICAR accumulation and histidine starvation, suggesting that a step in histidine biosynthesis could be inhibited by AICAR. The reason why we did not isolate any mutant affecting the inhibited step is unclear and could reflect the fact that more than one step is affected. It would be interesting to find out whether inhibition of the histidine pathway by AICAR takes place under physiological conditions.
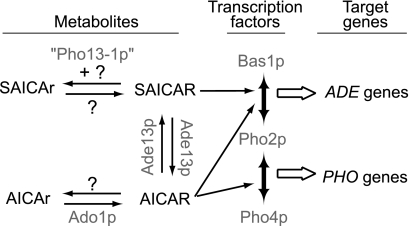
Our genetic study allowed us to show that adenosine kinase is responsible for phosphorylation of AICAr to AICAR in yeast as found previously in mammalian cells (31). We also show that SAICAr is not a good substrate for the adenosine kinase because the SAICAr concentration was only slightly higher in the ado1 suppressor strains, which accumulate a high level of AICAr (Fig. 3A). Our data do not provide any evidence for the existence of a yeast enzyme able to synthesize SAICAR from SAICAr (Fig. 7). The reverse dephosphorylation reaction catalyzed by the Pho13 mutant enzyme was very efficient for SAICAR, whereas AICAR was a very poor substrate (Fig. 4A). Importantly, the wild-type Pho13 enzyme was far less efficient in vitro (Fig. 4D) and could not restore histidine prototrophy when overexpressed (Fig. 4C). Thus, SAICAR does not appear to be a natural substrate for Pho13, the physiological role of which is not known. The putative enzymes responsible for physiological dephosphorylation of AICAR and SAICAR in the wild-type strain have not yet been identified (Fig. 7). This work clearly establishes that the monophosphate forms, AICAR and SAICAR, are detoxified via their dephosphorylation. In humans, adenylosuccinate lyase- and AICAR transformylase inosine monophosphate cyclohydrolase-deficient patients excrete high levels of riboside derivatives in their body fluids. An emerging conclusion of our work is that the concentration of the riboside forms most likely reflects detoxification; their level should therefore be considered with caution to establish correlations with the gravity of syndromes associated with these diseases.
FIGURE 7.
Schematic representation of (S)AICAr and (S)AICAR metabolism and effects in yeast. Question marks indicate that corresponding enzymatic activities are either catalyzed by an unidentified protein or absent in yeast.
This work strongly reinforces the proposed role of AICAR and SAICAR as regulators of transcription. These regulatory effects appear to be highly specific because our transcriptome analysis clearly shows that the riboside form, AICAr, does not substitute for AICAR and plays no major role in yeast transcriptional regulation. Whether AICAR and/or SAICAR plays other physiological roles besides transcriptional regulation remains to be investigated. Interestingly, although addition of extracellular AICAr has multiple effects on mammalian cells, it is not known whether intracellular AICAR naturally produced from the purine de novo pathway plays some physiological roles in these cells. Our work on yeast establishes for the first time that AICAR and SAICAR concentrations vary under physiological conditions (i.e. in the presence or absence of adenine and/or histidine). It would therefore not be surprising if in pluricellular organisms intracellular concentrations of these metabolites varied under physiological conditions in a cell type-specific way. Additionally, we previously reported a significant increase in the AICAR concentration when quiescent yeast cells were transferred to glucose, suggesting a wider role of this metabolite (18). Further understanding of the multiple roles of AICAR will necessitate the identification and study of its protein targets.
This work was supported in part by grants from the Association Française contre les Myopathies, Conseil Régional d'Aquitaine, and Université Bordeaux 2.
- AICAR
- 5-amino-4-imidazole carboxamide ribonucleotide 5′-phosphate
- AICAr
- 5-amino-4-imidazole carboxamide ribonucleoside
- SAICAR
- 5-amino-4-imidazole N-succinocarboxamide ribonucleotide 5′-phosphate
- SAICAr
- 5-amino-4-imidazole N-succinocarboxamide ribonucleoside
- Ade
- adenine
- rec
- recessive suppressor
- DOM
- dominant suppressor.
