Abstract
Background
Volatile anesthetics have a dual effect on cell survival dependent on caveolin expression. The effect of volatile anesthetics on cancer cell survival and death after anesthetic exposure has not been well investigated. The authors examined the effects of isoflurane exposure on apoptosis and its regulation by caveolin-1 (Cav-1).
Methods
The authors exposed human colon cancer cell lines to isoflurane and proapoptotic stimuli and assessed what role Cav-1 plays in cell protection. They evaluated apoptosis using assays for nucleosomal fragmentation, cleaved caspase 3 expression, and caspase activity assays. To test the mechanism, they used pharmacologic inhibitors (i.e., pertussis toxin) and assessed changes in glycolysis.
Results
Apoptosis as measured by nucleosomal fragmentation was enhanced by isoflurane (1.2% in air) in HT29 (by 64% relative to control, P < 0.001) and decreased in HCT116 (by 23% relative to control, P < 0.001) cells. Knockdown of Cav-1 in HCT116 cells increased the sensitivity to apoptotic stimuli but not with scrambled small interfering RNA (siRNA) treatment (19.7 ± 0.4 vs. 20.0 ± 0.6, P =0.7786 and 19.7 ± 0.5 vs. 16.3 ± 0.4, P =0.0012, isoflurane vs. control in Cav-1 small interfering RNA vs. scrambled small interfering RNA treated cells, respectively). The protective effect of isoflurane with various exposure times on apoptosis was enhanced in HT29 cells overexpressing Cav-1 (P <0.001 by two-way ANOVA). Pertussis toxin effectively blocked the antiapoptotic effect of isoflurane exhibited by Cav-1 in all cell lines. Cav-1 cells had increased glycolysis with isoflurane exposure; however, in the presence of tumor necrosis factor-related apoptosis-inducing ligand, this increase in glycolysis was maintained in HT29-Cav-1 but not control cells.
Conclusion
Brief isoflurane exposure leads to resistance against apoptosis via a Cav-1–dependent mechanism.
Volatile anesthetics have effects on cell death and survival. Isoflurane, one of the most commonly used volatile anesthetics, has been shown to induce apoptosis in different cell types, where anesthetic exposure leads to neuronal cell death via apoptosis in the developing rodent brain, and prolonged isoflurane has been reported to induce apoptosis in cancer cells.1–12 Volatile anesthetics have been shown to have effects on tumorigenesis and natural killer cell activity13; however, direct effects of volatile anesthetics on the ability to modulate the efficacy of chemotherapeutic agents has not been examined.
Isoflurane may induce apoptosis through Bcl-2 family proteins, mitochondrial reactive oxygen species-associated apoptosis, or by causing abnormal calcium release from the endoplasmic reticulum via excessive activation of inositol trisphosphate receptors.12,14 In contrast, brief isoflurane exposure to adult neurons results in protection against subsequent ischemia-reperfusion injury.15,16 Volatile anesthetics also protect the heart from ischemia-reperfusion injury. Importantly, we have shown that protection in both the neuronal and cardiac system is caveolin-dependent.17–20 Caveolins are structural components of caveolae, which are small membrane invaginations also enriched in glycosphingolipids and cholesterol.21,22 Three isoforms of caveolin, caveolin (Cav)-1, -2, and -3, exist within caveolae and interact with signaling molecules via a scaffolding domain to regulate a variety of cellular signaling processes. In nonmuscle tissues, Cav-1 is considered to be the predominant isoform. The expression of Cav-1 is increased in highly metastatic prostate cancer cell lines23 and in multidrug-resistant cancer cells.24 Given the role of caveolin in anesthetic-induced protection, the potential exists that caveolin status (i.e., presence or lack of caveolin expression) may be an important clinical determinant of the response of cancer cells to volatile anesthetics and subsequent postoperative anticancer therapy. Therefore, we sought to clarify the role of Cav-1 in regulating tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis after isoflurane exposure in colon cancer cell lines.
Materials and Methods
Reagents
Reagents were purchased from the following suppliers: polyclonal antibody to Cav-1, polyclonal antibody to actin (I-19), human Cav-1 siRNA, Control siRNA-A, and siRNA, Transfection Medium, Santa Cruz Biotechnology (Santa Cruz, CA); polyclonal antibody to cleaved caspase 3 and caspase 3, Cell Signaling (Danvers, MA); recombinant human TRAIL/Apo2 l, PeproTech Inc. (Rocky Hill, NJ); Lipofectamine 2000 and Opti-MEM Reduced Serum Medium, Invitrogen (Carlsbad, CA); and G418 Solution, Roche Diagnostics (Mannheim, Germany).
Cell Lines
We purchased human colon cancer cell lines, HCT116 and HT29, from ATCC (Rockville, MD). Both cell lines were cultured in McCoy’s 5a medium containing fetal bovine serum (10%), and penicillin/streptomycin (1%) in a 95% air, 5% CO2 humidified atmosphere at 37°C. Stably transfected HT29 cells were supplemented with 800 μg/ml G418.
Isoflurane Exposure and Treatment with Recombinant Human TRAIL/Apo2 l
Cancer cells were plated on 6- or 12-well plates, allowed to incubate overnight, and then subjected to various experimental conditions at 37°C. After reducing the media volume in each well to 0.5 ml for 12-well plates or 1 ml for 6-well plates, respectively, cells were exposed to air vehicle with or without isoflurane at 2 l/min in a metabolic chamber (Co-lumbus Instruments, Columbus, OH) for varying times. Immediately after isoflurane exposure, cells were treated with media containing TRAIL/Apo2 l (TRAIL) at the indicated final concentrations for 24 h. During the isoflurane exposure, the isoflurane concentration was verified continuously by sampling exhaust gas with a Datex Capnomac (SOMA Technology Inc., Cheshire, CT). Initial studies were done to determine concentration response of isoflurane (0.6, 1.2, or 2.4%), and all subsequent experiments were done with 1.2% isoflurane.
A subset of cells were also pretreated with an inhibitory G-protein–coupled receptor inhibitor, pertussis toxin (200 ng/ml; Sigma; Saint Louis, MO) for 2 h, before isoflurane and TRAIL exposure.
Detection of Apoptosis
After the 24-h treatment with TRAIL, apoptosis was quantified by nucleosomal fragmentation (Cell Death Detection ELISA PLUS; Roche Applied Science, Indianapolis, IN). The absorbance values were normalized to those from control-treated cells to derive a nucleosomal enrichment factor at all concentrations, according to the manufacturer’s protocol.
