Abstract
Background
Prenatal programming by maternal dietary protein deprivation and prenatal dexamethasone result in a reduction in nephron number and hypertension when the offspring are studied as adults.
Methods
To determine whether prenatal dietary protein deprivation results in a reduction in nephron number and hypertension in offspring by exposure to maternal glucocorticoids, we administered metyrapone to rats fed either a 6% or 20% protein diet to inhibit glucocorticoid production and compared the offspring to rats that were the product of mothers fed either a 6% or 20% protein diet during the last half of pregnancy.
Results
Male offspring from the 6% group had elevated systolic blood pressure (149 ± 2 vs. 130 ± 5 mm Hg, P < 0.05) and a reduction in glomeruli compared to the 20% group (22,111 ± 627 vs. 29,666 ± 654 glomeruli/kidney, P < 0.001). Maternal metyrapone administration did not affect the blood pressure in the 20% group but ameliorated the increase in blood pressure in the 6% male group to values comparable to the 20% control group (138 ± 6 vs. 130 ± 5 mm Hg). Male offspring of the 6% group that received metyrapone had an increase in the number of glomeruli compared to the vehicle-treated 6% group (26,780 ± 377 vs. 22,111 ± 627 glomeruli/kidney, P < 0.001), but less glomeruli compared to the 20% protein control group (26,780 ± 377 vs. 29,666 ± 654 glomeruli/kidney, P = 0.01).
Conclusions
The reduction in nephron number and hypertension induced by maternal protein deprivation in male offspring is ameliorated by inhibition of glucocorticoid production.
Keywords: blood pressure, fetal glucocorticoid levels, glomerular number, hypertension, low protein diet, metyrapone, prenatal glucocorticoids
Barker and colleagues were the first to show that small-for-gestational-age infants were at increased risk for the development of hypertension in later life independent of other risk factors.1 These observations have been confirmed in many populations and geographical areas. Importantly, a low protein intake relative to carbohydrate intake during pregnancy is associated with elevated blood pressure in offspring when studied as adults.2,3
In addition to hypertension, small-for-gestational-age infants have a reduction in nephron number compared to infants born with a normal birth weight.4,5 Although it is not clear to what extent a paucity of glomeruli is related to the hypertension with prenatal programming, low birth weight infants have been shown to have an increased risk of developing albuminuria, an early sign of renal injury6,7 and reduced renal function as young adults.8,9 Indeed, studies have shown that low birth weight infants (<2.5 kg) have an approximately 1.5-fold increased risk for having end-stage renal disease compared to infants with birth weights between 3 and 3.5 kg.10,11 Patients with low birth weight who develop proteinuria have recently been shown to have focal and segmental glomerulosclerosis.12
Animal studies have confirmed that prenatal insults result in a reduced number of nephrons and hypertension when animals are studied as adults. Rats whose mothers ingested a low protein diet during pregnancy have a reduction in nephron number when studied as adults.13,14 Maternal dietary protein restriction results in not only fewer but also less well-differentiated nephrons when rats are studied immediately after birth.15,16 Rats born to mothers that ingested a low protein diet during pregnancy develop hypertension.13,14,17–22 A reduction in nephron number and hypertension has also been found in the offspring of rats that received dexamethasone between days 15 and 18 of gestation, a time of active nephrogenesis.23–26
Previous studies have provided indirect evidence that maternal dietary protein deprivation causes prenatal changes that lead to programming of hypertension and renal disease by exposing the fetus to the relatively higher concentration of maternal glucocorticoids. Normally, the fetus is protected from maternal glucocorticoids by placental 11 β-hydroxysteroid dehydrogenase. This enzyme converts physiological maternal glucocorticoids to inactive metabolites.27–31 Indeed, pregnant rats that were fed a low protein diet have reduced placental 11 β-hydroxysteroid dehydrogenase activity27,29 Small-for-gestational-age children have been shown to have lower placental 11 β-hydroxysteroid dehydrogenase activity than those of normal weight for gestational age.32 Finally, prenatal administration of dexamethasone, a glucocorticoid that is not metabolized by 11 β-hydroxysteroid dehydrogenase, results in a similar reduction in nephron number and elevated blood pressure in offspring as maternal dietary protein deprivation.23–27,33 The purpose of this study was to determine whether inhibition of maternal glucocorticoid synthesis would prevent the prenatal programming of hypertension and reduction in glomerular number due to maternal dietary protein deprivation.
