Abstract
Heterotrimeric G proteins are critical transducers of cellular signaling. In addition to their classic roles in relaying signals from G protein-coupled receptors (GPCRs), heterotrimeric G proteins also mediate physiological functions from non-GPCRs. Previously, we have shown that Gα13, a member of the heterotrimeric G proteins, is essential for growth factor receptor-induced actin cytoskeletal reorganization such as dynamic dorsal ruffle turnover and cell migration. These Gα13-mediated dorsal ruffle turnover and cell migration by growth factors acting on their receptor tyrosine kinases (RTKs) are independent of GPCRs. However, the mechanism by which RTKs signal to Gα13 is not known. Here, we show that cholinesterase-8A (Ric-8A), a nonreceptor guanine nucleotide exchange factor for some heterotrimeric G proteins, is critical for coupling RTKs to Gα13. Down-regulation of Ric-8A protein levels in cells by RNA interference slowed down platelet-derived growth factor (PDGF)-induced dorsal ruffle turnover and inhibited PDGF-initiated cell migration. PDGF was able to increase the activity of Ric-8A in cells. Furthermore, purified Ric-8A proteins interact directly with purified Gα13 protein in a nucleotide-dependent manner. Deficiency of Ric-8A prevented the translocation of Gα13 to the cell cortex. Hence, Ric-8A is critical for growth factor receptor-induced actin cytoskeletal reorganization.
Keywords: Cell Migration, Cytoskeleton, G Protein-coupled Receptors (GPCR), G Proteins, Growth Factors, Signal Transduction
Introduction
Heterotrimeric G proteins are essential for the transmembrane signaling by G protein-coupled receptors (GPCRs).2 A structurally diverse repertoire of ligands activates GPCRs to elicit their physiological functions (1). Ligand-bound GPCRs function as guanine nucleotide exchange factors (GEFs) catalyzing the exchange of GDP bound on the Gα subunit with GTP in the presence of Gβγ. This leads to the dissociation of the Gα subunit from the Gβγ dimer to form two functional units (Gα and Gβγ) (2). Both Gα and Gβγ subunits signal to various cellular pathways. Based on the sequence and functional homologies, G proteins are grouped into four families: Gs, Gi, Gq, and G12 (3). Among these four subfamilies of G proteins, the physiological function of the G12 subfamily is less well understood. In this family, there are two members, G12 and G13. Gα12 knock-out mice appeared normal (4). Gα13 knock-out mice displayed embryonic lethality (∼E9.5) (5). The molecular basis that underlies the vascular defect observed in Gα13−/− mouse embryos has not been defined.
In addition to their classic roles in GPCR signaling, heterotrimeric G proteins have been genetically demonstrated to play important roles in GPCR-independent signaling (6). The best examples are in the mitotic spindle positioning and orientation (in the establishment of cell polarity) during asymmetric division in Caenorhabditis elegans embryos and in Drosophila neuroblasts (7–11). In these processes, Gαi/o-GDP binds to a protein with the tetratricopeptide-GoLoco domain (such as GPR-1/2 in C. elegans and Pins in Drosophila) and disrupts intramolecular tetratricopeptide-GoLoco interactions. Then, the tetratricopeptide-GoLoco protein binds a coiled-coil protein (LIN-5 in C. elegans and Mud in Drosophila). The formation of the complex of these three proteins, Gαi/o-GPR-1/2-LIN-5 in C. elegans and Gαi-Pins-Mud in Drosophila, is required for spindle orientation (12). A nonreceptor GEF, Ric-8, has been implicated in these GPCR-independent processes (13–19).
Ric-8 (synembryn) was originally identified in C. elegans through genetic analysis (20). Ric-8 functions upstream of Gαq in regulating neurotransmitter secretion (20). Ric-8 also acts upstream of Gαo and GPA16 (another Gα subunit in C. elegans) during asymmetric cell division of one-cell stage C. elegans embryos (13, 21, 22). In Drosophila, Ric-8 is also required for Gα-mediated spindle orientation and cell polarity during asymmetric cell division (16–18). In Ric-8 mutants, Gαi failed to localize at the cell cortex (16–18). There are two distinct mammalian Ric-8-like genes, Ric-8A and Ric-8B. Ric-8A was identified in yeast two-hybrid screens of a rat brain embryonic cDNA library as a protein that interacted with a Gαo bait (23). The Ric-8A prey clone interacted with Gαo, Gαi1, Gαq, and Gα13, but not Gαs baits in pairwise two-hybrid interaction studies. In vitro biochemical studies have shown that Ric-8A is a GEF for Gαq, Gαi, Gαo, Gα12, Gα13, but not Gαs (23, 24). On the other hand, Ric-8B interacts with Gαs and Gαq (23, 24). Mechanistically, Ric-8A binds to GDP-bound Gα proteins, promotes rapid GDP release, and forms a stable nucleotide-free transition state complex with the Gα that is disrupted upon GTP binding, thus leading to the formation of Gα-GTP. Furthermore, although Ric-8A mRNA is expressed in a variety of tissues, Ric-8B mRNA is mainly expressed in the olfactory epithelium (25). Moreover, Ric-8A−/− mouse embryos died in the early stages of embryonic development (26).
Recently, we have discovered that G13 is essential for growth factor-induced cell migration, independent of GPCRs (27). This represents a novel cellular signal aspect and physiological function of heterotrimeric G proteins (28). Our finding that G proteins could transduce signals from non-GPCRs, in addition to GPCRs, reinforces the integrative nature of cellular signaling. Because heterotrimeric G proteins are usually activated by GPCRs, the GPCR-independent function of Gα13 in PDGFR-induced dorsal ruffle turnover and cell migration immediately begs the question of how PDGFR signal is relayed to Gα13. Here, we tested a hypothesis that Ric-8A is involved in PDGFR signaling to Gα13. The reason for testing Ric-8A is the following. First, Drosophila Ric-8 has recently been genetically shown to play a role in gastrulation and is involved in the fog-concertina pathway (17). Concertina is the Drosophila homolog of Gα13 (29). Fog (folded gastrulation) is an extracellular polypeptide growth factor (29). Thus, Ric-8 is involved in Gα13-mediated signaling in Drosophila. Second, in vitro biochemical studies with mammalian Ric-8A have shown that Ric-8A is capable of catalyzing the nucleotide exchange on Gα13 (23, 24). Hence, it is possible that Ric-8A is involved in the PDGFR-Gα13 pathway and functions as the GEF for GPCR-independent activation of Gα13 in this PDGF-initiated pathway. Indeed, we have shown here that down-regulation of Ric-8A in cells impaired PDGF-induced dorsal ruffle turnover and decreased PDGF-initiated cell migration. PDGF treatment increased the activity of Ric-8A in cells. Moreover, deficiency of Ric-8A impairs the translocation of Gα13 to the cell cortex. Therefore, Ric-8A plays a critical role in PDGFR-induced actin cytoskeletal reorganization.