Plasmid Vector Constructs and Generation of Stable Cell Lines
Mouse Cav-1 (456bp) sequence was cloned into an expression vector pEGFP-N1 (CLONTECH Laboratories, Inc., Palo Alto, CA), and enhanced green fluorescent protein sequence was deleted. The original vector containing enhanced green fluorescent protein was used as a control. Cells (5 × 105/well) were seeded on 24-well plates without antibiotics and cultured overnight. The next day, 0.8 μg pEGFP-N1 or pEGFP-N1-Cav-1 (enhanced green fluorescent protein deleted) plasmid was transfected into HT29 cells using 2 μl Lipofectamine 2000 in Opti-MEM Reduced Serum Medium following the manufacturer’s instructions. Transfected cells were selected with G418 at 800 μg/ml for 4 weeks, and the expression of Cav-1 protein in selected cell clones was determined by Western blotting.
Small Interfering RNA and Transient Transfection
HCT116 cells were transfected with commercially available Cav-1 siRNA following the manufacturer’s protocol. In brief, 1 day before transfection, 2 × 105 cells per well in 6-well plates were seeded in McCoy’s 5a medium without antibiotics and incubated until 60~80% confluence for optimum transfection. After the cells were washed, they were transfected with Cav-1 siRNA or control siRNA-A diluted to 30 nM/l in transfection medium for 7 h, after which normal medium containing 20% fetal bovine serum and 2% penicillin-streptomycin was added. The transfected cells were maintained for 48 h and then used for additional analysis.
Immunoblot Analysis
Cell lines were first trypsinized (5 min) and then pelleted at 1,000 rpm. Cells were then lysed in 150 mM NaCO3 pH 11.0. Protein concentration was quantified via Bradford reagent (BioRad; Hercules, CA) and analyzed via spectrophotometry at wavelength of 595 nm on a Infinite M200 plate reader (Tecan; San Jose, CA).
Protein was separated by 10% polyacrylamide precast gels (Invitrogen) and transferred to a polyvinylidene difluoride membrane by electroelution. Membranes were blocked in tris-buffered saline and 1% Tween containing 3% bovine serum albumin solution and incubated with primary antibody (1:2,000 dilution) overnight at 4°C. Bound primary antibodies were visualized using secondary antibodies (1: 5,000 dilution) conjugated with horseradish peroxidase from Santa Cruz Biotechnology and enhanced chemiluminescent reagent from Amersham Biosciences (Piscataway, NJ). All displayed bands migrated at the appropriate size, as determined by comparison to molecular weight standards (Santa Cruz Biotechnology).
Quantitative Caspase 3 Activity Assay
Caspase 3 activity was detected by using the Caspase 3/CPP32 Colorimetric Assay Kit (Biovision, Palo Alto, CA) according to the manufacturer’s instructions. Briefly, 1 × 106 cells were counted and incubated with 50 μl chilled lysis buffer on ice for 10 min. After 10,000g centrifugation, supernatant was collected. Protein (150 μg) in a total volume of 50 μl was added to 50 μl 2× reaction buffer and 5 μl N-Acetyl-Asp-Glu-Val-Asp-pNA substrate (200 μM final concentrations). After incubation (1–2 h, 37°C), N-Acetyl-Asp-Glu-Val-Asp-pNA cleavage was monitored by detecting enzyme-catalyzed release of pNA at 405 nm using a micro-plate reader.
Analysis of Intact Cell Respiration Using a Seahorse XF96 Analyzer
HT29 cells were plated in growth media at 4 × 104 cells/well in polystyrene XF96 Seahorse (Seahorse Bioscience, Billerica, MA) plates the day before experimentation. Cells were then exposed to designated experimental conditions. One hour before analysis, which was performed essentially as suggested by the manufacturer, the growth media was changed to unbuffered Dulbecco modified eagle medium supplemented with 10 mM glucose, 10 mM sodium pyruvate, and 2 mM glutamine. Endogenous rates of respiration and extra-cellular acidification were measured, followed by addition of 2 μM oligomycin to obtain state 4 rates, and then 300 nM FCCP (carbonyl cyanide 4-[trifluoromethoxy]phenylhydra-zone) to obtain maximal rates.
Quantitative Real-time Polymerase Chain Reaction Analysis
Total RNA was isolated from cell lines using an RNeasy Mini Kit (Qiagen, Valencia, CA). First-strand complementary DNA synthesis (iScript complementary DNA synthesis kit; Bio-Rad, Hercules, CA) was performed using random hex-amers on 1–2 μg total RNA. The concentration of complementary DNA was determined via NanoPhotometer Pearl (Implen; Westlake Village, CA) and adjusted to 50 ng/μl for real-time polymerase chain reaction analysis, which was performed on a BioRad CFX96 C1000 cycler in duplicate using the IQ SYBR Green Supermix (Bio-Rad) with 100 ng complementary DNA and 1× forward/reverse primer mix in 20 μl final reaction volume. Human BCL2, Caspase 3 and 8, actin, and Fas QuantiTect Primers (Qiagen) were used. Thermal cycler conditions were as follows: 94°C 10 min (1 cycle); 94°C 20 s, 55°C 20 s, and 72°C 30s (40 cycles). The resulting polymerase chain reaction products were confirmed by melt curve analysis. Analysis of cycle threshold was performed using CFX Manager software (Bio-Rad); normalized values were obtained for each group by subtracting matched actin cycle threshold values.
Statistical Analysis
GraphPad Prism 4 software (GraphPad Software, Inc., San Diego, CA) was used for all statistical analysis. Statistical analyses were performed by unpaired Student t test (two-tailed testing), one-way ANOVA followed by a Bonferroni post hoc test, and two-way ANOVA (where the factors in the analysis were time of exposure and exposure stimulus with and without TRAIL or were treatment to analysis under basal and oligomycin treatment on the Seahorse machine). None of the two-way ANOVA analyses were repeated measures. All data are expressed as mean ±SD. Statistical significance was defined as P < 0.05. Individuals making all endpoint measurements were blinded to identification of treatment group.