Methods
Animals
Pregnant Sprague– Dawley rats were divided into four groups. Control rats were fed a 20% protein intake (control diet). The low protein group was fed a 6% protein isocaloric diet from the 13th day of pregnancy. The diets were purchased from Harlan Teklad (Madison, WI), and all rats had free access to food and water. To determine whether maternal dietary protein deprivation causes hypertension and reduced nephron number in the offspring by exposure of maternal steroids, rats were administered metyrapone from day 13 to term using the same dose, time of initiation, and duration of treatment as described by Smith and Waddell.34–36 Metyrapone (500 μg/ml) inhibits glucocorticoid synthesis by inhibiting 11 β-hydroxylase; thus, the pregnant rats were administered isotonic saline in their drinking water to prevent volume depletion.34–36 Metyrapone was also administered to a group of pregnant rats that were fed a 20% protein diet as a control. Control adult rats that were anesthetized for blood collection had a corticosterone level of 512.8 ± 56.0 ng/ml compared to 285.9 ± 76.5 ng/ml in rats given metyrapone in their drinking water (P < 0.05). Thus, this dose of metyrapone reduces glucocorticoid production.
To ensure that all of the effects found were from changes in the prenatal environment, the litter size from all four groups were reduced to 10 to ensure adequate maternal milk. In addition, all the offspring were reared by foster control dams after delivery to abolish the effect of different prenatal diets on lactation and rearing. The pregnant rats were weighed starting at the 13th day of gestation and daily until delivery to see the effect of metyrapone and low protein diet on maternal weight gain. The animals studied were from at least three different pregnancies in all groups. The pups were weighed on the day of birth. Only male animals were studied as they have been shown to have more significant increases in blood pressure with prenatal maternal dietary protein deprivation.14,37 These studies were in accordance with the APS's Guiding Principles in the Care and Use of Animals and were approved by the IACUC at the University of Texas Southwestern Medical Center.
Blood pressure
The blood pressures of all groups were measured between 60 and 80 days of age. Males were studied separately as there have been sex differences in blood pressure shown in the past.14,21 Rats underwent acclimatization to the restraint and cuff for 3 days, and blood pressure was measured on the fourth day. There were 6–8 blood pressure readings using IITC model no. 179 blood pressure analyzer (IITC, Woodland Hills, CA), and the mean was used as the blood pressure for that rat. The person who measured the blood pressure was blinded and did not know the origin of the rats being studied.
Glomerular number
Glomerular number was measured between 60 and 80 days of life using methods previously described with slight modifications.14,23 The rats were anesthetized with 100 mg/kg of body weight Inactin (Sigma Chemical, St Louis, MO). The abdomen was shaved and the rat restrained on the operating platform. A femoral artery catheter was inserted and advanced to a level above the renal artery. Five percent Alcian blue (Sigma Chemical Company) dissolved in isotonic saline was infused at a dose of 0.2–0.3 ml/100 g body weight over 30 s.23 After 5 min, a second dose of 0.2–0.3 ml/100 g body weight Alcian blue was given as a bolus. Five minutes after the second bolus, the kidney was harvested, the capsule was removed, and the kidney placed in 1% ammonium chloride solution (Sigma Chemical Company) where it was minced using a single-edge razor blade and incubated at room temperature for 5 min. The tissue was then incubated in 5 ml of 50% HCl (6N) in a water bath at 37°C with gentle agitation for 90 min. The sample was then vigorously mixed until all pieces were dissolved. The sample was then centrifuged for 10 min at 3,000 rpm. The supernatant was discarded and pellet was dissolved in 50 ml of distilled water. The glomeruli were counted in 30 samples of 10 μl each. The total number of glomeruli in the kidney was then extrapolated. The tube containing the glomeruli was gently shaken before pipetting each sample to ensure that the glomeruli were dispersed uniformly. The investigator that counted the glomeruli was blinded from the origin of the samples.
Statistical analysis
Data are presented as mean ± standard error of the mean. Comparisons were made using analysis of variance followed by a post hoc Student–Newman–Keuls test. A level of 0.05 was considered statistically significant.
Results
In our first series of experiments, we examined whether an isocaloric low protein diet affected maternal or fetal glucocorticoid levels. There was no difference between maternal glucocorticoid levels in 20-day-old pregnant rats that were fed a 6% vs. a 20% protein diet (166.7 ± 35.1 ng/ml vs. 205.2 ± 46.9 ng/ml, n = 4). However, maternal dietary protein deprivation caused a significant increase in 20-day fetal glucocorticoid serum levels compared to the 20% group (355.4 ± 18.8 ng/ml vs. 259.3 ± 23.9 ng/ml, n = 12, P < 0.01). Thus, dietary protein deprivation increases fetal glucocorticoid exposure.
