Abstract
Acid-solubilized collagen type I (COL1) can form highly organized, three-dimensional scaffolds for a wide variety of bioengineering and cell culture applications. A rapid COL1 isolation method would be a valuable tool for both basic and translational researchers because conventional techniques require days or weeks to complete, typically use nonhuman animal tissues as a source material, and do not efficiently purify autologous COL1 from small samples. Here, we describe a 3-h method to isolate COL1 from rabbit, lamb, and human skin in sufficient quantities for fabrication of autologously derived tissues by using a rapid agitation technique and incorporating centrifugal filtration units into a traditional isolation procedure. We demonstrate that the purified product is comparable to traditional preparations using polyacrylamide gel electrophoresis, transmission electron microscopy, and collagen content assays. In addition, our COL1 is able to support myogenic cell growth and direct orientation of these cells in vitro. Importantly, this ultrarapid COL1 isolation procedure increases the feasibility of autologous COL1 use in humans as well as overall safety for clinical patients.
Introduction
Collagen type I (COL1) is found in many tissues, including cartilage, bone, cornea, muscle, and tendon. It is the principal structural component of skin and the most abundant protein in the body.1,2 Decades ago, investigators discovered that at neutral pH, acid-solubilized collagen aggregated into fibrils composed of the same cross-striated patterns observed in native collagen.3,4 This attribute promoted its use as a scaffold material capable of supporting cells for innumerable bioengineering applications. Solubilized COL1 can be mixed with cells, growth factors, and other biomaterials while a liquid and then cast into a mold to create engineered tissue of virtually any desired size and shape.5
Cellularized collagen constructs have been subjected to tensile strain regimens that alter the stiffness and gene expression profiles of cells and can be optimized to form individualized tendon grafts.6 Three-dimensional (3D) collagen scaffolds seeded with fibroblasts and keratinocytes can generate a stratum corneum similar to that found in human skin.7 In addition to fabricated tissue, collagen-based matrices have also been increasingly utilized to control the differentiation and orientation of various cell populations in vitro and in vivo. Micro patterned collagen has been used to direct bone formation, manage smooth muscle cell morphology, and to control mesenchymal stem cell attachment.8–10
Although acid-solubilized COL1 can be purchased from commercial vendors, it is costly, often derived from nonhuman sources, and quite variable in its ability to form highly organized hydrogels with consistent structural properties. Concurrently, traditional COL1 isolation procedures require days or even weeks to complete.11–13 If a less time-consuming method for COL1 extraction from clinically relevant sample sizes were available, the safety and utility of collagen-based hydrogels for regenerative medical applications may become more practical. The use of autologously derived COL1 would also circumvent implanted tissue inflammation and rejection as well as eliminate the possibility of xenobiotic disease transmission. Accordingly, we sought to establish a method for the reliable, efficient, and rapid isolation of COL1 from small dermal samples and then test its ability to form 3D cellular support scaffolds.
Materials and Methods
Preparation of dermal samples
Skin was removed from newborn lambs and adult rabbits, rinsed in dH2O, and placed at 4°C until purification. Flash frozen adult (38 years) human skin samples (2×2 cm or 8.75×8.75 cm) were purchased from AMS Biotechnology. Dermal samples were rinsed with ice-cold dH2O and any wool, fur, or hair was removed with depilatory cream (Church and Dwight). The samples were then scraped clean of connective tissues and fat before being rinsed extensively in ice-cold dH2O and sliced into 10×10 mm pieces with a razor blade.
Removal of noncollagenous solubilized material
The entire COL1 isolation was performed at 4°C and all of the reagents were equilibrated to this temperature before their use. Lamb, rabbit, and human dermal samples (5 g each) were placed into 50 mL conical tubes with 30 mL of ice-cold 0.5 M sodium acetate to remove noncollagenous soluble proteins and polysaccharides.12 Tubes were mixed for 1 min at 6 m/s using the appropriate tube adapters for the FastPrep™ system (MP Biomedicals). Supernatants were discarded and replenished with fresh 0.5 M sodium acetate and repeated a total of seven cycles. Residual sodium acetate was removed by rinsing the samples in ice-cold dH2O. The samples were subsequently compressed to remove any excess liquid and placed into fresh conical tubes for further processing.