Results
Isoflurane Exposure Does Not Induce Apoptosis in Cancer Cell Lines with Variable Expression of Cav-1
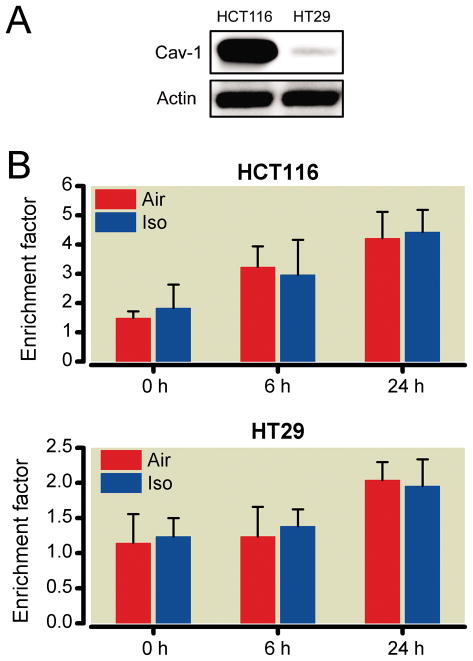
We confirmed the expression of Cav-1 protein in the human colon cancer cell lines HCT116 and HT29. Immunoblots revealed that Cav-1 protein was highly expressed in HCT116 cells but not HT29 cells (fig. 1A). HCT116 and HT29 cells were exposed to isoflurane (1.2%) for 30 min, and then apoptosis was evaluated at 0, 6, and 24 h after treatment. Isoflurane exposure did not enhance apoptosis at any time point compared with the vehicle (air) in either cell type (fig. 1B).
Fig. 1.
The effects of brief isoflurane exposure in the human colon cancer cell lines HCT116 and HT29. Cav-1 is up-regulated in HCT116 cells and down-regulated in HT29 cells. Actin was used as a loading control (A). Cells were exposed to isoflurane (1.2%, 30 min) and enrichment factor, a measure for apoptosis, was evaluated at 0, 6, and 24 h after treatment with isoflurane (B). Brief isoflurane alone did not show proapoptotic effect at any time point relative to vehicle (five independent experiments were performed). Cav-1 = caveolin-1.
Isoflurane Protects HCT116 Cells from TRAIL-induced Apoptosis
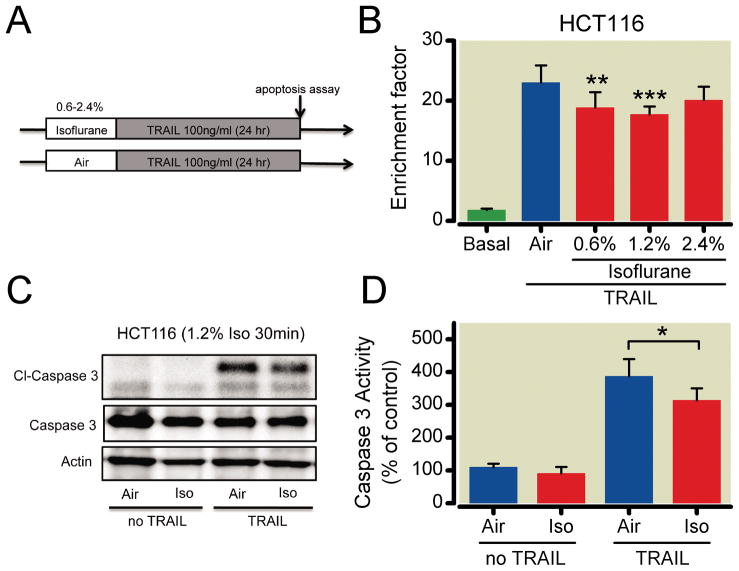
We examined the effects of isoflurane exposure on cell survival using TRAIL-induced apoptosis to mimic the cellular response to chemotherapy (fig. 2A). HCT116 (Cav-1 expressing) cells were treated with different concentrations of isoflurane (0, 0.6, 1.2, 2.4%) for 30 min and then treated with TRAIL for 24 h (fig. 2B). In HCT116 cells, isoflurane pretreatment protected against TRAIL-induced apoptosis, with isoflurane (1.2%) being optimal (fig. 2B) and a potential biphasic effect, where toxicity may be likely at 2.4%. Western blot analysis also revealed a significant decrease in cleaved caspase 3 expression (fig. 2C). Caspase 3 activity was also significantly less after isoflurane (1.2%) and TRAIL, verifying the protective effects of the anesthetic (fig. 2D).
Fig. 2.
Human colon cancer HCT116 cells that express high basal levels of caveolin-1 (Cav-1) are protected against tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis after isoflurane exposure. Schematic illustration of experimental protocol using TRAIL (A). HCT-116 cells were treated with air (vehicle) or air with isoflurane (0.6, 1.2, or 2.4% vol/vol) for 30 min, followed by treatment with TRAIL for 24 h before Western blot analysis and caspase 3 activity assay. Isoflurane reduced DNA fragmentation at 0.6 and 1.2%. However, these protective effects were not observed at 2.4% (n = 6 for all groups except Air and 1.2%, which was n = 9) (B). Western blot analysis after 30 min exposure of isoflurane (1.2%) revealed no effect on cleaved caspase 3 (Cl-Caspase 3; major product of apoptosis) in the absence of TRAIL. However, in the presence of isoflurane and TRAIL, Cl-Caspase 3 was significantly decreased (C). Western blot results were verified by a quantitative caspase 3 activity assay (n = 5) (D). *P < 0.05; **P < 0.01; ***P < 0.001.
Knockdown of Cav-1 Reverses Isoflurane-induced Protection from TRAIL-induced Apoptosis in HCT116 Cells
To explore the relationship between Cav-1 expression and isoflurane protection from TRAIL-induced apoptosis, we knocked down Cav-1 in HCT116 cells that normally have high caveolin expression. Cav-1 siRNA reduced the expression of Cav-1 at 72 h after transfection to 50% of basal and scrambled siRNA control (fig. 3A). Because these are highly proliferative cells, we were unable to observe a reduction in Cav-1 expression of less than 50%. In HCT116 cells treated with scrambled siRNA, we continued to observe isoflurane-induced protection against TRAIL-induced apoptosis, whereas this protection was lost in Cav-1 siRNA-treated cells (fig. 3, B and C). Similar effects for scrambled and Cav-1 siRNA were observed with respect to cleaved caspase 3 expression and activity (fig. 3, D and E).
Fig. 3.
Caveolin-1 (Cav-1) knockdown in HCT-116 cells abolishes protection against tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Cav-1 expression was decreased ~50% from basal levels 72 h after the small interfering RNA (siRNA) transfection (n = 4 for scrambled siRNA and n = 3 for Cav-1 siRNA). *P < 0.05 (A). HCT116 cells treated with control scrambled siRNA were still resistant to TRAIL-induced apoptosis after isoflurane (n = 5) (B). However, Cav-1 knockdown reversed this effect (n = 5) (C). Western blot analysis and caspase activity assays confirmed these findings and revealed attenuated caspase 3 activation in cells treated with scrambled siRNA and exposed to isoflurane and TRAIL, but this protective effect was attenuated by Cav-1 siRNA (n = 5) (D and E). *P < 0.05. ISO = isoflurane.