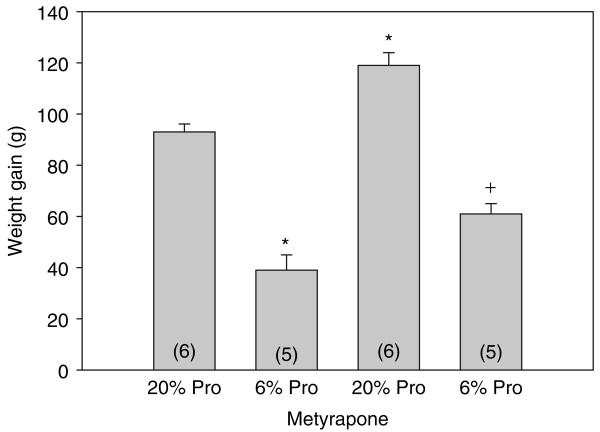
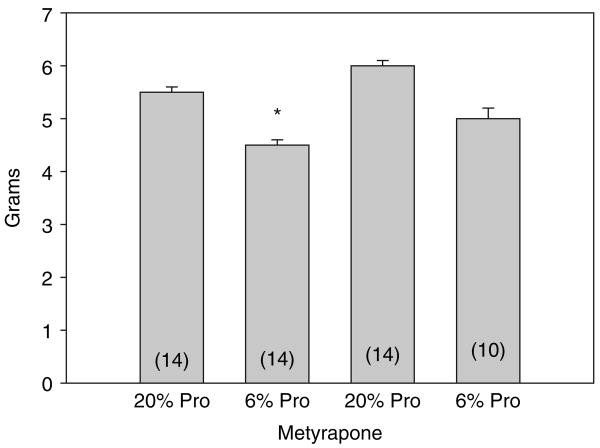
We next compared maternal weight gain from day 13 of their pregnancy until the day of birth. As shown in Figure 1, pregnant rats that were fed a 6% protein diet, whether they were on metyrapone or not, gained less weight than those on a 20% protein diet. The rats that were administered metyrapone in their drinking water gained more weight than the rats fed a comparable diet but not given metyrapone. In Figure 2, the birth weights of the rats are shown. All groups had significantly different weights from each other. Importantly, the 6% protein group whose mothers were given metyrapone, weighed more than the 6% protein group without metyrapone, but less than the 20% control group. Thus, metyrapone attenuates the effect of a maternal low protein diet on birth weight but does not increase the weight to that of controls. The body weights at the time of study are shown in Table 1. The 6% protein group weighed less than all other groups. Thus, there was catch-up weight gain in the 6% protein metyrapone group but not in the 6% protein group.
Figure 1.
Effect of metyrapone on maternal weight gain. Maternal weight gain was measured in grams from day 13 until day 21. Rats were treated with a control 20% diet or a 6% isocaloric low protein diet with or without the glucocorticoid synthesis inhibitor metyrapone. Metyrapone groups gained more weight than the group eating a comparable protein diet without metyrapone. *P < 0.001 vs. all groups. +P ≤ 0.005 vs. 20% protein and 20% protein metyrapone. Pro, protein.
Figure 2.
Weight of 1-day-old neonates: neonatal rats were weighed on the first day of life. We could not tell males from females at this age, so the results reflect total neonates. *All weights were different from each other at P < 0.05. Pro, protein.
Table 1. Kidney and body weight, and glomerular number/gram of kidney weight at the time of study.
| 20% Protein (n) | 6% Protein (n) | 20% Protein/metyrapone (n) | 6% Protein/metyrapone (n) | |
|---|---|---|---|---|
| Kidney weight | 1.57 ± 0.06 (10) | 1.33 ± 0.03* (9) | 1.49 ± 0.06 (9) | 1.38 ± 0.05* (8) |
| Body weight | 346.7 ± 5.3 (9) | 285.6 ± 11.8** (9) | 321.3 ± 7.7 (8) | 324.9 ± 7.1 (10) |
| Glomerular number/gram kidney weight | 18,870 ± 1,030† (9) | 16,504 ± 700*** (9) | 20,178 ± 974 (9) | 19,621 ± 796(9) |
All weights are in grams measured at time of death for glomerular counting.
Different from control at P < 0.05.
Different than all other groups.
Different from control metyrapone and low protein metyrapone.
20% vs. 6% P = 0.07.