COL1 extraction
The dermal samples were washed twice with 2 mL/g 0.075 M sodium citrate buffer for 1 min at 6 m/s. Supernatants were discarded and replenished with fresh buffer. Samples were then subjected to six sequential 1 min cycles (6 m/s) of agitation without removing the sodium citrate buffer. At this point, thick, clear supernatants indicative of efficient COL1 extraction were observed and collected. Another 1 mL/g 0.075 M sodium citrate buffer was added to each dermal sample and a final agitation cycle was performed. These supernatants were combined with the previous extraction. The COL1 samples were centrifuged at 3200 g for 10 min at 4°C to remove remaining particulates, and the clarified samples were transferred to 100,000 MWCO Amicon Ultra-15 centrifugal filter devices (Millipore). After additional centrifugation at 3200 g for 30 min at 4°C, the purified COL1 preparations from the top compartments were placed in conical tubes and stored at 4°C.
Ultrarapid isolation of COL1 from rat tails
For whole tail COL1 preparations, adult rat tails were sliced off of deceased animals 1 cm from the base, chopped into 1 cm slices, and then placed into a 50 mL vial (one tail per vial) and rinsed with cold dH2O. The ultrarapid COL1 isolation procedure was then performed through the sodium citrate buffer extraction step. For rat tail tendon COL1 preparations, adult rat tails were sliced off of deceased animals 1 cm from the base and the skin was detached. Fascia on the surface of the tendon bundles was removed and the fibers were dissected from the tail. Each tail's tendons were weighed and then placed into a 50 mL vial (one tail per vial) containing 5 mL of Lysing Matrix D (MP Biomedicals) and rinsed with cold dH2O. The rapid COL1 isolation procedure was performed as previously described with 1 modification. After the first citrate buffer wash/shake/rinse there were a total of three citrate buffer extractions that were performed. Each of the three extractions contained 15 mL of citrate buffer and included four 1-min, 6-m/s agitation cycles.
COL1 yield determination
All collagen concentrations were assessed using the Sircol™ assay (Biocolor).
Nucleic acid detection
Direct protein measurements using a NanoDrop ND-1000 spectrophotometer were performed every COL1 preparation to assess the 260:280 ratios. These ratios were compared to a standard curve made with 260:280 ratios obtained from known quantities of genomic DNA diluted into a purified commercially available collagen solution.
Protein gel electrophoresis
The total protein content in each COL1 sample was determined using the Pierce BCA Protein Assay (Thermo Scientific). The indicated amounts of protein were electrophoresed on 1.5-mm-thick 8.0% Tris-glycine protein gels (Invitrogen) at 120 V for 2 h. The gels were then either stained using the Silver stain kit for proteins (Sigma) or immunoblotting was performed using a COL1-specific antibody (Sigma-Aldrich). The primary COL1 antibody was detected with a horseradish peroxidase-labeled secondary antibody and the ECL kit (Amersham).
Results and Discussion
Dramatic reduction in COL1 isolation time
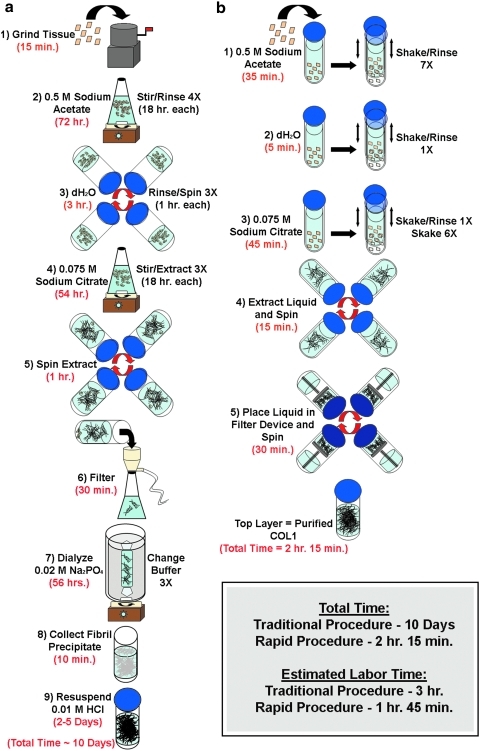
We have developed a rapid method to isolate COL1 from skin that is based upon the same purification and extraction concepts first presented by Seifter and Gallop.12 While our laboratory has successfully used this traditional procedure for many years, it has become evident that a strategy to reduce the total isolation time would increase the convenience of COL1 use both in research as well as clinical settings. The traditional procedure typically requires 10 days to complete (Fig. 1a). By incorporating steps that (1) forcefully mix the purification and extraction buffers with the skin samples and (2) make use of modern centrifugal concentrator units, we have been able to reduce the time of isolation to just over 2 h (Fig. 1b). In addition to reducing the total isolation time, our ultrarapid procedure has fewer total steps (six instead of nine). Although completing the rapid protocol requires 1 h and 45 min of hands-on time, the absolute amount of labor is less than that of the traditional procedure, which requires approximately 3 h of work.