Stable Expression of Cav-1 in HT29 Cells Confers Protection from TRAIL-induced Apoptosis after Isoflurane Exposure
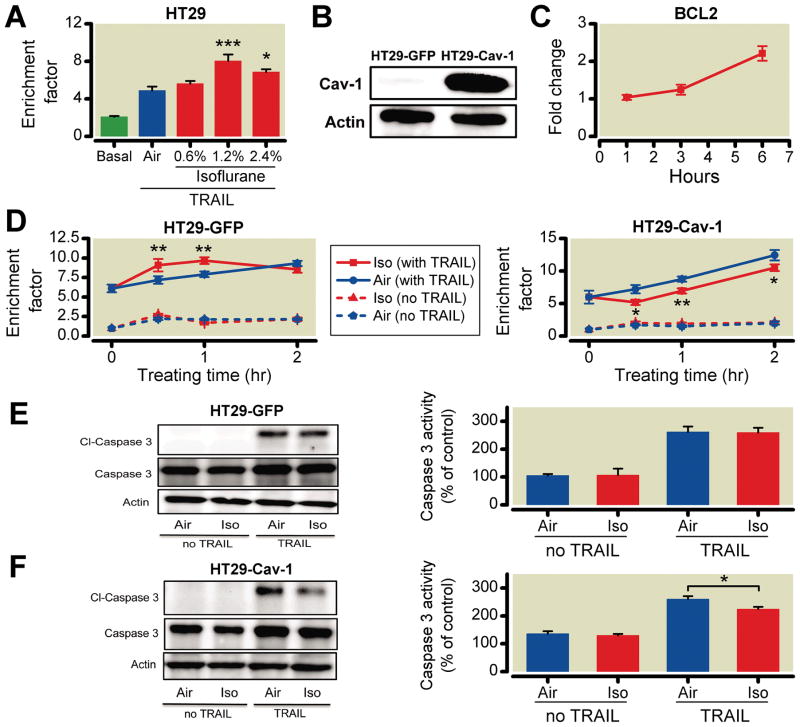
HT29 cells (which express virtually no Cav-1) when exposed to isoflurane are sensitive to TRAIL-induced apoptosis (fig. 4A). To address mechanisms for the role of Cav-1 in protection by isoflurane in TRAIL-induced apoptosis, HT29 cells were genetically engineered to stably express Cav-1 (fig. 4B). As a control, HT29 cells stably expressing enhanced green fluorescent protein were generated (HT29-GFP). HT29-Cav-1 cells had increased messenger RNA expression of BCL2, an antiapoptotic gene, after isoflurane exposure compared with HT29-GFP control cells (fig. 4C), but no increase in pro-apoptotic gene expression (data not shown). HT29-GFP cells were not protected against TRAIL-induced apoptosis; however, stable expression of Cav-1 in HT29 cells resulted in protection against TRAIL-induced apoptosis with increasing exposure time to isoflurane to 2 h (fig. 4D). Cleaved caspase 3 expression and activity also were reduced in HT29-Cav-1 relative to GFP-expressing cells (fig. 4, E and F).
Fig. 4.
Isoflurane sensitizes colon cancer HT29 cells (low caveolin-1 [Cav-1] expressing) to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis, but Cav-1 overexpression effectively protects against TRAIL. HT29-Cav-1 cells are sensitized to isoflurane exposure, resulting in increased DNA fragmentation with increased isoflurane concentrations (n = 6 for all groups except basal and 0.6%, which was n = 9) (A). Stable HT29 cells expressing enhanced green fluorescent protein (HT29-GFP) and caveolin-1 (HT29-Cav-1) cells were cloned (n = 4) (B). Real-time polymerase chain reaction revealed that HT29-Cav-1 cells had nearly a 2.5-fold increase in the antiapoptotic gene BCL2 6 h after a 30-min exposure to isoflurane (n = 4) (C). Isoflurane continued to sensitize HT29-GFP cells to TRAIL-induced apoptosis over 2 h. However, HT29-Cav-1 cells showed significant protection against TRAIL at all time points. These results were confirmed via Western blot analysis for Cl-caspase 3 and caspase 3 activity, which revealed enhanced protection in HT29-Cav-1 cells (n = 5 for all groups) (D, E, and F). *P < 0.05; **P < 0.01; ***P < 0.001.
Cav-1- and Isoflurane-induced Protection against TRAIL-induced Apoptosis Is Mediated by Heterotrimeric G-proteins
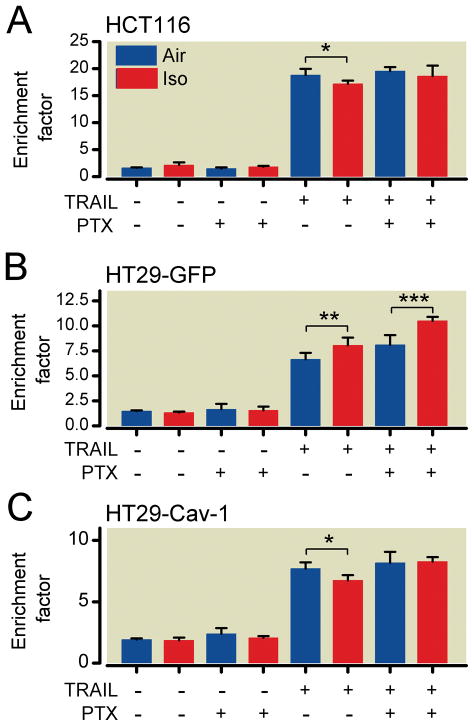
To investigate a potential mechanism of isoflurane-induced protection observed in HCT116 and HT29-Cav-1 cells, an inhibitor of inhibitory G-protein–coupled receptors, pertussis toxin, was added to cell media before isoflurane exposure. Pertussis toxin had no effect on both cell lines in the absence of TRAIL. However, in the presence of TRAIL, pertussis toxin was effective at inhibiting isoflurane-induced protection in HCT116 cells after isoflurane exposure (fig. 5A).
Fig. 5.
Signaling through inhibitory G-protein–coupled receptors is essential to caveolin-1 (Cav-1)–dependent isoflurane-induced protection against tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis. HCT116 cells were incubated with 200 ng/ml pertussis toxin (PTX) for 2 h before isoflurane exposure. PTX inhibited isoflurane-induced protection (A). HT29-green fluorescent protein (HT29-GFP) cells conferred no protective effect, and PTX enhanced DNA fragmentation in the presence of TRAIL (B). Similar to HCT116 cells, the protection observed in HT29-Cav-1 cells was abolished by PTX (n = 5 for all groups) (C). *P < 0.05; **P < 0.01; ***P < 0.001.