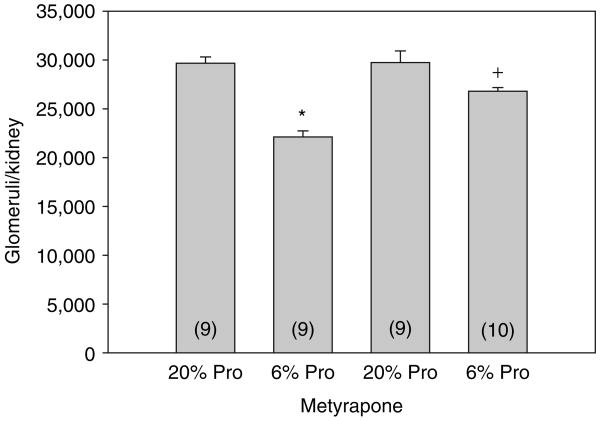
The effects of maternal protein intake and metyrapone on glomerular number are shown in Figure 3. Males that were the product of mothers fed a low protein diet had a significant reduction in glomerular number. Although the number of glomeruli in the 6% metyrapone group was less than that in either the 20% control or 20% metyrapone group, it was significantly greater than the 6% group without metyrapone. We also compared glomerular number factored for gram of kidney weight as shown in Table 1. The 6% group with metyrapone was also greater than the 6% group without metyrapone when factored for kidney weight. Thus, prenatal metyrapone attenuated the nephron deficit caused by a low protein diet. The control kidneys weighed significantly more than the low protein and low protein plus metyrapone groups (Table 1). This was also true of the control metyrapone group, but this did not reach statistical significance. Interestingly, the kidney weights were not different in the low protein and low protein plus metyrapone groups, though the numbers of glomeruli were different.
Figure 3.
Effect of maternal dietary protein and metyrapone on glomerular number in males: the number of glomeruli was assessed in male rats that were the product of mothers that were fed a 6% or 20% protein diet with or without metyrapone in the drinking water during the last half of pregnancy. Rats were cross-reared to control rats that were fed a 20% protein diet.The rats that were the products of mothers that were fed a low protein diet had fewer glomeruli.The 6% protein–metyrapone group had significantly more glomeruli than the 6% group not treated with metyrapone. *P < 0.001 vs. all groups. +P < 0.05 vs. all groups. Pro, protein.
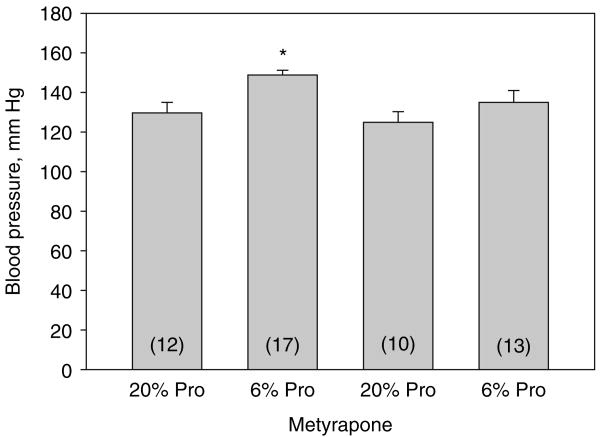
In the final series of experiments, we examined the effect of maternal dietary protein deprivation on blood pressure on male rat offspring when studied as adults. The blood pressures of male rats were higher in the 6% protein group as compared to the 20% protein group with or without metyrapone as shown in Figure 4. Maternal ingestion of metyrapone resulted in a reduction in blood pressure in the 6% protein group to a level that is not different than the 20% control group. The blood pressure of the 6% protein group was higher than the 6% protein group that received metyrapone. Thus, metyrapone did not affect the blood pressure in rats whose mothers were fed a 20% protein diet but attenuated the increase in blood pressure in male rat offspring of rats whose mothers ingested a 6% protein diet.
Figure 4.
Effect of maternal dietary protein and metyrapone on systolic blood pressure in males: systolic blood pressure was assessed in male rats that were the product of mothers that were fed a 6% or 20% protein diet with or without metyrapone in the drinking water during the last half of pregnancy. Rats were cross-reared to control rats that were fed a 20% protein diet.The 6% group not treated with metyrapone had elevated blood pressure compared to the other groups.The rats that were the products of mothers that were fed a low protein diet but treated with metyrapone had normalization of their blood pressure as adults. *P < 0.05 vs. all groups. Pro, protein.
Discussion
The present study examined whether the effect of a low protein diet on offspring could be attenuated by administration of metyrapone to the pregnant mother. Compared to the mothers that ate a low protein diet without metyrapone, the mothers that ate a low protein diet with metyrapone gained more weight and had heavier offspring. However, the 6% metyrapone group did not gain as much weight as the 20% group with or without metyrapone, and had offspring that weighed less than the 20% group. Maternal metyrapone administration to the 6% protein group attenuated the nephron deficit and lowered the blood pressure in adult male offspring compared to the 6% group without metyrapone.