EXPERIMENTAL PROCEDURES
RNA Interference
RNA interference of Ric-8A was performed in mouse embryonic fibroblast (MEF) cells as described previously (30). The shRNA target sequence of Ric-8A used in most of experiments here was 5′-CCATGAAGCTAGTGAACAT-3′ in pSUPER vector (Oligoengine). Four additional targeting sequences were used to confirm the effects of RNA interference. The targeting sequences were selected with the Dharmacon siDesign program. Control shRNA was an shRNA that targets a LacZ sequence. Stable Ric-8A knock-down cell lines and control shRNA-treated cell lines were obtained by selecting puromycin-resistant colonies. Transient Ric-8A RNAi knock-down was performed using the ON-TARGET plus SMART pool from Dharmacon. The efficiency of RNAi knock-down was examined by Western blotting. RNA interference of Ric-8A in human breast cancer cell line MDA-MB-231 was done with ON-TARGETplus SMARTpool siRNA against human Ric-8A from Dharmacon. The targeting sequences were GGGAGAUGCUGCGGAACA, AGAACUUUCCAUACGAGUA, CAGGAUGCCAUGUGCGAGA, and CAGAGGAGUUCCACGGCCA.
Wound Healing Assay
Wild-type, LacZ shRNA, and Ric-8A shRNA-treated MEF cells in Dulbecco's modified Eagle's medium (DMEM) (Invitrogen) containing 10% FBS were seeded into wells of 24-multiwell plates (BD Biosciences) (31, 32). After they grew to confluence, wounds were made with sterile pipette tips. Cells were washed with phosphate-buffered saline (PBS) and refreshed with medium containing 20 ng/ml platelet-derived growth factor (PDGF-BB). After ∼16-h incubation at 37 °C, cells were fixed and photographed (with 100 × magnification).
Boyden Chamber Cell Migration Assay
Cell migration was assayed using Boyden chambers (8.0-μm pore size polyethylene terephthalate membrane, FALCON cell culture insert (BD Biosciences)) (31–34). Cells were trypsinized and counted. 200 μl of 5–10 × 104 cells in serum-free medium was added to the upper chamber, and 500 μl of appropriate medium with 20 ng/ml PDGF-BB was added to the lower chamber. PDGF receptor inhibitor AG1296 was purchased from EMD Chemicals. Epidermal Growth Factor (EGF) was obtained from Sigma-Aldrich. The concentration of AG1296 used was 10 μm. The concentration of EGF was 20 ng/ml. Transwells were incubated for 6 h at 37 °C. Cells on the upper surface of the membrane were removed with a cotton swab, and cells on the under surface of the membrane were fixed with 3.7% formaldehyde and stained. Photographs of three random regions (10 × objective) were taken, and the number of cells was counted to calculate the average number of cells that had transmigrated.
Fluorescence Microscopy
Staining and observation of actin filaments were performed as described previously (35). Cells were plated onto coverslips coated with gelatin. Cells were then fixed with 3.7% formaldehyde. The fixed cells were then permeabilized in 0.1% Triton X-100 for 5 min. After washing in PBS, phalloidin conjugated to rhodamine (Molecular Probes) in a solution containing PBS and 1% BSA was added to stain actin. After incubation for 30 min at room temperature, the cells were washed extensively to reduce nonspecific interactions. The coverslips were then fixed onto slides and imaged using a Zeiss fluorescence microscope. For Gα13 staining, polyclonal anti-Gα13 antibody was from NewEast Biosciences. The immunostaining was done as described previously (35).
Protein Purification
GST-tagged Ric-8A, His6-tagged Gα13/i-5, and His6-tagged Gαi1 proteins were purified from Escherichia coli as described previously (27). BL21 (DE3) E. coli cells harboring pET28a-Gα13/i-5 plasmids were grown to A600 = 0.5–0.6 in LB medium at 37 °C and induced with 30 μm isopropyl 1-thio-β-d-galactopyranoside at 30 °C overnight (36). Cells were harvest and lysed in 20 mm Tris-HCl, pH 8.0, 300 mm NaCl, 5 mm β-mercaptoethanol, 1% Triton X-100, proteinase inhibitors, and purified with Ni-nitrilotriacetic acid beads (Qiagen). Gαi1 proteins were induced with 1 mm isopropyl 1-thio-β-d-galactopyranoside at A600 = 0.5 for 18 h at 16 °C, then purified with Ni-nitrilotriacetic acid beads. GST-tagged Ric-8A protein was purified as described (37).
Ric-8A Activity Assay
Ten 10-cm plates of MEF cells were starved in DMEM without serum overnight and then treated with or without 20 ng/ml PDGF-BB for 10 min. Cells were harvest and lysed in 50 mm Na-HEPES, pH 7.4, 100 mm NaCl, 1% Triton X-100, 1 mm PMSF, 2.5 mm sodium pyrophosphate, 1 mm β-glycerophosphate, and 1 mm Na3VO4. Ric-8A proteins were precipitated with rabbit polyclonal anti-Ric-8A antibodies (Millipore). 50 μl of precipitated proteins was mixed with 50 μl of 2 μm His6-Gi1 or Gα13/i-5 proteins contained in GTPγS loading buffer (50 mm HEPES, pH 8.0, 100 mm NaCl, 100 mm MgCl2, 1 mm dithiothreitol, 20 μm GTPγS, and 1 μm [35S]GTPγS). Aliquots were removed after 2 min, and binding of GTPγS was stopped by the addition of ice-cold buffer containing 20 mm Tris-HCl, pH 7.7, 100 mm NaCl, 2 mm MgSO4, 1 mm GTP, and 0.02% C12E10. The quenched reactions were passed through BA-85 nitrocellulose filters and washed with 20 mm Tris-HCl, pH 8.0, 100 mm NaCl, 25 mm MgCl2, and subjected to scintillation counting. Assays with purified GST-Ric-8A were performed as described (37).