Isoflurane enhanced TRAIL-induced apoptosis in HT29-GFP cells, which express low basal levels of Cav-1. Pertussis toxin administration further enhanced TRAIL-induced apoptosis after isoflurane (fig. 5B). In HT29-Cav-1 cells, the protective effect of isoflurane observed with Cav-1 expression was abolished by pertussis toxin (fig. 5C).
Cav-1 Enhances Glycolysis in Response to Isoflurane and TRAIL in HT29 Cells
Enhanced glycolysis is a mechanism that allows cancer cells to survive under hypoxic conditions.25 To further explore a potential role of enhanced glycolysis versus oxidative phosphorylation in Cav-1’s role in isoflurane protection from TRAIL-induced apoptosis, we investigated glycolysis and respiration after isoflurane and TRAIL in HT29-Cav-1 cells. As a measure of glycolysis, we assessed the extracellular acidification rate in the presence and absence of oligomycin to inhibit adenosine triphosphate synthase. As a measure of cellular respiration, we assessed the oxygen consumption rate. HT29-Cav-1 cells showed enhanced glycolysis after isoflurane and TRAIL compared with untreated cells, and no differences were observed in oxygen consumption rate (fig. 6A and 6B).
Fig. 6.
Caveolin-1 (Cav-1) maintains isoflurane-mediated glycolysis after tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). Extracellular acidification rate, a measure of glycolysis, and oxygen consumption rate were assessed in HT29-GFP cells. Isoflurane exposure increased extracellular acidification rate basally and after oligomycin but not after TRAIL. No differences were observed in oxygen consumption rate (A). Extracellular acidification rate and oxygen consumption rate were measured in HT29-Cav-1 cells. Isoflurane exposure increased the extracellular acidification rate basally and after oligomycin. This increased glycolysis was maintained after TRAIL in HT29-Cav-1 cells. No differences were observed in oxygen consumption rate (n = 10 for all groups) (B). *P < 0.05; **P < 0.01; ***P < 0.001. ECAR = extracellular acidification rate; GFP = green fluorescent protein; ISO = isoflurane; OCR = oxygen consumption rate.
Discussion
In the current study, we observed three novel findings: (1) exposure of certain cancer cells to isoflurane can result in sensitization to TRAIL-induced apoptosis via a G-protein–coupled receptor mechanism, (2) the expression of Cav-1 in cancer cells alters glycolysis and determines whether the cell can be sensitized by isoflurane to TRAIL-induced apoptosis, and (3) manipulation of Cav-1 expression within the cancer cell can influence the apoptotic response to TRAIL after isoflurane exposure. This is the first study to describe the effects of isoflurane pretreatment on subsequent chemotherapy.
Volatile anesthetics used in the clinical management of anesthesia have beneficial or adverse secondary effects dependent on the cell type or developmental stage of the cell. Recently, the fact that anesthetic exposure leads to neuronal apoptotic cell death in the developing rodent brain2–5,7,12 has raised concerns regarding the safety of pediatric anesthesia, although the mechanisms by which anesthetics produce neuronal death are not well understood. In addition, the use of volatile anesthetics in anesthesia for cancer patients has been discussed.26 In the clinical setting, the choice of the anesthetic technique may affect metastatic capability and growth of preexisting tumor cells in cancer patients. In fact, several retrospective studies have reported an association between anesthetic technique and risk of cancer recurrence.27–30 However, clinical studies in humans are difficult to carry out because of numerous variables and the multiple drugs to which patients are exposed. Recent studies have reported that isoflurane may induce apoptosis through Bcl-2 family proteins and reactive-oxygen-species–associated mitochondrial pathway of apoptosis12 or by causing abnormal calcium release from the endoplasmic reticulum via excessive activation of inositol trisphosphate receptors.14
No previous studies have described the impact of volatile anesthetics on anticancer therapy. The current in vitro study simulated the potential postoperative situation, in which anticancer drugs are administered after cancer surgery. Research suggests that caveolin proteins have a significant role in cancer biology31–34; however, a link among volatile anesthetic exposure, caveolin expression, and cancer cell response to chemotherapeutic agents has not been examined. Caveolins, structural proteins found in caveolae, serve as scaffolds and regulators of signaling proteins, including G-proteins.35–38 Cav-1 can regulate multiple cancer-associated cellular processes and function as a growth inhibitory protein.33,39,40 In contrast, Cav-1 also promotes tumor growth in a variety of human cancer cell lines,41 and its presence is a marker of poor prognosis in several human cancers.42–44 The functional role of Cav-1 appears to be linked to tumor type, as well as the nature and potency of the apoptosis inducers used.45 In cardiac ischemia-reperfusion injury, protection is mediated by inhibitory G-protein– coupled receptor stimulation.19,46,47 Our pertussis toxin results suggest localization of inhibitory G-protein– coupled receptors and subsequent activation via anesthetics may be facilitated by Cav-1 to produce a protected state in cancer cells. Thus, Cav-1 may serve a role in stabilizing stress adaptation to TRAIL-induced apoptosis.
It has been hypothesized that cancer cells are able to survive stress by enhancing glycolysis during aerobic conditions, a mechanism known as the Warburg effect.25 Cav-1 has also been shown to enhance membrane targeting of glycolytic enzymes by facilitating membrane compartmentalization.48 Our findings suggest that Cav-1 overexpression increases glycolysis after isoflurane and TRAIL. Under the stress of TRAIL-induced apoptosis, Cav-1– expressing cells showed a glycolytic rate increased from basal but not different from isoflurane alone, suggesting an enhanced Warburg effect, which likely allows for the protection from apoptosis.
In noncancer cells we have shown that isoflurane increases the activation of cardiac protective proteins, such as sarcoma kinase.18 In addition, brief isoflurane exposure to neurons has been shown to exhibit protection against subsequent ischemia/reperfusion injury.15,16 Moreover, volatile anesthetics exert biphasic cardiac protection,49–51 and we have also reported that the protection observed in the heart is caveolin-dependent.18–20 Collectively, these data implicate caveolins as an essential component in the temporal and spatial organization of cardiac-protective signaling molecules. In this regard, these data complement our findings in cancer cells, which show an association between Cav-1 expression and survival against chemotherapy after isoflurane exposure.