Previous studies have examined the role of placental 11 β-hydroxysteroid dehydrogenase on the fetus. Carbenoxolone, an inhibitor of 11 β-hydroxysteroid dehydrogenase, was administered to pregnant rats to expose the fetus to maternal glucocorticoids. The neonates were small for gestational age and developed hypertension when they were studied as adults.38,39 The effect of carbenoxolone on the prenatal programming of hypertension was abrogated by maternal adrenalectomy prior to pregnancy, consistent with the conclusion that maternal glucocorticoid exposure results in the programming of hypertension.38
A previous study examined the effect of inhibition of maternal steroid synthesis with metyrapone on prenatal programming of hypertension due to maternal dietary protein deprivation.39 In this study, metyrapone was administered during the first half of pregnancy, and pregnant rats were fed a low protein diet throughout pregnancy. Although offspring of the metyrapone group whose mothers were fed a low protein diet had a normalization in blood pressure at 7 weeks of age, it is hard to interpret these data because these offspring failed to thrive and weighed 40% less than the control group. There were a number of major differences between this study and the present one. First and most important, metyrapone was injected into pregnant rats only during the first half of pregnancy. This is at a time before nephrogenesis occurs in the rat, and previous studies have shown that dietary protein deprivation in the first half of pregnancy does not program hypertension.40 In addition, the mothers that received metyrapone were not administered saline water and thus likely had severe volume depletion during the first half of pregnancy. The rats in the present study were cross-fostered at birth to eliminate any difference in postnatal rearing and nutrition caused by maternal dietary protein deprivation during pregnancy. Finally, the number of glomeruli was not determined in the previous study using metyrapone.39
It is possible that the improvement in blood pressure with metyrapone in the low protein group was indirect and not related in reduced fetal steroid exposure. In elegant studies by the Woods Laboratory, maternal dexamethasone resulted in a decrease in maternal and fetal weight gain.21 The offspring of mothers administered prenatal dexamethasone developed hypertension as did offspring of mothers that did not receive dexamethasone but were pair-fed to receive the same food intake as the prenatal dexamethasone group. There was not a maternal dose of dexamethasone that would cause prenatal programming of hypertension without a reduction in maternal weight gain.20 Thus, an alternative interpretation of our data is that the increased maternal weight in the metyrapone group that was administered a low protein diet compared to the low protein group may have protected the neonates from hypertension by preventing caloric deprivation independent of maternal steroid exposure.
Although we find that maternal metyrapone administration to the 6% protein group attenuated the nephron deficit and lowered the blood pressure in adult male offspring compared to the 6% group without metyrapone, we did not find comparable increases in glomerular number or lowering of the blood pressure in the 20% metyrapone group compared to the 20% control group. The 20% metyrapone group had a higher birth weight but was comparable to the 20% control thereafter. These data and studies from prenatal dexamethasone administration are consistent with increased glucocorticoid exposure having adverse effects on the developing fetus; however, lowering fetal glucocorticoid exposure does not program a change in blood pressure or nephron endowment in the control group.
The mechanism of how prenatal exposure to dietary deprivation and fetal steroid exposure cause hypertension has recently been reviewed.41 Excessive glucocorticoid exposure decreases the number of nephrons that is likely due to impaired branching nephrogenesis. Culturing rat metanephroi in the presence of 10−5 mol/l dexamethasone decrease nephron branching and alters expression of several genes important for renal development.26 However, the reduction in nephron endowment in most programming studies is 10–25% of normal and thus unlikely to be a major factor in postnatal hypertension. Prenatal programming of hypertension is associated with alterations in the renin-angiotensin system, renal sodium transport, and renal nerve activity that are likely to be the primary factors in the generation and maintenance of hypertension.14,25,41–46
The preponderance of evidence is consistent with the hypothesis that maternal dietary protein deprivation leads to increased exposure to maternal glucocorticoids. In pregnant rats that had a 50% decrease in caloric intake from days 14–21 of pregnancy, maternal glucocorticoid levels were elevated. Direct measurement of maternal corticosterone levels of food-deprived dams showed that they had significantly higher corticosterone levels than controls on days 19 and 21 of gestation compared to control rats.47 The food-deprived rats had lower levels of placental 11 β-hydroxysteroid dehydrogenase mRNA levels and likely lower activity as shown by others.27,29,47 Indeed, 21-day-old fetuses from food-restricted dams had a 30% higher serum corticosterone level than control fetuses.48 In the present study, we directly demonstrate that although dietary protein deprivation did not affect maternal serum glucocorticoid levels, it increased the glucocorticoid levels in the 20-day fetus. How prenatal glucocorticoid exposure results in prenatal programming of a reduction in nephron number and hypertension in later life is unclear at present.