GST Pulldown Assay
Purified His6-tagged Gα13/i-5 proteins were preloaded with 100 μm GDP or GTPγS at room temperature for 30 min. 10 μg of the nucleotide loaded Gα13/i-5 proteins were added into 1 ml of binding buffer containing 20 mm Tris-HCl, pH 8.0, 300 mm NaCl, 1% Triton X-100, 1 mm PMSF, 0.25 μm GST-Ric-8A or GST protein and 20 μl of glutathione beads. The mixtures were rotated at 4 °C for 2 h and washed three times with 1 ml of binding buffer. The beads were boiled with SDS buffer, resolved by PAGE, and blotted with anti-His6 antibody.
Confocal Microscopy
Cells were seeded on acid-washed glass coverslips and allowed to grow overnight. After appropriate treatments, the cells were fixed for 10 min at room temperature with 3.7% formaldehyde in PBS, permeabilized with PBS containing 0.1% Triton X-100 for 5 min, and then washed three times with PBS. The fixed cells were incubated for 30 min with PBS containing 1% BSA to block nonspecific binding and then incubated overnight at 4 °C with rabbit polyclonal antibody against Gα13 from NewEast Biosciences at 1:100 dilution. The cells were washed three times with PBS and incubated with fluorescence-conjugated secondary antibody (Molecular Probes) for 1 h. Actin polymer staining was performed using fluorescent-labeled phalloidin (Molecular Probes). The coverslips were mounted onto glass slides and imaged with a Zeiss LSM 510 laser scanning confocal microscope.
Statistical Analysis
Data are expressed as mean ± S.D. and analyzed by one-way ANOVA followed by Dunnett's Multiple Comparison test with significance defined as p < 0.05.
RESULTS
Ric-8A Is Involved in PDGF-BB-induced Dorsal Ruffle Turnover
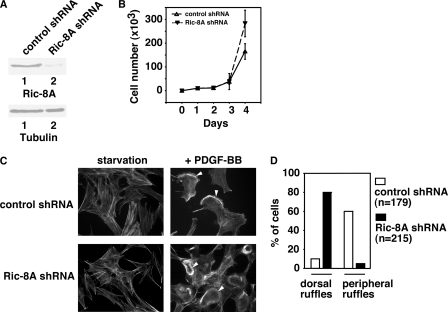
First, we investigated whether Ric-8A is involved in PDGF receptor-induced dorsal ruffle formation and cell migration. The earliest ultrastructural changes of cells treated with growth factors are the intensive bursts of ruffling of the dorsal surface plasma membranes as seen under the phase-contrast microscope (38–40). The physiological functions of dorsal ruffles, including macropinocytosis, cell migration, and invasion, are continually expanding (41–44). It has been suggested that one major function of dorsal ruffles is to reorganize the actin cytoskeleton to prepare a static cell for motility (45). We used RNA interference to down-regulate the protein levels of Ric-8A in MEF cells (Fig. 1A). We transfected plasmid DNAs carrying shRNAs either against Ric-8A or against LacZ (as control) into MEF cells and selected stable cell lines expressing these shRNAs. Although Ric-8A shRNA decreased the protein level of Ric-8A in MEF cells, LacZ shRNA did not (Fig. 1A). Decrease of Ric-8A protein levels had no effects on MEF cell proliferation within the first 48 h of cell culture after splitting the cells (Fig. 1B), although it increased cell proliferation after 72 h. Because our experiments (described below) were done within 1 h (dorsal ruffle turnover), 6 h (Boyden chamber cell migration assays), or 16 h (wound-healing assays), cell proliferation was not a contributing factor here.
FIGURE 1.
Ric-8A is required for PDGF-BB-induced dorsal ruffle turnover. A, upper panel, Western blot with anti-Ric-8A antibody showing the knock-down of Ric-8A protein levels in Ric-8A shRNA-treated MEF cells, but not in the control LacZ shRNA-treated MEF cells. Lower panel, Western blot with anti-tubulin antibody showing the whole cell lysate loading. B, cell proliferation assay. LacZ shRNA-treated and Ric-8A shRNA-treated cells were cultured for days, and the number of cells in the plate was counted every day. Results are mean ± S.D. (error bars) of three plates. C, actin filament staining. LacZ shRNA-treated and Ric-8A shRNA-treated MEF cells were starved overnight or treated with 20 ng/ml PDGF-BB. Cells were fixed and stained with phalloidin-rhodamine. Arrowheads indicate either peripheral membrane ruffles (upper) or dorsal ruffles (lower). D, distribution of peripheral membrane ruffles and dorsal ruffles in control LacZ shRNA-treated cells (n = 179) or in Ric-8A shRNA-treated cells (n = 215). Data are representative of three to five experiments.