Our findings should be interpreted within the constraints of potential limitations. HT29 cells are considered to contain a p53 mutant. Therefore, it was necessary to avoid commonly used chemotherapeutic drugs that use a p53-dependent induction of apoptosis. TRAIL is an anticancer agent that induces apoptosis via the intrinsic and extrinsic pathway,52,53 independent of p53, and without causing toxicity to normal cells.54 TRAIL currently is undergoing clinical trials as a treatment for certain cancers.55
In summary, these results demonstrate that isoflurane exposure leads to resistance against TRAIL-induced apoptosis via Cav-1–dependent mechanisms. In addition, modulating Cav-1 expression alters the response to TRAIL-induced apoptosis after isoflurane exposure. Our results may have clinical relevance to patients undergoing surgical removal of cancers. Isoflurane inadvertently may decrease sensitivity to chemotherapeutic agents depending on the caveolin expression status of the cancer. The expression of Cav-1 may be a consideration in the anesthetic management of patients scheduled to undergo postoperative chemotherapy. Furthermore, manipulation of Cav-1 may be a unique way to enhance postanesthetic anticancer chemotherapy in certain cancers.
What We Already Know about This Topic
Volatile anesthetics can be cytotoxic or cytoprotective, but their effects on tumor cell apoptosis and sensitivity to chemo-therapeutic drugs is unknown
What This Article Tells Us That Is New
Isoflurane pretreatment enhanced resistance to apoptotic cell death of colon cancer cells after exposure to the potential anticancer drug tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), suggesting a possible anticancer drug resistance after anesthesia
Acknowledgments
Supported by National Institutes of Health grants HL091071 (to Dr. Patel) and HL081400 (to Dr. Roth) from the United States Public Health Service, Bethesda, Maryland, and BX000783 from the Veterans Affairs Biomedical Laboratory Research and Development (to Dr. Roth) from the Department of Veterans Affairs, Washington, D.C.
Footnotes
Information on purchasing reprints may be found at www.anesthesiology.org or on the masthead page at the beginning of this issue. Anesthesiology’s articles are made freely accessible to all readers, for personal use only, 6 months from the cover date of the issue.
References
- 1.Aravindan N, Cata JP, Hoffman L, Dougherty PM, Riedel BJ, Price KJ, Shaw AD. Effects of isoflurane, pentobarbital, and urethane on apoptosis and apoptotic signal transduction in rat kidney. Acta Anaesthesiol Scand. 2006;50:1229–37. doi: 10.1111/j.1399-6576.2006.01102.x. [DOI] [PubMed] [Google Scholar]
- 2.Brambrink AM, Evers AS, Avidan MS, Farber NB, Smith DJ, Zhang X, Dissen GA, Creeley CE, Olney JW. Isoflurane-induced neuroapoptosis in the neonatal rhesus macaque brain. Anesthesiology. 2010;112:834–41. doi: 10.1097/ALN.0b013e3181d049cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Head BP, Patel HH, Niesman IR, Drummond JC, Roth DM, Patel PM. Inhibition of p75 neurotrophin receptor attenuates isoflurane-mediated neuronal apoptosis in the neonatal central nervous system. Anesthesiology. 2009;110:813–25. doi: 10.1097/ALN.0b013e31819b602b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jevtovic-Todorovic V, Hartman RE, Izumi Y, Benshoff ND, Dikranian K, Zorumski CF, Olney JW, Wozniak DF. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J Neurosci. 2003;23:876–82. doi: 10.1523/JNEUROSCI.23-03-00876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson SA, Young C, Olney JW. Isoflurane-induced neuro-apoptosis in the developing brain of nonhypoglycemic mice. J Neurosurg Anesthesiol. 2008;20:21–8. doi: 10.1097/ANA.0b013e3181271850. [DOI] [PubMed] [Google Scholar]
- 6.Kvolik S, Glavas-Obrovac L, Bares V, Karner I. Effects of inhalation anesthetics halothane, sevoflurane, and isoflurane on human cell lines. Life Sci. 2005;77:2369–83. doi: 10.1016/j.lfs.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 7.Loepke AW, Istaphanous GK, McAuliffe JJ, 3rd, Miles L, Hughes EA, McCann JC, Harlow KE, Kurth CD, Williams MT, Vorhees CV, Danzer SC. The effects of neonatal isoflurane exposure in mice on brain cell viability, adult behavior, learning, and memory. Anesth Analg. 2009;108:90–104. doi: 10.1213/ane.0b013e31818cdb29. [DOI] [PubMed] [Google Scholar]
- 8.Loop T, Dovi-Akue D, Frick M, Roesslein M, Egger L, Humar M, Hoetzel A, Schmidt R, Borner C, Pahl HL, Geiger KK, Pannen BH. Volatile anesthetics induce caspase-dependent, mitochondria-mediated apoptosis in human T lymphocytes in vitro. Anesthesiology. 2005;102:1147–57. doi: 10.1097/00000542-200506000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Wei H, Liang G, Yang H, Wang Q, Hawkins B, Madesh M, Wang S, Eckenhoff RG. The common inhalational anesthetic isoflurane induces apoptosis via activation of inositol 1,4,5-trisphosphate receptors. Anesthesiology. 2008;108:251–60. doi: 10.1097/01.anes.0000299435.59242.0e. [DOI] [PubMed] [Google Scholar]