FIG. 1.
Schematic comparison of collagen type I (COL1) isolation techniques. (a) Illustration of the steps and time required for COL1 isolation using the traditional procedure. (b) Illustration of the steps and time required for COL1 isolation using the ultrarapid procedure.
COL1 isolation yields and scalability
Soluble, helical collagen concentrations were assessed using the Sircol™ assay that is based upon the specific affinity of Sirius Red dye for the intact triple helical structure of collagen. The averaged COL1 yield per gram of starting material for lamb, rabbit, and human samples was 0.26±0.031, 0.31±0.26 and 0.305±0.069, respectively (Fig. 2a). To assess the procedure's scalability, COL1 was also isolated from 1, 5, 10, and 20 g lamb skin samples and a proportionate increase in yield was observed in relationship to weight (Fig. 2b).
FIG. 2.
Ultrarapid isolation yields highly pure, scalable COL1. (a) Bar graph showing the average COL1 yields from lamb, rabbit, and human skin per 1 g of starting material (n=4 per group) based upon the Sircol Sirius Red dye binding assay (Biocolor; Cat No. S1000) (mean±standard error). (b) Average total COL1 yields from preparations (n=3–4) starting with 1, 5, 10, or 20 g of lamb skin demonstrates scalability at a higher starting skin weight (mean±standard error). (c) Silver stained 1.5 mm, 8% Tris-glycine gel (Invitrogen SilverXpress Cat No. LC6100) showing collagen α1, α2, β1.1 (dimer consisting of 2 α1 chains), β1.2 (dimer consisting of 1 α1 and 1 α2 chain), and γ (trimer of 2 α1 chains and 1 α2 chain) from lanes loaded with 0.25 μg of either lamb skin (La.), rabbit skin (Ra.) or human skin (Hu.) derived COL1.15 (d) Corresponding representative immunoblot showing 0.25 μg of each COL1 sample loaded per lane and a mouse primary antibody against COL1 (Sigma C2456). (e) Silver stained 8% Tris-glycine gels showing COL1 bands from 0.20 μg COL1: 1, BD Biosciences collagen (Cat No. 354231); 2, lamb skin derived COL1 that was isolated using a 2-week-long modified version of a COL1 isolation procedure originally described by Seifter and Gallop12; 3, COL1 derived from human skin using our rapid procedure; 4, COL1 derived from lamb skin using our procedure. The lack of background staining in both preparations isolated using the rapid technique demonstrates the higher purity achieved with this method as compared to traditional COL1 isolation methods.
COL1 purity
The purity of these preparations was assessed by silver staining of protein gels containing lamb, rabbit, and human samples (Fig. 2c). Protein bands of the expected sizes representing the monomer, dimer, and trimer forms of collagen fibers were observed. Immunoblotting was also performed with a COL1-specific antibody to ensure that these bands represented COL1 (Fig. 2d). The more intense reactivity of the antibody with the γ band in immunoblots was due to the antibody's increased binding efficiency to the triple helix of intact COL1.
Vendor-supplied COL1 (BD Biosciences), lamb COL1 isolated using a traditional procedure described by Seifter and Gallop,12 human skin COL1 isolated using the rapid technique, and lamb skin COL1 isolated using the rapid technique were electrophoresed on protein gels. Silver staining showed the characteristic α1 and α2 monomer bands as well as β1,2 and β1,1 dimer bands. Reduced background staining was observed between the α and β bands in the rapid isolation preparations as compared with either the vendor-supplied COL1 or lamb skin COL1 isolated using the traditional procedure indicating less protein degradation and a more highly pure product (Fig. 2e).