In serum-starved fibroblasts, PDGF-BB induces at least two types of membrane ruffles: peripheral membrane ruffles (or lamellipodia) and dorsal ruffles (46). Dorsal ruffles are dynamic structures. They form and disassemble rapidly (47, 48). Previously, we have reported that, in MEF cells, dorsal ruffles formed within ∼5 min after PDGF-BB (20 ng/ml) treatment (33). These dorsal ruffles were disassembled ∼10 min after PDGF-BB treatment. Dorsal ruffles formed only one time after PDGF-BB stimulation. After the disassembly of these dorsal ruffles, protrusion of large peripheral membrane ruffles was observed (33). Without PDGF-BB treatment, actin filaments were uniformly distributed in MEF cells (Fig. 1C). After PDGF-BB treatment for 10 min, control cells (treated with LacZ shRNA) showed dorsal ruffles in <10% of all cells and peripheral membrane ruffles in ∼60% of cells (Fig. 1, C and D). On the other hand, in Ric-8A shRNA-treated cells, dorsal ruffles could still be observed in ∼80% of cells and peripheral membrane ruffles in <5% of the cells (Fig. 1, C and D). These data imply that deficiency of Ric-8A delayed the dorsal ruffle turnover, similar to our previous observation with Gα13 deficiency (33). These data demonstrate that Ric-8A is involved in PDGF-BB-initiated dynamic dorsal ruffle turnover.
Ric-8A Is Required for RTK-initiated Cell Migration
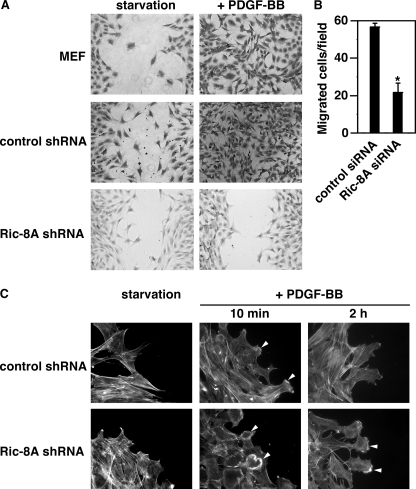
Next, we used cell migration as the second model to investigate the function of Ric-8A in actin cytoskeletal reorganization. Although some believe that dorsal ruffle turnover is part of the cell migration process and indeed required for cell migration, this notion is still under debate. Therefore, here, we treated these as two events of actin cytoskeletal reorganization. To investigate a possible role of Ric-8A in PDGF-BB-initiated cell migration, we have used two approaches to compare the migration of Ric-8A-down-regulated cells and control cells. One approach is the qualitative in vitro wound-healing assay, the other the quantitative Boyden chamber assay (31, 32). For the wound-healing assay, control LacZ shRNA-treated cells and Ric-8A shRNA-treated cells were grown to confluence. A wound (small scratch) was made in the middle of the tissue culture plate with a pipette tip. After ∼24 h in the presence of PDGF-BB, control cells migrated and covered the wound, yet Ric-8A shRNA-treated cells did not (Fig. 2A). Therefore, PDGF-BB-induced migration of Ric-8A-down-regulated cells was markedly reduced compared with the migration of control cells. These results were confirmed with Boyden chamber assays (Fig. 2B). Different cell proliferation rates of control MEF cells and Ric-8A shRNA-treated cells might affect their migratory rates in wound-healing assays. However, because we have shown that proliferation of Ric-8A shRNA-treated cells was faster than control cells after 72 h of culture (Fig. 1B), the slower rate of migration exhibited by Ric-8A shRNA-treated cells is not due to a slower proliferation rate. Furthermore, to investigate the dynamic turnover of dorsal ruffles and peripheral ruffles in cells at the wound edge, we examined the actin cytoskeletal reorganization of LacZ-shRNA-treated and Ric-8A shRNA-treated cells at the wound edge (Fig. 2C). After a 10-min treatment with PDGF-BB, cells treated with control (LacZ) shRNA displayed lamellipodia at the edges facing the wound (65% of the cells), indicating that the dorsal ruffles had turned over, similar to the nonmigrating cells in Fig. 1C. On the other hand, cells treated with Ric-8A shRNA exhibited dorsal ruffles (70% of the cells) (Fig. 2C), confirming a delayed dorsal ruffle turnover in Ric-8A down-regulated cells. After PDGF-BB treatment for 2 h, LacZ shRNA-treated cells showed weaker lamellipodial staining than Ric-8A shRNA-treated cells (∼80% of the cells) (Fig. 2C). It is interesting to note that, even though Ric-8A shRNA-treated cells did not migrate, they still formed lamellipodia, albeit delayed. Together, these data demonstrate that Ric-8A is required for PDGF-BB-induced cell migration.
FIGURE 2.
Ric-8A is needed for PDGF-BB-initiated cell migration. A, wound-healing assay of cell migration. MEF cells, LacZ shRNA-treated control cells, and Ric-8A shRNA-treated cells were assayed in the absence (starvation) or presence of 20 ng/ml PDGF-BB for 16 h. Representative images are shown. B, Boyden chamber assay of cell migration of LacZ siRNA-treated and Ric-8A siRNA-treated MEF cells in the presence of 20 ng/ml PDGF-BB. The number of migrated cells in each microscopic field in the absence of PDGF-BB was subtracted from the number in the presence of PDGF-BB. Results are mean ± S.D. (error bars; n = 3, p < 0.05). C, actin filament staining of cells at the wound edge. LacZ shRNA-treated and Ric-8A shRNA-treated MEF cells at the edges of wounds were starved overnight or treated with PDGF-BB. Cells were fixed and stained with phalloidin-rhodamine. Arrowheads indicate either peripheral membrane ruffles or dorsal ruffles. Data are representative of three to five experiments.
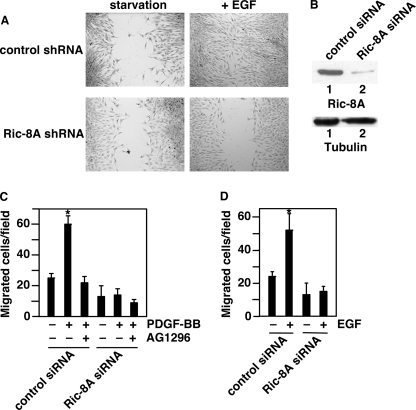
Furthermore, Ric-8A is also required for EGF-induced migration of MEF cells and human breast tumor MDA-MB-231 cells (Fig. 3). Although EGF induced the migration of MEF cells treated with control (LacZ) shRNA, EGF-induced migration was impaired in MEF cells treated with Ric-8A shRNA (Fig. 3A). In addition, we have investigated the effect of Ric-8A in human breast tumor MDA-MB-231 cells (Fig. 3, B–D). Both PDGF-BB and EGF induced the migration of MDA-MB-231 cells treated with control siRNAs (Fig. 3, B–D). However, PDGF-BB- or EGF-induced migration of MDA-MB-231 cells treated with Ric-8A siRNAs was impaired (Fig. 3, C and D). Pretreatment with a PDGFR inhibitor AG1296 prevented the effect of PDGF-BB on cell migration (Fig. 3C). Together, these data indicate that Ric-8A plays roles in RTK-induced cell migration.