- 10.Xie Z, Dong Y, Maeda U, Alfille P, Culley DJ, Crosby G, Tanzi RE. The common inhalation anesthetic isoflurane induces apoptosis and increases amyloid beta protein levels. Anesthesiology. 2006;104:988–94. doi: 10.1097/00000542-200605000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Xie Z, Dong Y, Maeda U, Moir RD, Xia W, Culley DJ, Crosby G, Tanzi RE. The inhalation anesthetic isoflurane induces a vicious cycle of apoptosis and amyloid beta-protein accumulation. J Neurosci. 2007;27:1247–54. doi: 10.1523/JNEUROSCI.5320-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Dong Y, Wu X, Lu Y, Xu Z, Knapp A, Yue Y, Xu T, Xie Z. The mitochondrial pathway of anesthetic isoflurane-induced apoptosis. J Biol Chem. 2010;285:4025–37. doi: 10.1074/jbc.M109.065664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitsuhata H, Shimizu R, Yokoyama MM. Suppressive effects of volatile anesthetics on cytokine release in human peripheral blood mononuclear cells. Int J Immunopharmacol. 1995;17:529–34. doi: 10.1016/0192-0561(95)00026-x. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Liang G, Hawkins BJ, Madesh M, Pierwola A, Wei H. Inhalational anesthetics induce cell damage by disruption of intracellular calcium homeostasis with different potencies. Anesthesiology. 2008;109:243–50. doi: 10.1097/ALN.0b013e31817f5c47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao P, Peng L, Li L, Xu X, Zuo Z. Isoflurane preconditioning improves long-term neurologic outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology. 2007;107:963–70. doi: 10.1097/01.anes.0000291447.21046.4d. [DOI] [PubMed] [Google Scholar]
- 16.Sasaoka N, Kawaguchi M, Kawaraguchi Y, Nakamura M, Konishi N, Patel H, Patel PM, Furuya H. Isoflurane exerts a short-term but not a long-term preconditioning effect in neonatal rats exposed to a hypoxic-ischaemic neuronal injury. Acta Anaesthesiol Scand. 2009;53:46–54. doi: 10.1111/j.1399-6576.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- 17.Head BP, Patel HH, Tsutsumi YM, Hu Y, Mejia T, Mora RC, Insel PA, Roth DM, Drummond JC, Patel PM. Caveolin-1 expression is essential for N-methyl-D-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB J. 2008;22:828–40. doi: 10.1096/fj.07-9299com. [DOI] [PubMed] [Google Scholar]
- 18.Horikawa YT, Patel HH, Tsutsumi YM, Jennings MM, Kidd MW, Hagiwara Y, Ishikawa Y, Insel PA, Roth DM. Caveolin-3 expression and caveolae are required for isoflurane-induced cardiac protection from hypoxia and ischemia/reperfusion injury. J Mol Cell Cardiol. 2008;44:123–30. doi: 10.1016/j.yjmcc.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel HH, Tsutsumi YM, Head BP, Niesman IR, Jennings M, Horikawa Y, Huang D, Moreno AL, Patel PM, Insel PA, Roth DM. Mechanisms of cardiac protection from ischemia/reperfusion injury: A role for caveolae and caveolin-1. FASEB J. 2007;21:1565–74. doi: 10.1096/fj.06-7719com. [DOI] [PubMed] [Google Scholar]
- 20.Tsutsumi YM, Kawaraguchi Y, Horikawa YT, Niesman IR, Kidd MW, Chin-Lee B, Head BP, Patel PM, Roth DM, Patel HH. Role of caveolin-3 and glucose transporter-4 in isoflurane-induced delayed cardiac protection. Anesthesiology. 2010;112:1136–45. doi: 10.1097/ALN.0b013e3181d3d624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kathuria H, Cao YX, Ramirez MI, Williams MC. Transcription of the caveolin-1 gene is differentially regulated in lung type I epithelial and endothelial cell lines: A role for ETS proteins in epithelial cell expression. J Biol Chem. 2004;279:30028–36. doi: 10.1074/jbc.M402236200. [DOI] [PubMed] [Google Scholar]
- 22.Eberspächer E, Werner C, Engelhard K, Pape M, Laacke L, Winner D, Hollweck R, Hutzler P, Kochs E. Long-term effects of hypothermia on neuronal cell death and the concentration of apoptotic proteins after incomplete cerebral ischemia and reperfusion in rats. Acta Anaesthesiol Scand. 2005;49:477–87. doi: 10.1111/j.1399-6576.2005.00649.x. [DOI] [PubMed] [Google Scholar]
- 23.Yang G, Addai J, Ittmann M, Wheeler TM, Thompson TC. Elevated caveolin-1 levels in African-American versus white-American prostate cancer. Clin Cancer Res. 2000;6:3430–3. [PubMed] [Google Scholar]
- 24.Lavie Y, Fiucci G, Liscovitch M. Up-regulation of caveolae and caveolar constituents in multidrug-resistant cancer cells. J Biol Chem. 1998;273:32380–3. doi: 10.1074/jbc.273.49.32380. [DOI] [PubMed] [Google Scholar]
- 25.Kim JW, Dang CV. Cancer’s molecular sweet tooth and the Warburg effect. Cancer Res. 2006;66:8927–30. doi: 10.1158/0008-5472.CAN-06-1501. [DOI] [PubMed] [Google Scholar]
- 26.Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105:106–15. doi: 10.1093/bja/aeq164. [DOI] [PubMed] [Google Scholar]
- 27.Biki B, Mascha E, Moriarty DC, Fitzpatrick JM, Sessler DI, Buggy DJ. Anesthetic technique for radical prostatectomy surgery affects cancer recurrence: A retrospective analysis. Anesthesiology. 2008;109:180–7. doi: 10.1097/ALN.0b013e31817f5b73. [DOI] [PubMed] [Google Scholar]
- 28.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–4. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gottschalk A, Ford JG, Regelin CC, You J, Mascha EJ, Sessler DI, Durieux ME, Nemergut EC. Association between epidural analgesia and cancer recurrence after colorectal cancer surgery. Anesthesiology. 2010;113:27–34. doi: 10.1097/ALN.0b013e3181de6d0d. [DOI] [PubMed] [Google Scholar]
- 30.Wuethrich PY, Hsu Schmitz SF, Kessler TM, Thalmann GN, Studer UE, Stueber F, Burkhard FC. Potential influence of the anesthetic technique used during open radical prostatectomy on prostate cancer-related outcome: A retrospective study. Anesthesiology. 2010;113:570–6. doi: 10.1097/ALN.0b013e3181e4f6ec. [DOI] [PubMed] [Google Scholar]
- 31.Park J, Bae E, Lee C, Yoon SS, Chae YS, Ahn KS, Won NH. RNA interference-directed caveolin-1 knockdown sensitizes SN12CPM6 cells to doxorubicin-induced apoptosis and reduces lung metastasis. Tumour Biol. 2010;31:643–50. doi: 10.1007/s13277-010-0081-1. [DOI] [PubMed] [Google Scholar]
- 32.Tirado OM, MacCarthy CM, Fatima N, Villar J, Mateo-Lozano S, Notario V. Caveolin-1 promotes resistance to chemotherapy-induced apoptosis in Ewing’s sarcoma cells by modulating PKCalpha phosphorylation. Int J Cancer. 2010;126:426–36. doi: 10.1002/ijc.24754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams TM, Lisanti MP. Caveolin-1 in oncogenic transformation, cancer, and metastasis. Am J Physiol Cell Physiol. 2005;288:C494–506. doi: 10.1152/ajpcell.00458.2004. [DOI] [PubMed] [Google Scholar]