On the whole, the rapid COL1 isolation procedure results in a less acidic preparation (pH=4.2 [rapid], 2.1 [traditional]), a similar final volume per initial weight (1.84 mL/g [rapid], 1.67 mL/g [traditional]) and a higher ratio of triple helical COL1 to degraded COL1/residual protein (0.53 [rapid], 0.36 [traditional]) when compared to the traditional isolation method. Protein measurements using a spectrophotometer were performed on these preparations to assess the 260:280 ratios. When the ratios were compared with known quantities of genomic DNA diluted into purified commercially available collagen solution, the rapid COL1 preparation showed no detectible nucleic acid contamination. Therefore, the rapid COL1 isolation method generates a highly pure product that is comparable or superior to that obtained from the traditional procedure.
Rapid and traditional COL1 preparations form comparable sheets, fibrils and matrices
Five different COL1 samples—vendor-supplied COL1, lamb skin COL1 isolated using the traditional method as well as lamb, rabbit, and human skin COL1 isolated using the ultrarapid isolation procedure—were stained with the Sircol Dye Reagent and then imaged using phase contrast (Fig. 3a–e) and light microscopy (Fig. 3f–j). The phase-contrast microscopy images confirm that stained COL1 fibrils from each of the five different collagens form thin sheets that are similar in appearance. The light microscopy images depict areas of twisted COL1 within the samples and highlight the Sircol dye-bound fibrils of which the thin sheets are comprised. Unstained COL1 samples were solidified by addition of NaHCO3 and imaged (Fig. 3k–o). These images illustrate the dense fibril meshwork that forms upon solidification of COL1 and provide further evidence that samples isolated using the ultrarapid technique are comparable to both vendor-supplied COL1 as well as that isolated by the traditional method.
FIG. 3.
COL1 isolated using the ultrarapid isolation technique solidifies into sheets, fibrils, and matrices that are similar to those created with both vendor supplied and traditionally isolated COL1. Vendor supplied COL1 (BD Biosciences) (a, f, k), lamb skin COL1 isolated using a traditional method (b, g, l), and lamb (c, h, m), rabbit (d, i, n), and human (e, j, o) skin COL1 isolated using our ultrarapid method were solidified and stained with the Sircol Dye Reagent then imaged using phase-contrast microscopy at 20×magnification (a–e) and light microscopy at 100×magnification (f–j). Unstained COL1 samples were solidified by addition of NaHCO3 and imaged using phase-contrast microscopy at 40×magnification (k–o).
Traditional and ultrarapid preparations compared: 3D constructs and myogenic engineered tissue
Traditional and rapid COL1 preparations were used to create 3D gels. In over 400 measurements performed by transmission electron microscopy, no statistically significant difference between COL1 fibril diameters from either isolation method was observed (Fig. 4a, b). Light microscopy images were also acquired and show the similar matrices that form from polymerized COL1 fibrils from each preparation (Fig. 4c, d). To confirm that our COL1 isolations are able to form scaffolds capable of supporting cell cultures, myogenic engineered tissue constructs were cast as droplets or into linear molds as previously described.14 The droplets were imaged by differential interference contrast microscopy and showed elongated cells embedded within the COL1 fibril networks (Fig. 4e, f). After 3 and 7 days of incubation viability assays demonstrated highly viable cellular networks throughout these constructs (Fig. 4g–j). Cells were also seeded onto tissue culture plates that were coated with either vendor supplied, traditional, or rapid COL1 preparations. All displayed a similar cell phenotype and high viability after 7 days of 2D culture (Fig. 4k–m).
FIG. 4.
Comparisons between traditional and ultrarapid COL1 isolation products. Lamb COL1 isolations using either the traditional method (left) or rapid method (right) were solidified through the addition of NaHCO3 followed by incubation at 37°C. Electron microscopy images of COL1 fibrils were acquired at 30,000×(a, b) and light microscopy at 100×(c, d). Cells were cast in each COL1 preparation and imaged at 100×(e, f). Viability assays were performed on constructs using calcein (Molecular Probes Cat. No. C3100MP) (green), which infiltrates live cells, propidium iodide (MP Biomedicals 195458) (red), which infiltrates dead cells, and Hoechst Dye 33258 (Molecular Probes H-3569) (blue) to identify nuclei and were imaged at 20×magnification at 3 days (g, h) and 7 days (i, j). Cells were also seeded into wells coated with 50 μg/mL of vendor-supplied, traditional, or rapid isolation COL1 and imaged using phase-contrast microscopy at 7 days (k, l, m).