In summary, our data show that maternal low protein diet results in hypertension and a reduction in nephron number in males that are cross-fostered at birth. Administration of metyrapone attenuated the reduction in nephron number in adult male offspring of mothers fed a low protein diet during the last half of pregnancy. Thus, the reduction in nephron number and hypertension in males that were the product of mothers that ate a low protein diet was at least in part due to exposure to maternal glucocorticoids.
Acknowledgments
This work was supported by NIH grant DK078596 to M.B., T32 DK07257, and the O'Brien Center P30DK079328.
Footnotes
Disclosure:The authors declared no conflict of interest.
References
- 1.Barker DJ, Bull AR, Osmond C, Simmonds SJ. Fetal and placental size and risk of hypertension in adult life. BMJ. 1990;301:259–262. doi: 10.1136/bmj.301.6746.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell DM, Hall MH, Barker DJ, Cross J, Shiell AW, Godfrey KM. Diet in pregnancy and the offspring's blood pressure 40 years later. Br J Obstet Gynaecol. 1996;103:273–280. doi: 10.1111/j.1471-0528.1996.tb09718.x. [DOI] [PubMed] [Google Scholar]
- 3.Roseboom TJ, van der Meulen JH, van Montfrans GA, Ravelli AC, Osmond C, Barker DJ, Bleker OP. Maternal nutrition during gestation and blood pressure in later life. J Hypertens. 2001;19:29–34. doi: 10.1097/00004872-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Hughson M, Farris AB, 3rd, Douglas-Denton R, Hoy WE, Bertram JF. Glomerular number and size in autopsy kidneys: the relationship to birth weight. Kidney Int. 2003;63:2113–2122. doi: 10.1046/j.1523-1755.2003.00018.x. [DOI] [PubMed] [Google Scholar]
- 5.Hinchliffe SA, Lynch MR, Sargent PH, Howard CV, Van Velzen D. The effect of intrauterine growth retardation on the development of renal nephrons. Br J Obstet Gynaecol. 1992;99:296–301. doi: 10.1111/j.1471-0528.1992.tb13726.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoy WE, Rees M, Kile E, Mathews JD, Wang Z. A new dimension to the Barker hypothesis: low birthweight and susceptibility to renal disease. Kidney Int. 1999;56:1072–1077. doi: 10.1046/j.1523-1755.1999.00633.x. [DOI] [PubMed] [Google Scholar]
- 7.Hoy WE, Mathews JD, McCredie DA, Pugsley DJ, Hayhurst BG, Rees M, Kile E, Walker KA, Wang Z. The multidimensional nature of renal disease: rates and associations of albuminuria in an Australian Aboriginal community. Kidney Int. 1998;54:1296–1304. doi: 10.1046/j.1523-1755.1998.00099.x. [DOI] [PubMed] [Google Scholar]
- 8.Hallan S, Euser AM, Irgens LM, Finken MJ, Holmen J, Dekker FW. Effect of intrauterine growth restriction on kidney function at young adult age: the Nord Trøndelag Health (HUNT 2) Study. Am J Kidney Dis. 2008;51:10–20. doi: 10.1053/j.ajkd.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Keijzer-Veen MG, Schrevel M, Finken MJ, Dekker FW, Nauta J, Hille ET, Frölich M, van der Heijden BJ. Dutch POPS-19 Collaborative Study Group Microalbuminuria and lower glomerular filtration rate at young adult age in subjects born very premature and after intrauterine growth retardation. J Am Soc Nephrol. 2005;16:2762–2768. doi: 10.1681/ASN.2004090783. [DOI] [PubMed] [Google Scholar]
- 10.Lackland DT, Bendall HE, Osmond C, Egan BM, Barker DJ. Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch Intern Med. 2000;160:1472–1476. doi: 10.1001/archinte.160.10.1472. [DOI] [PubMed] [Google Scholar]
- 11.Vikse BE, Irgens LM, Leivestad T, Hallan S, Iversen BM. Low birth weight increases risk for end-stage renal disease. J Am Soc Nephrol. 2008;19:151–157. doi: 10.1681/ASN.2007020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hodgin JB, Rasoulpour M, Markowitz GS, D'Agati VD. Very low birth weight is a risk factor for secondary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2009;4:71–76. doi: 10.2215/CJN.01700408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langley-Evans SC, Welham SJ, Sherman RC, Jackson AA. Weanling rats exposed to maternal low-protein diets during discrete periods of gestation exhibit differing severity of hypertension. Clin Sci. 1996;91:607–615. doi: 10.1042/cs0910607. [DOI] [PubMed] [Google Scholar]