- 34.Zhao X, Liu Y, Ma Q, Wang X, Jin H, Mehrpour M, Chen Q. Caveolin-1 negatively regulates TRAIL-induced apoptosis in human hepatocarcinoma cells. Biochem Biophys Res Commun. 2009;378:21–6. doi: 10.1016/j.bbrc.2008.10.123. [DOI] [PubMed] [Google Scholar]
- 35.Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP. Identification of peptide and protein ligands for the caveolin-scaffolding domain: Implications for the interaction of caveolin with caveolae-associated proteins. J Biol Chem. 1997;272:6525–33. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- 36.Feron O, Dessy C, Opel DJ, Arstall MA, Kelly RA, Michel T. Modulation of the endothelial nitric-oxide synthase-caveolin interaction in cardiac myocytes: Implications for the autonomic regulation of heart rate. J Biol Chem. 1998;273:30249–54. doi: 10.1074/jbc.273.46.30249. [DOI] [PubMed] [Google Scholar]
- 37.Feron O, Saldana F, Michel JB, Michel T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. J Biol Chem. 1998;273:3125–8. doi: 10.1074/jbc.273.6.3125. [DOI] [PubMed] [Google Scholar]
- 38.Head BP, Patel HH, Roth DM, Lai NC, Niesman IR, Farquhar MG, Insel PA. G-protein-coupled receptor signaling components localize in both sarcolemmal and intracellular caveolin-3-associated microdomains in adult cardiac myocytes. J Biol Chem. 2005;280:31036–44. doi: 10.1074/jbc.M502540200. [DOI] [PubMed] [Google Scholar]
- 39.Capozza F, Williams TM, Schubert W, McClain S, Bouzahzah B, Sotgia F, Lisanti MP. Absence of caveolin-1 sensitizes mouse skin to carcinogen-induced epidermal hyperplasia and tumor formation. Am J Pathol. 2003;162:2029–39. doi: 10.1016/S0002-9440(10)64335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee SW, Reimer CL, Oh P, Campbell DB, Schnitzer JE. Tumor cell growth inhibition by caveolin re-expression in human breast cancer cells. Oncogene. 1998;16:1391–7. doi: 10.1038/sj.onc.1201661. [DOI] [PubMed] [Google Scholar]
- 41.Ross DT, Scherf U, Eisen MB, Perou CM, Rees C, Spellman P, Iyer V, Jeffrey SS, Van de Rijn M, Waltham M, Pergamenschikov A, Lee JC, Lashkari D, Shalon D, Myers TG, Weinstein JN, Botstein D, Brown PO. Systematic variation in gene expression patterns in human cancer cell lines. Nat Genet. 2000;24:227–35. doi: 10.1038/73432. [DOI] [PubMed] [Google Scholar]
- 42.Di Vizio D, Morello M, Sotgia F, Pestell RG, Freeman MR, Lisanti MP. An absence of stromal caveolin-1 is associated with advanced prostate cancer, metastatic disease and epithelial Akt activation. Cell Cycle. 2009;8:2420–4. doi: 10.4161/cc.8.15.9116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rödel F, Capalbo G, Rödel C, Weiss C. Caveolin-1 as a prognostic marker for local control after preoperative chemoradiation therapy in rectal cancer. Int J Radiat Oncol Biol Phys. 2009;73:846–52. doi: 10.1016/j.ijrobp.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 44.Zhang ZB, Cai L, Zheng SG, Xiong Y, Dong JH. Overexpression of caveolin-1 in hepatocellular carcinoma with metastasis and worse prognosis: Correlation with vascular endothelial growth factor, microvessel density and unpaired artery. Pathol Oncol Res. 2009;15:495–502. doi: 10.1007/s12253-008-9144-7. [DOI] [PubMed] [Google Scholar]
- 45.Goetz JG, Lajoie P, Wiseman SM, Nabi IR. Caveolin-1 in tumor progression: The good, the bad and the ugly. Cancer Metastasis Rev. 2008;27:715–35. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 46.Patel HH, Head BP, Petersen HN, Niesman IR, Huang D, Gross GJ, Insel PA, Roth DM. Protection of adult rat cardiac myocytes from ischemic cell death: Role of caveolar microdomains and delta-opioid receptors. Am J Physiol Heart Circ Physiol. 2006;291:H344–50. doi: 10.1152/ajpheart.01100.2005. [DOI] [PubMed] [Google Scholar]
- 47.Schultz JE, Hsu AK, Barbieri JT, Li PL, Gross GJ. Pertussis toxin abolishes the cardioprotective effect of ischemic preconditioning in intact rat heart. Am J Physiol. 1998;275:H495–500. doi: 10.1152/ajpheart.1998.275.2.H495. [DOI] [PubMed] [Google Scholar]
- 48.Raikar LS, Vallejo J, Lloyd PG, Hardin CD. Overexpression of caveolin-1 results in increased plasma membrane targeting of glycolytic enzymes: The structural basis for a membrane associated metabolic compartment. J Cell Biochem. 2006;98:861–71. doi: 10.1002/jcb.20732. [DOI] [PubMed] [Google Scholar]
- 49.Cason BA, Gamperl AK, Slocum RE, Hickey RF. Anesthetic-induced preconditioning: Previous administration of isoflurane decreases myocardial infarct size in rabbits. Anesthesiology. 1997;87:1182–90. doi: 10.1097/00000542-199711000-00023. [DOI] [PubMed] [Google Scholar]
- 50.Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: Reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–70. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- 51.Tonkovic-Capin M, Gross GJ, Bosnjak ZJ, Tweddell JS, Fitzpatrick CM, Baker JE. Delayed cardioprotection by isoflurane: Role of K(ATP) channels. Am J Physiol Heart Circ Physiol. 2002;283:H61–8. doi: 10.1152/ajpheart.01040.2001. [DOI] [PubMed] [Google Scholar]
- 52.Corazza N, Jakob S, Schaer C, Frese S, Keogh A, Stroka D, Kassahn D, Torgler R, Mueller C, Schneider P, Brunner T. TRAIL receptor-mediated JNK activation and Bim phosphorylation critically regulate Fas-mediated liver damage and lethality. J Clin Invest. 2006;116:2493–9. doi: 10.1172/JCI27726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ravi R, Bedi A. Requirement of BAX for TRAIL/Apo2L-induced apoptosis of colorectal cancers: Synergism with sulin-dac-mediated inhibition of Bcl-x(L) Cancer Res. 2002;62:1583–7. [PubMed] [Google Scholar]
- 54.Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bellail AC, Qi L, Mulligan P, Chhabra V, Hao C. TRAIL agonists on clinical trials for cancer therapy: The promises and the challenges. Rev Recent Clin Trials. 2009;4:34–41. doi: 10.2174/157488709787047530. [DOI] [PubMed] [Google Scholar]