COL1 isolation from rat tail
To determine whether or not the ultrarapid isolation technique could be employed to purify COL1 from rat tails two experiments were performed: one with sliced whole rat tails and one in which the tendons were extracted from the tails and used as the starting material (Fig. 5). When sliced whole rat tail samples were subjected to the sodium citrate buffer step of the ultrarapid purification procedure a very thick gelatin was extracted from the tails that could not be further purified using the centrifugal filter devices. Although this did contain COL1 that was able to be bound by Sircol dye (Fig. 5a, c), it also included a large amount of contamination that could not be easily removed due to the highly viscous nature of the preparation (Fig. 5e). When the rat tail tendons were used as the starting material, the final step yielded a product that was more similar in nature to that acquired using skin from lamb, rabbit, or human (Fig. 5b, d, f). The rat tail tendons essentially dissolved during the sodium citrate buffer extraction. A total of 3–15 mL sodium citrate buffer extractions were necessary (Fig. 5g) and when these were combined the average total COL1 yield per tail was 1.95±0.55 mg. While rat tail COL1 extractions are not of direct interest for our primary research in developing completely autologous engineered tissue for humans, the ability to rapidly extract COL1 from rat tails would be of great use to other researchers performing basic studies in the biomedical engineering field.
FIG. 5.
Ultrarapid isolation of COL1 from rat tails. COL1 preparations isolated from either whole rat tails (a, c, e) or rat tail tendons (b, d, f) were compared. The relative abilities of each preparation to bind Sircol dye is shown by phase-contrast microscopy at 20×magnification (a, b) and light microscopy at 100×magnification (c, d). A sample from each preparation was solidified by addition of NaHCO3 and incubation at 37°C. Phase-contrast images of the polymerized fibrils were acquired at 40×magnification (e, f). The relative yields acquired from each of the three sodium citrate buffer extractions necessary for rat tail tendon preparations is shown (g).
Variable results between sample sources
These experiments were carried out using lamb and rabbit skin due to the utility of those species as tissue engineering models. To establish clinical applicability, we also tested and confirmed that the procedure is capable of rapidly isolating human COL1. While our procedure does not effectively purify COL1 from whole rat tails, we have shown that it is an efficient method to isolate COL1 from excised rat tail tendons. Neither rat nor pig skin showed efficient extraction of COL1 after many cycles of agitation. For these reasons, our rapid procedure may require modifications before use for certain species. Age would also impact the efficiency of COL1 isolation due to increased covalent intermolecular cross-linking. Although we have not observed a significant difference in the amount or purity of COL1 isolated from lamb, adult rabbit, or adult human skin, it may be advantageous to perform isolations on younger skin samples. We performed our procedure on vendor supplied human skin (68 years old) and obtained a final yield of 0.092 mg/g starting material, which is approximately 1/3 the amount isolated from the younger (38 year old) human sample.
Conclusions
The dramatic increase in the rate of COL1 isolation as well as the enhanced purity of the final product has been achieved through the incorporation of multiple agitation cycles and rinses at several key points in the protocol. The sodium acetate agitation/rinse cycles are far more capable of cleaning samples before extraction than the traditional technique of stirring for several days. Likewise, integrating agitation cycles into the sodium citrate buffer elution step has proven to be more highly efficient at releasing COL1 than the traditional stirring technique. Finally, traditional isolation procedures typically require a tedious dialysis step that can cause highly concentrated preparations of COL1 to amalgamate. It then becomes necessary to re-dissolve the COL1 preparations into an acidic solution as a final step. The ultrarapid isolation procedure described here circumvents that time-consuming problem by including the use of a centrifugal filter unit which concentrates the COL1 and further aids in purification through removal of all contaminants that are smaller than the 100,000 kDa molecular weight cut off.
All of these modifications culminate into a COL1 isolation method that could easily be translated into the clinic and integrated into procedures currently being performed. This ultrarapid technique will be of great use for routine autologous collagen injections designed for cosmetic or reconstructive purposes as well as enabling the development of more complex procedures involving the fabrication of autologous tissue (skin, cartilage, bone, cornea, muscle, vasculature, tendons, etc.). Essentially, any clinical application involving the use of COL1 could be enhanced by the use of an autologous scaffold that can be reliably established in a matter of hours.