FIGURE 3.
Ric-8A is needed for EGF-initiated cell migration. A, wound-healing assay of cell migration. LacZ shRNA-treated control cells and Ric-8A shRNA-treated MEF cells were assayed in the absence (starvation) or presence of EGF for 16 h. Representative images are shown. B, upper, Western blot with anti-Ric-8A antibody showing the knock-down of Ric-8A protein levels in Ric-8A siRNA-treated MDA-MB-231 cells, but not in the control siRNA-treated MDA-MB-231 cells. Lower, Western blotting with anti-tubulin antibody to show the whole cell lysate loading. C and D, Boyden chamber assay of cell migration of control siRNA-treated and Ric-8A siRNA-treated MDA-MB-231 cells in the presence of 20 ng/ml PDGF-BB (C) or EGF (D). The number of migrated cells in each microscopic field in the absence of PDGF-BB or EGF was subtracted from the number in the presence of PDGF-BB or EGF. Results are mean ± S.D. (error bars; n = 3, p < 0.05). Data are representative of three to five experiments.
PDGFR Increases Ric-8A Activity in Cells
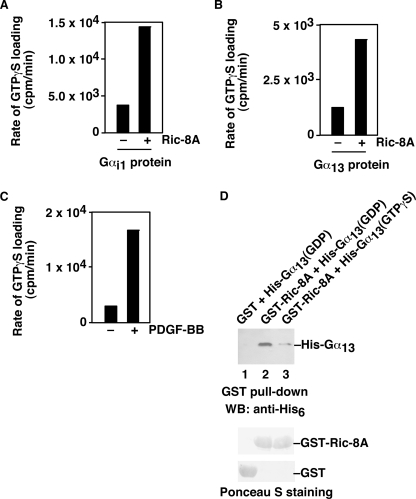
To investigate the mechanism by which PDGF receptors signal through Ric-8A to induce actin cytoskeletal reorganization, we examined the effect of PDGFR on the activity of Ric-8A. First, we showed that purified Ric-8A protein could increase the rates of [35S]GTPγS loading onto purified Gαi (Fig. 4A) and purified Gα13 (Fig. 4B), demonstrating the working of the assay conditions in our hands and confirming the GEF activity of Ric-8A for Gαi and Gα13. Next, we treated wild-type MEF cells with PDGF-BB. Whole cell lysates were prepared. Ric-8A protein was immunoprecipitated. The GEF activity of Ric-8A was measured by following the [35S]GTPγS binding assays with purified Gαi. As shown in Fig. 4C, PDGF-BB treatment increased the Ric-8A activity by ∼5-fold. Moreover, purified Ric-8A protein could bind directly to purified Gα13 protein, preferably bound with GDP (Fig. 4D). These data indicate that PDGFR is able to activate Ric-8A in cells.
FIGURE 4.
PDGF-BB increases the GEF activity of Ric-8A in cells. A, in vitro assay of the GEF activity of purified Ric-8A on purified Gαi1 proteins. [35S]GTPγS was used to monitor the loading of GTP to Gαi1 by Ric-8A. The initial rate of [35S]GTPγS loading was compared without or with Ric-8A. B, in vitro assay of the GEF activity of purified Ric-8A on purified Gα13 proteins. [35S]GTPγS was used to monitor the loading of GTP to Gα13 by Ric-8A. The initial rate of [35S]GTPγS loading was compared without or with Ric-8A. C, PDGF-BB treatment increased the GEF activity of Ric-8A in cells. MEF cells were treated with or without 20 ng/ml PDGF-BB. Ric-8A proteins were immunoprecipitated from whole cell lysates. These immunoprecipitated Ric-8A proteins were used for in vitro [35S]GTPγS loading assays with purified Gαi1 proteins. The initial rate of [35S]GTPγS loading was compared without or with PDGF-BB treatment. Representatives of three similar experiments are shown. D, direct interaction of Ric-8A and Gα13. Purified GST-Ric-8A proteins (lanes 2 and 3) or control GST proteins (lane 1) were used to pull down purified His-tagged Gα13 proteins, in the presence of GDP (lane 2) or GTPγS (lane 3). The pull-downed Gα13 was shown by anti-His6 antibody (upper panel). Lower panels are Ponceau S staining showing the used GST-Ric-8A proteins or GST proteins. Data are representative of three to five experiments.