REFERENCES
- 1. Narkar V. A., Downes M., Yu R. T., Embler E., Wang Y. X., Banayo E., Mihaylova M. M., Nelson M. C., Zou Y., Juguilon H., Kang H., Shaw R. J., Evans R. M. (2008) Cell 134, 405–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tang Y. C., Williams B. R., Siegel J. J., Amon A. (2011) Cell 144, 499–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rattan R., Giri S., Singh A. K., Singh I. (2005) J. Biol. Chem. 280, 39582–39593 [DOI] [PubMed] [Google Scholar]
- 4. Sullivan J. E., Carey F., Carling D., Beri R. K. (1994) Biochem. Biophys. Res. Commun. 200, 1551–1556 [DOI] [PubMed] [Google Scholar]
- 5. Sullivan J. E., Brocklehurst K. J., Marley A. E., Carey F., Carling D., Beri R. K. (1994) FEBS Lett. 353, 33–36 [DOI] [PubMed] [Google Scholar]
- 6. Jacobs R. L., Lingrell S., Dyck J. R., Vance D. E. (2007) J. Biol. Chem. 282, 4516–4523 [DOI] [PubMed] [Google Scholar]
- 7. Kuo C. L., Ho F. M., Chang M. Y., Prakash E., Lin W. W. (2008) J. Cell. Biochem. 103, 931–940 [DOI] [PubMed] [Google Scholar]
- 8. López J. M., Santidrián A. F., Campàs C., Gil J. (2003) Biochem. J. 370, 1027–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pinson B., Vaur S., Sagot I., Coulpier F., Lemoine S., Daignan-Fornier B. (2009) Genes Dev. 23, 1399–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rébora K., Desmoucelles C., Borne F., Pinson B., Daignan-Fornier B. (2001) Mol. Cell. Biol. 21, 7901–7912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rébora K., Laloo B., Daignan-Fornier B. (2005) Genetics 170, 61–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marie S., Heron B., Bitoun P., Timmerman T., Van Den Berghe G., Vincent M. F. (2004) Am. J. Hum. Genet. 74, 1276–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tibbetts A. S., Appling D. R. (2000) J. Biol. Chem. 275, 20920–20927 [DOI] [PubMed] [Google Scholar]
- 14. Sherman F. (1991) Methods Enzymol. 194, 3–21 [DOI] [PubMed] [Google Scholar]
- 15. Garí E., Piedrafita L., Aldea M., Herrero E. (1997) Yeast 13, 837–848 [DOI] [PubMed] [Google Scholar]
- 16. Daignan-Fornier B., Fink G. R. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 6746–6750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Canelas A. B., ten Pierick A., Ras C., Seifar R. M., van Dam J. C., van Gulik W. M., Heijnen J. J. (2009) Anal. Chem. 81, 7379–7389 [DOI] [PubMed] [Google Scholar]
- 18. Laporte D., Lebaudy A., Sahin A., Pinson B., Ceschin J., Daignan-Fornier B., Sagot I. (2011) J. Cell Biol. 192, 949–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanko V. P., Heckenberg A., Rohrer J. S. (2004) J. Biomol. Tech. 15, 317–324 [PMC free article] [PubMed] [Google Scholar]
- 20. Lecoq K., Konrad M., Daignan-Fornier B. (2000) Genetics 156, 953–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deleted in proof.
- 22. Pinson B., Sagot I., Borne F., Gabrielsen O. S., Daignan-Fornier B. (1998) Nucleic Acids Res. 26, 3977–3985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Breton A., Pinson B., Coulpier F., Giraud M. F., Dautant A., Daignan-Fornier B. (2008) Genetics 178, 815–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lemoine S., Combes F., Servant N., Le Crom S. (2006) BMC Bioinformatics 7, 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mizunuma M., Miyamura K., Hirata D., Yokoyama H., Miyakawa T. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 6086–6091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lecoq K., Belloc I., Desgranges C., Daignan-Fornier B. (2001) Yeast 18, 335–342 [DOI] [PubMed] [Google Scholar]
- 27. Kaneko Y., Toh-e A., Banno I., Oshima Y. (1989) Mol. Gen. Genet. 220, 133–139 [DOI] [PubMed] [Google Scholar]
- 28. Ndubuisil M. I., Kwok B. H., Vervoort J., Koh B. D., Elofsson M., Crews C. M. (2002) Biochemistry 41, 7841–7848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gauthier S., Coulpier F., Jourdren L., Merle M., Beck S., Konrad M., Daignan-Fornier B., Pinson B. (2008) Mol. Microbiol. 68, 1583–1594 [DOI] [PubMed] [Google Scholar]
- 30. Guetsova M. L., Lecoq K., Daignan-Fornier B. (1997) Genetics 147, 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sabina R. L., Patterson D., Holmes E. W. (1985) J. Biol. Chem. 260, 6107–6114 [PubMed] [Google Scholar]