In summary, we have successfully developed a method to rapidly isolate highly purified COL1 from the skin of three different species as well as rat tail tendons. The isolation time has been reduced from weeks to less than 3 h and the purified products efficiently support 3D cell network formation, thereby making it a useful procedure for fabricating autologously derived engineered tissue. This rapid isolation technique greatly augments the feasibility of producing autologous COL1 for use in a multitude of biological applications in humans and increases the overall safety of these procedures for patients.
Acknowledgments
We would like to acknowledge Bjoern Sill and Mark Kelly for their assistance in acquiring materials necessary for these experiments as well as Maria Ericsson for preparation of transmission electron microscopy samples. This work is supported by research grants from the National Institutes of Health (HL068915 and HL088206 to D.B.C.), a New Researcher Award from the Thrasher Research Fund (to C.A.P.), and donations to the Children's Hospital Boston Cardiac Conduction Fund, the Ryan Family Endowment, and by David Pullman.
Disclosure Statement
The authors have no competing financial interests to declare.
References
- 1.Kuhn K. The classical collagens: types I, II, and III. In: Mayne R., editor; Burgeson R.E., editor. Structure and Function of Collagen Types. Orlando, FL: Academic Press, Inc.; 1987. pp. 1–37. [Google Scholar]
- 2.Pratt B.M. Madri J.A. Immunolocalization of type IV collagen and laminin in nonbasement membrane structures of murine corneal stroma. A light and electron microscopic study. Lab Invest. 1985;52:650. [PubMed] [Google Scholar]
- 3.Schmitt F.O. J. Cell. Comp Physiol. 1942;20:11. [Google Scholar]
- 4.Nageotte C.R. Hebd. Seances Acad Sci. 1927;184:115. [Google Scholar]
- 5.Choi Y.H. Stamm C. Hammer P.E. Kwaku K.F. Marler J.J. Friehs I. Jones M. Rader C.M. Roy N. Eddy M.T. Triedman J.K. Walsh E.P. McGowan F.X., Jr. del Nido P.J. Cowan D.B. Cardiac conduction through engineered tissue. Am J Pathol. 2006;169:72. doi: 10.2353/ajpath.2006.051163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chokalingam K. Juncosa-Melvin N. Hunter S.A. Gooch C. Frede C. Florert J. Bradica G. Wenstrup R. Butler D.L. Tensile stimulation of murine stem cell-collagen sponge constructs increases collagen type I gene expression and linear stiffness. Tissue Eng Part A. 2009;15:2561. doi: 10.1089/ten.tea.2008.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahn S. Yoon H. Kim G. Kim Y. Lee S. Chun W. Designed three-dimensional collagen scaffolds for skin tissue regeneration. Tissue Eng Part C. 2010;16:813. doi: 10.1089/ten.tec.2009.0511. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y. Sun S. Singha S. Cho M.R. Gordon R.J. 3D femtosecond laser patterning of collagen for directed cell attachment. Biomaterials. 2005;26:4597. doi: 10.1016/j.biomaterials.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 9.Ber S. Torun Kose G. Hasirci V. Bone tissue engineering on patterned collagen films: an in vitro study. Biomaterials. 2005;26:1977. doi: 10.1016/j.biomaterials.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Thakar R.G. Ho F. Huang N.F. Liepmann D. Li S. Regulation of vascular smooth muscle cells by micropatterning. Biochem Biophys Res Commun. 2003;307:883. doi: 10.1016/s0006-291x(03)01285-3. [DOI] [PubMed] [Google Scholar]
- 11.Rajan N. Habermehl J. Cote M.F. Doillon C.J. Mantovani D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat Prot. 2006;1:2753. doi: 10.1038/nprot.2006.430. [DOI] [PubMed] [Google Scholar]
- 12.Seifter S., editor; Gallop P., editor. Preparation and Properties of Soluble Collagens. New York: Academic Press; 1963. [Google Scholar]
- 13.Cliche S. Amiot J. Avezard C. Gariepy C. Extraction and characterization of collagen with or without telopeptides from chicken skin. Poultry Sci. 2003;82:503. doi: 10.1093/ps/82.3.503. [DOI] [PubMed] [Google Scholar]
- 14.Pacak C.A. Cowan D.B. Fabrication of myogenic engineered tissue constructs. J Vis Exp. 2009;27:1137. doi: 10.3791/1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deyl Z. Miksi'ik I. Separation of collagen type I chain polymers by electrophoresis in non-cross-linked polyacrylamide-filled capillaries. J Chromatogr A. 1995;698:369. [Google Scholar]