Ric-8A Is Required for Gα13 Translocation to Cell Cortex
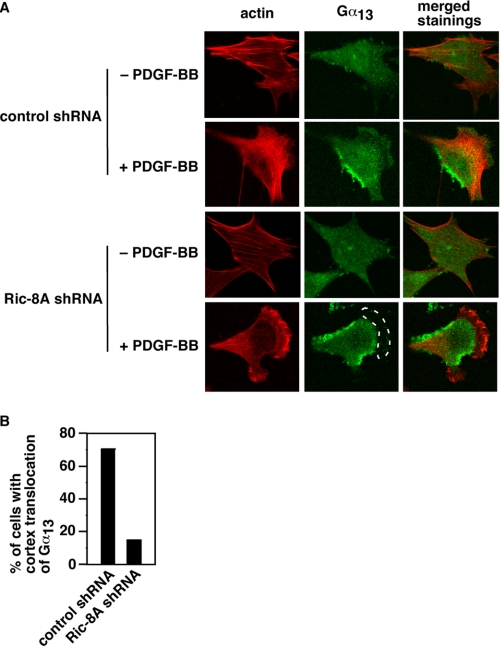
The above data demonstrate that Ric-8A is critical for PDGFR signal to dorsal ruffle turnover and cell migration. Previously, we have also shown that Gα13 is essential for PDGF-BB-initiated dorsal ruffle turnover and cell migration. Do Ric-8A and Gα13 act in the same pathway or in parallel pathways? Because Ric-8A interacts directly with Gα13 (Fig. 4D) and to catalyze guanine nucleotide exchange on Gα13 (Fig. 4B), it is likely that Ric-8A and Gα13 work in the same pathway and that Ric-8A functions upstream of Gα13. It would be helpful to investigate the activation of Gα13 by PDGF-BB in cells in the presence or absence of Ric-8A. However, for technical reasons, it is rather difficult to measure Gα13 activation in cells. Because one common effect of Ric-8A on heterotrimeric G proteins in C. elegans and in Drosophila is to mediate the cell cortex translocation of heterotrimeric G proteins (even on Gα proteins that Ric-8 has no GEF activity), we examined the cell cortex translocation of Gα13 in response to PDGF-BB, in the presence or absence of Ric-8A. Without PDGF-BB treatment, Gα13 was uniformly distributed in cells (Fig. 5A). Phalloidin staining showed actin stress fibers, in both control shRNA-treated and Ric-8A shRNA-treated cells (Fig. 5A). PDGF-BB treatment led to lamellipodial formation with strong cell cortex actin filament staining in both control shRNA-treated and Ric-8A shRNA-treated cells (Fig. 5A). However, although Gα13 was translocated to the cell cortex in control shRNA-treated cells, Gα13 was absent in the cortex in Ric-8A shRNA-treated cells (Fig. 5A, dashed line circled area). As summarized in Fig. 5B, ∼70% of LacZ shRNA-treated controls (n = 109) showed Gα13 staining on the cell cortex after PDGF-BB treatment. On the other hand, only ∼15% of Ric-8A shRNA-treated cells (n = 111) displayed cell cortex staining of Gα13. These data show that Ric-8A is needed for Gα13 cell cortex translocation in response to PDGF-BB and imply that Ric-8A functions upstream of Gα13 in PDGFR signaling.
FIGURE 5.
Role of Ric-8Ain PDGF-BB-induced cell cortex translocation of Gα13. A, representative images of cells stably expressing LacZ shRNA (top two panels) or Ric-8A shRNA (bottom two panels) after stimulation with 20 ng/ml PDGF-BB. The images in the left column were phalloidin-stained actin filaments (shown in red color). The images in the middle column were stained with anti-Gα13 antibody (shown in green color). The images in the right column were merged stainings of actin filaments and Gα13. The dashed lined circle highlights the absence of Gα13 signal in the cell cortex. B, quantification of cells with Gα13 cell cortex localization after PDGF-BB treatment. LacZ shRNA-treated and Ric-8A shRNA-treated cells were treated with 20 ng/ml PDGF-BB. After fixation and staining with phalloidin and anti-Gα13 antibody, the number of cells with Gα13 cell cortex localization was counted. Data are representative of three to five experiments.
DISCUSSION
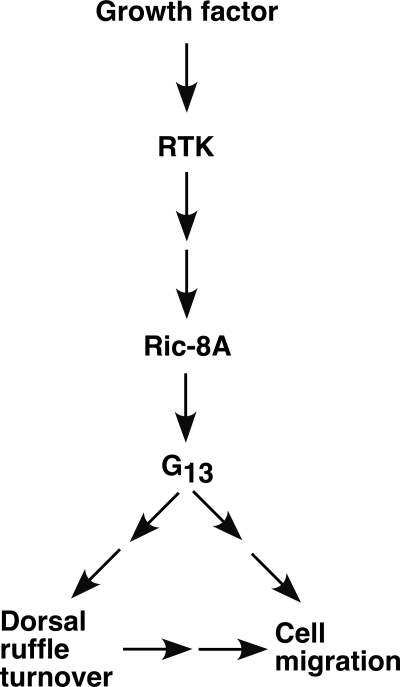
We have shown that a nonreceptor GEF, Ric-8A, plays a critical role in PDGFR-induced actin cytoskeletal reorganization. Down-regulation of Ric-8A in cells decreased PDGF-BB-induced dorsal ruffle turnover and cell migration. PDGFR is able to activate Ric-8A in cells. Ric-8A is required for Gα13 cell cortical translocation in response to PDGF-BB stimulation. Thus, Ric-8A links PDGFR signal to Gα13 in this pathway (Fig. 6).
FIGURE 6.
Diagram of a model for the participation of Ric-8A in PDGF-BB signaling. Growth factors, such as PDGF-BB, act on their RTKs, such as PDGF receptor, to increase the GEF activity of Ric-8A. Ric-8A, in turn, regulates Gα13 cell cortex translocation and activates Gα13. Activated Gα13 regulates the dynamic turnover of dorsal ruffles and cell migration.
Our data could provide a molecular mechanism by which RTKs, and possibly other non-GPCR receptors, use heterotrimeric G proteins (in addition to Gα13) to signal and to regulate various physiological functions. Although Ric-8 has been implicated in receptor-independent activation of heterotrimeric G proteins in asymmetric division, our report is the first to reveal that Ric-8A functions downstream of a RTK. Thus, this might provide a molecular mechanism for linking RTKs to heterotrimeric G proteins. Indeed, previously, heterotrimeric G proteins have been shown to play roles in RTK signaling. Various approaches including toxins, inhibitors, and antisense constructs have been used to inhibit the function or to reduce the level of heterotrimeric G proteins (49–52). These treatments led to impairment of RTK cellular signaling (53). Although in some cases a direct interaction between the Gα subunit and a RTK had been proposed, in most instances the mechanism was not known. Future experiments will be directed to investigate the molecular mechanism by which RTKs regulate the activity of Ric-8A.