- 14.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001;59:238–245. doi: 10.1046/j.1523-1755.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 15.Hall SM, Zeman FJ. Kidney function of the progeny of rats fed a low protein diet. J Nutr. 1968;95:49–54. doi: 10.1093/jn/95.1.49. [DOI] [PubMed] [Google Scholar]
- 16.Zeman FJ. The effect of prenatal protein-calorie malnutrition on kidney development in the rat. Prog Clin Biol Res. 1983;140:309–338. [PubMed] [Google Scholar]
- 17.Gardner DS, Jackson AA. Langley-Evans SC Maintenance of maternal diet-induced hypertension in the rat is dependent on glucocorticoids. Hypertension. 1997;30:1525–1530. doi: 10.1161/01.hyp.30.6.1525. [DOI] [PubMed] [Google Scholar]
- 18.Manning J, Beutler K, Knepper MA, Vehaskari VM. Upregulation of renal BSC1 and TSC in prenatally programmed hypertension. Am J Physiol Renal Physiol. 2002;283:F202–F206. doi: 10.1152/ajprenal.00358.2001. [DOI] [PubMed] [Google Scholar]
- 19.Manning J, Vehaskari VM. Low birth weight-associated adult hypertension in the rat. Pediatr Nephrol. 2001;16:417–422. doi: 10.1007/s004670000560. [DOI] [PubMed] [Google Scholar]
- 20.Woods LL. Maternal glucocorticoids and prenatal programming of hypertension. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1069–R1075. doi: 10.1152/ajpregu.00753.2005. [DOI] [PubMed] [Google Scholar]
- 21.Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. Am J Physiol Regul Integr Comp Physiol. 2005;289:R955–R962. doi: 10.1152/ajpregu.00455.2004. [DOI] [PubMed] [Google Scholar]
- 22.Woods LL. Fetal origins of adult hypertension: a renal mechanism? Curr Opin Nephrol Hypertens. 2000;9:419–425. doi: 10.1097/00041552-200007000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz LA, Quan A, Weinberg A, Baum M. Effect of prenatal dexamethasone on rat renal development. Kidney Int. 2001;59:1663–1669. doi: 10.1046/j.1523-1755.2001.0590051663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003;41:328–334. doi: 10.1161/01.hyp.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dagan A, Gattineni J, Cook V, Baum M. Prenatal programming of rat proximal tubule Na+/H+ exchanger by dexamethasone. Am J Physiol Regul Integr Comp Physiol. 2007;292:R1230–R1235. doi: 10.1152/ajpregu.00669.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh RR, Moritz KM, Bertram JF, Cullen-McEwen LA. Effects of dexamethasone exposure on rat metanephric development: in vitro and in vivo studies. Am J Physiol Renal Physiol. 2007;293:F548–F554. doi: 10.1152/ajprenal.00156.2007. [DOI] [PubMed] [Google Scholar]
- 27.Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339–341. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- 28.Seckl JR, Benediktsson R, Lindsay RS, Brown RW. Placental 11 beta-hydroxysteroid dehydrogenase and the programming of hypertension. J Steroid Biochem Mol Biol. 1995;55:447–455. doi: 10.1016/0960-0760(95)00193-x. [DOI] [PubMed] [Google Scholar]
- 29.Langley-Evans SC, Phillips GJ, Benediktsson R, Gardner DS, Edwards CR, Jackson AA, Seckl JR. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996;17:169–172. doi: 10.1016/s0143-4004(96)80010-5. [DOI] [PubMed] [Google Scholar]
- 30.Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. 11 beta-Hydroxysteroid dehydrogenases: key enzymes in determining tissue-specific glucocorticoid effects. Steroids. 1996;61:263–269. doi: 10.1016/0039-128x(96)00033-5. [DOI] [PubMed] [Google Scholar]
- 31.Edwards CR, Benediktsson R, Lindsay RS, Seckl JR. Dysfunction of placental glucocorticoid barrier: link between fetal environment and adult hypertension? Lancet. 1993;341:355–357. doi: 10.1016/0140-6736(93)90148-a. [DOI] [PubMed] [Google Scholar]
- 32.Kajantie E, Dunkel L, Turpeinen U, Stenman UH, Wood PJ, Nuutila M, Andersson S. Placental 11 beta-hydroxysteroid dehydrogenase-2 and fetal cortisol/cortisone shuttle in small preterm infants. J Clin Endocrinol Metab. 2003;88:493–500. doi: 10.1210/jc.2002-021378. [DOI] [PubMed] [Google Scholar]