It remains unclear how Gα13 controls the dynamic turnover of dorsal ruffles. Previously, we have shown that, in wild-type fibroblast cells, dorsal ruffles form and disappear quickly (33). However, in the absence of Gα13, the dorsal ruffles stay much longer (33). Because dorsal ruffles take up much of the actin polymers, a slow disassembly of dorsal ruffles would slow down the formation of other actin polymer-based structures such as peripheral membrane ruffles that are required for cell migration. Given that the formation of these dorsal ruffles is controlled by Ras, Rac, and Rab5 small GTPases (43, 48, 54, 55), one way to slow down the disassembly of dorsal ruffles (in the absence of Gα13) would be to slow the conversion of Ras (or Rac, Rab5) from its active GTP-bound state to the inactive GDP-bound state, i.e. to slow the GTP hydrolysis. In other words, the presence of Gα13 could accelerate the conversion. It is interesting to note that we have previously shown that Gα12 could activate a specific Ras GTPase-activating protein, Gap1m, to shorten the duration of activated Ras in cells (56). Furthermore, in a yeast two-hybrid assay, Ras-GAP III (Gap1IP4BP, related to Gap1m) was shown to interact with Gαo (23). Moreover, Rap1GAP has also been shown to interact directly with Gαo, Gαi, and Gαz (57–59). Thus, Gα13 could act through a GAP for Ras, Rac, or Rab5 to accelerate the actin cytoskeletal turnover and, hence, cell migration.
Acknowledgments
We thank Dr. S. R. Sprang for the Ric-8A and for the Gα13/i-5 plasmids and D. McGarrigle and S. Li for technical assistance with some experiments. We thank members of our laboratory for critically reading the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant HL91525. This work was also supported by the 111 Project of China Grant B06018 and the National Science Foundation of China Grants 31028015 and 30921001.
- GPCR
- G protein-coupled receptor
- GEF
- guanine nucleotide exchange factor
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- MEF
- mouse embryonic fibroblast
- Ric
- resistance to inhibitors of cholinesterase
- RTK
- receptor tyrosine kinase.
REFERENCES
- 1. Gilman A. G. (1987) Annu. Rev. Biochem. 56, 615–649 [DOI] [PubMed] [Google Scholar]
- 2. Bourne H. R., Sanders D. A., McCormick F. (1990) Nature 348, 125–132 [DOI] [PubMed] [Google Scholar]
- 3. Simon M. I., Strathmann M. P., Gautam N. (1991) Science 252, 802–808 [DOI] [PubMed] [Google Scholar]
- 4. Gu J. L., Müller S., Mancino V., Offermanns S., Simon M. I. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 9352–9357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Offermanns S., Mancino V., Revel J. P., Simon M. I. (1997) Science 275, 533–536 [DOI] [PubMed] [Google Scholar]
- 6. Manning D. R. (2003) Sci. STKE 2003, pe35. [DOI] [PubMed] [Google Scholar]
- 7. Zwaal R. R., Ahringer J., van Luenen H. G., Rushforth A., Anderson P., Plasterk R. H. (1996) Cell 86, 619–629 [DOI] [PubMed] [Google Scholar]
- 8. Gotta M., Ahringer J. (2001) Nat. Cell Biol. 3, 297–300 [DOI] [PubMed] [Google Scholar]
- 9. Srinivasan D. G., Fisk R. M., Xu H., van den Heuvel S. (2003) Genes Dev. 17, 1225–1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Colombo K., Grill S. W., Kimple R. J., Willard F. S., Siderovski D. P., Gönczy P. (2003) Science 300, 1957–1961 [DOI] [PubMed] [Google Scholar]
- 11. Gotta M., Dong Y., Peterson Y. K., Lanier S. M., Ahringer J. (2003) Curr. Biol. 13, 1029–1037 [DOI] [PubMed] [Google Scholar]
- 12. Siller K. H., Doe C. Q. (2009) Nat. Cell Biol. 11, 365–374 [DOI] [PubMed] [Google Scholar]
- 13. Afshar K., Willard F. S., Colombo K., Johnston C. A., McCudden C. R., Siderovski D. P., Gönczy P. (2004) Cell 119, 219–230 [DOI] [PubMed] [Google Scholar]
- 14. Couwenbergs C., Spilker A. C., Gotta M. (2004) Curr. Biol. 14, 1871–1876 [DOI] [PubMed] [Google Scholar]
- 15. Afshar K., Willard F. S., Colombo K., Siderovski D. P., Gönczy P. (2005) Development 132, 4449–4459 [DOI] [PubMed] [Google Scholar]
- 16. David N. B., Martin C. A., Segalen M., Rosenfeld F., Schweisguth F., Bellaïche Y. (2005) Nat. Cell Biol. 7, 1083–1090 [DOI] [PubMed] [Google Scholar]
- 17. Hampoelz B., Hoeller O., Bowman S. K., Dunican D., Knoblich J. A. (2005) Nat. Cell Biol. 7, 1099–1105 [DOI] [PubMed] [Google Scholar]
- 18. Wang H., Ng K. H., Qian H., Siderovski D. P., Chia W., Yu F. (2005) Nat. Cell Biol. 7, 1091–1098 [DOI] [PubMed] [Google Scholar]
- 19. Woodard G. E., Huang N. N., Cho H., Miki T., Tall G. G., Kehrl J. H. (2010) Mol. Cell. Biol. 30, 3519–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Miller K. G., Emerson M. D., McManus J. R., Rand J. B. (2000) Neuron 27, 289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miller K. G., Rand J. B. (2000) Genetics 156, 1649–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hess H. A., Röper J. C., Grill S. W., Koelle M. R. (2004) Cell 119, 209–218 [DOI] [PubMed] [Google Scholar]
- 23. Tall G. G., Krumins A. M., Gilman A. G. (2003) J. Biol. Chem. 278, 8356–8362 [DOI] [PubMed] [Google Scholar]
- 24. Tall G. G., Gilman A. G. (2004) Methods Enzymol. 390, 377–388 [DOI] [PubMed] [Google Scholar]
- 25. Von Dannecker L. E., Mercadante A. F., Malnic B. (2005) J. Neurosci. 25, 3793–3800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tõnissoo T., Kõks S., Meier R., Raud S., Plaas M., Vasar E., Karis A. (2006) Behav. Brain Res. 167, 42–48 [DOI] [PubMed] [Google Scholar]
- 27. Shan D., Chen L., Wang D., Tan Y. C., Gu J. L., Huang X. Y. (2006) Dev. Cell 10, 707–718 [DOI] [PubMed] [Google Scholar]
- 28. Dhanasekaran D. N. (2006) Sci. STKE 2006, pe31. [DOI] [PubMed] [Google Scholar]
- 29. Parks S., Wieschaus E. (1991) Cell 64, 447–458 [DOI] [PubMed] [Google Scholar]
- 30. Chen L., Yang S., Jakoncic J., Zhang J. J., Huang X. Y. (2010) Nature 464, 1062–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shan D., Chen L., Njardarson J. T., Gaul C., Ma X., Danishefsky S. J., Huang X. Y. (2005) Proc. Natl. Acad. Sci. U.S.A. 102, 3772–3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yang S., Huang X. Y. (2005) J. Biol. Chem. 280, 27130–27137 [DOI] [PubMed] [Google Scholar]
- 33. Wang D., Tan Y. C., Kreitzer G. E., Nakai Y., Shan D., Zheng Y., Huang X. Y. (2006) J. Biol. Chem. 281, 32660–32667 [DOI] [PubMed] [Google Scholar]
- 34. Guo D., Tan Y. C., Wang D., Madhusoodanan K. S., Zheng Y., Maack T., Zhang J. J., Huang X. Y. (2007) Cell 128, 341–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lowry W. E., Huang J., Ma Y. C., Ali S., Wang D., Williams D. M., Okada M., Cole P. A., Huang X. Y. (2002) Dev. Cell 2, 733–744 [DOI] [PubMed] [Google Scholar]
- 36. Chen Z., Singer W. D., Sternweis P. C., Sprang S. R. (2005) Nat. Struct. Mol. Biol. 12, 191–197 [DOI] [PubMed] [Google Scholar]
- 37. Thomas C. J., Tall G. G., Adhikari A., Sprang S. R. (2008) J. Biol. Chem. 283, 23150–23160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mellstroöm K., Höglund A. S., Nistér M., Heldin C. H., Westermark B., Lindberg U. (1983) J. Muscle Res. Cell Motil. 4, 589–609 [DOI] [PubMed] [Google Scholar]
- 39. Schliwa M., Nakamura T., Porter K. R., Euteneuer U. (1984) J. Cell Biol. 99, 1045–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mellström K., Heldin C. H., Westermark B. (1988) Exp. Cell Res. 177, 347–359 [DOI] [PubMed] [Google Scholar]
- 41. Warn R., Brown D., Dowrick P., Prescott A., Warn A. (1993) Symp. Soc. Exp. Biol. 47, 325–338 [PubMed] [Google Scholar]
- 42. Dowrick P., Kenworthy P., McCann B., Warn R. (1993) Eur. J. Cell Biol. 61, 44–53 [PubMed] [Google Scholar]
- 43. Suetsugu S., Yamazaki D., Kurisu S., Takenawa T. (2003) Dev. Cell 5, 595–609 [DOI] [PubMed] [Google Scholar]
- 44. Bretscher M. S. (1996) Cell 87, 601–606 [DOI] [PubMed] [Google Scholar]
- 45. Krueger E. W., Orth J. D., Cao H., McNiven M. A. (2003) Mol. Biol. Cell 14, 1085–1096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Buccione R., Orth J. D., McNiven M. A. (2004) Nat. Rev. Mol. Cell Biol. 5, 647–657 [DOI] [PubMed] [Google Scholar]
- 47. Hedberg K. M., Bengtsson T., Safiejko-Mroczka B., Bell P. B., Lindroth M. (1993) Cell Motil. Cytoskeleton 24, 139–149 [DOI] [PubMed] [Google Scholar]
- 48. Dharmawardhane S., Sanders L. C., Martin S. S., Daniels R. H., Bokoch G. M. (1997) J. Cell Biol. 138, 1265–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Moxham C. M., Malbon C. C. (1996) Nature 379, 840–844 [DOI] [PubMed] [Google Scholar]
- 50. Alderton F., Rakhit S., Kong K. C., Palmer T., Sambi B., Pyne S., Pyne N. J. (2001) J. Biol. Chem. 276, 28578–28585 [DOI] [PubMed] [Google Scholar]
- 51. Waters C., Sambi B., Kong K. C., Thompson D., Pitson S. M., Pyne S., Pyne N. J. (2003) J. Biol. Chem. 278, 6282–6290 [DOI] [PubMed] [Google Scholar]
- 52. Waters C., Pyne S., Pyne N. J. (2004) Semin. Cell Dev. Biol. 15, 309–323 [DOI] [PubMed] [Google Scholar]
- 53. Sun H., Chen Z., Poppleton H., Scholich K., Mullenix J., Weipz G. J., Fulgham D. L., Bertics P. J., Patel T. B. (1997) J. Biol. Chem. 272, 5413–5420 [PubMed] [Google Scholar]
- 54. Bar-Sagi D., Feramisco J. R. (1986) Science 233, 1061–1068 [DOI] [PubMed] [Google Scholar]
- 55. Lanzetti L., Palamidessi A., Areces L., Scita G., Di Fiore P. P. (2004) Nature 429, 309–314 [DOI] [PubMed] [Google Scholar]
- 56. Jiang Y., Ma W., Wan Y., Kozasa T., Hattori S., Huang X. Y. (1998) Nature 395, 808–813 [DOI] [PubMed] [Google Scholar]
- 57. Jordan J. D., Carey K. D., Stork P. J., Iyengar R. (1999) J. Biol. Chem. 274, 21507–21510 [DOI] [PubMed] [Google Scholar]
- 58. Mochizuki N., Ohba Y., Kiyokawa E., Kurata T., Murakami T., Ozaki T., Kitabatake A., Nagashima K., Matsuda M. (1999) Nature 400, 891–894 [DOI] [PubMed] [Google Scholar]
- 59. Meng J., Glick J. L., Polakis P., Casey P. J. (1999) J. Biol. Chem. 274, 36663–36669 [DOI] [PubMed] [Google Scholar]