- 33.Celsi G, Kistner A, Aizman R, Eklöf AC, Ceccatelli S, de Santiago A, Jacobson SH. Prenatal dexamethasone causes oligonephronia, sodium retention, and higher blood pressure in the offspring. Pediatr Res. 1998;44:317–322. doi: 10.1203/00006450-199809000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Smith JT, Waddell BJ. Leptin distribution and metabolism in the pregnant rat: transplacental leptin passage increases in late gestation but is reduced by excess glucocorticoids. Endocrinology. 2003;144:3024–3030. doi: 10.1210/en.2003-0145. [DOI] [PubMed] [Google Scholar]
- 35.Smith JT, Waddell BJ. Leptin receptor expression in the rat placenta: changes in ob-ra, ob-rb, and ob-re with gestational age and suppression by glucocorticoids. Biol Reprod. 2002;67:1204–1210. doi: 10.1095/biolreprod67.4.1204. [DOI] [PubMed] [Google Scholar]
- 36.Smith JT, Waddell BJ. Increased fetal glucocorticoid exposure delays puberty onset in postnatal life. Endocrinology. 2000;141:2422–2428. doi: 10.1210/endo.141.7.7541. [DOI] [PubMed] [Google Scholar]
- 37.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1131–R1136. doi: 10.1152/ajpregu.00037.2003. [DOI] [PubMed] [Google Scholar]
- 38.Lindsay RS, Lindsay RM, Edwards CR, Seckl JR. Inhibition of 11-beta-hydroxysteroid dehydrogenase in pregnant rats and the programming of blood pressure in the offspring. Hypertension. 1996;27:1200–1204. doi: 10.1161/01.hyp.27.6.1200. [DOI] [PubMed] [Google Scholar]
- 39.Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. J Hypertens. 1997;15:537–544. doi: 10.1097/00004872-199715050-00010. [DOI] [PubMed] [Google Scholar]
- 40.Woods LL, Weeks DA, Rasch R. Programming of adult blood pressure by maternal protein restriction role of nephrogenesis. Kidney Int. 2004;65:1339–1348. doi: 10.1111/j.1523-1755.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 41.Baum M. Role of the kidney in the prenatal and early postnatal programming of hypertension. Am J Physiol Renal Physiol. 2010;298:F235–F247. doi: 10.1152/ajprenal.00288.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dagan A, Kwon HM, Dwarakanath V, Baum M. Effect of renal denervation on prenatal programming of hypertension and renal tubular transporter abundance. Am J Physiol Renal Physiol. 2008;295:F29–F34. doi: 10.1152/ajprenal.00123.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dagan A, Habib S, Gattineni J, Dwarakanath V, Baum M. Prenatal programming of rat thick ascending limb chloride transport by low-protein diet and dexamethasone. Am J Physiol Regul Integr Comp Physiol. 2009;297:R93–R99. doi: 10.1152/ajpregu.91006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dagan A, Gattineni J, Habib S, Baum M. Effect of prenatal dexamethasone on postnatal serum and urinary angiotensin II levels. Am J Hypertens. 2010;23:420–424. doi: 10.1038/ajh.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woods LL, Ingelfinger JR, Nyengaard JR, Rasch R. Maternal protein restriction suppresses the newborn renin-angiotensin system and programs adult hypertension in rats. Pediatr Res. 2001;49:460–467. doi: 10.1203/00006450-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Vehaskari VM, Woods LL. Prenatal programming of hypertension: lessons from experimental models. J Am Soc Nephrol. 2005;16:2545–2556. doi: 10.1681/ASN.2005030300. [DOI] [PubMed] [Google Scholar]
- 47.Lesage J, Blondeau B, Grino M, Bréant B, Dupouy JP. Maternal undernutrition during late gestation induces fetal overexposure to glucocorticoids and intrauterine growth retardation, and disturbs the hypothalamo-pituitary adrenal axis in the newborn rat. Endocrinology. 2001;142:1692–1702. doi: 10.1210/endo.142.5.8139. [DOI] [PubMed] [Google Scholar]
- 48.Blondeau B, Lesage J, Czernichow P, Dupouy JP, Bréant B. Glucocorticoids impair fetal beta-cell development in rats. Am J Physiol Endocrinol Metab. 2001;281:E592–E599. doi: 10.1152/ajpendo.2001.281.3.E592. [DOI] [PubMed] [Google Scholar]