Abstract
Background
Real-time PCR can be carried out using either probes or DNA dyes. SYBR Green has been used the most, but it suffers from several drawbacks. Numerous other DNA dyes are commercially available, but with limited structural information. Dye behavior in real time PCR is difficult to predict, so empirical data are needed. In the work described here, a panel of 23 different DNA dyes--including green, orange, and red SYTO dyes, EvaGreen, and SYBR Green--were evaluated with respect to their performance in real time PCR.
Findings
Data were analyzed for reaction inhibition, effects on amplicon melting temperature, fluorescent signal strength, and reaction efficiency. This is the first report of reaction efficiency using alternatives to SYBR Green. Results indicated substantial variation in performance even within the SYTO dye family. EvaGreen and the SYTO dyes 13, 16, 80, and 82 performed better than SYBR Green in general, and high reaction efficiencies can be achieved using these dyes.
Conclusions
Empirical data were generated for 23 DNA dyes. This paper confirms and extends previous findings that among commercially available DNA dyes, EvaGreen and certain SYTO dyes are the most desirable alternatives to the commonly used SYBR Green in real-time PCR.
Background
Real-time PCR can be carried out using sequence-specific oligonucleotide probes or non-sequence specific DNA dyes [1,2]. Dyes offer greater flexibility and reduced cost, and they allow dissociation (melt) curve analysis of PCR products, while the most commonly used probes do not [2-4]. In cases where template sequence tends to vary, dye-based detection helps prevent false negatives that might result from basepair mismatches in a sequence-specific probe binding region [5,6]. Despite the advantages of dye-based detection and the wide variety of commercially available fluorescent DNA dyes, most real-time PCR has been conducted using SYBR Green. SYBR Green exhibits a very strong fluorescent signal, but it has been shown to inhibit the PCR reaction and has a narrow dynamic range and lower reproducibility than other detection chemistries [2,3,7]. Melt curve analysis using SYBR Green is complicated by the dye's effect on melting temperature and by dye redistribution which occurs during melting [4]. This occurs because SYBR Green must be used at low nonsaturating concentrations to prevent reaction inhibition [8-10].
A previous investigation of DNA dyes from several families suggested that SYTO dyes are the most promising for real-time PCR applications [7]. The SYTO dyes constitute a large family of commercially available cyanine dyes. Interaction of cyanine dyes with DNA is complex and is influenced by electrostatic, van der Waals, hydrophobic, and steric interactions, all of which are governed by the dye's chemical structure. Dye binding appears to be cooperative and is affected by dye concentration and dye-to-basepair ratio [11]. SYTO dyes are more hydrophobic than other cyanine dyes. They bind DNA based mainly on charge and primarily in the minor groove. Within the SYTO dye family, there are variations in fluorescent enhancement on nucleic acid binding, excitation and emission spectra, DNA/RNA selectivity, binding mode, and binding affinity (Invitrogen/Molecular Probes, probes.invitrogen.com). All of these characteristics may affect performance in PCR reactions. EvaGreen is another DNA dye which is less inhibitory to PCR than SYBR Green and is marketed as an alternative [12]. Detailed structural information is generally not available for individual dyes, making it difficult to predict their behavior in real-time PCR. Published work has not included data on fluorescent signal strength or investigation of reaction efficiency, a critical aspect of real-time PCR experiments [7,13]. The most significant advantage of real-time PCR over standard end-point PCR is that it can be used quantitatively; high reaction efficiencies are imperative for accurate quantitation [2,14]. Inhibitory dyes such as SYBR Green are likely to have adverse effects on reaction efficiency.
The difficulties associated with predicting a dye's performance in real-time PCR require that researchers rely on empirical data. Relatively few studies have been published on alternatives to SYBR Green; this is especially true for the SYTO dyes despite their promising performance [7,13]. In the work described here, EvaGreen and a panel of 21 green, orange, and red SYTO dyes were evaluated for their performance in real-time PCR and compared to the commonly used SYBR Green. Data were analyzed with respect to reaction inhibition, effects on amplicon melting temperature, fluorescent signal strength, and reaction efficiency. The objectives of this work were 1) to generate empirical data for a larger panel of SYTO dyes than has previously been reported and 2) to test reaction efficiency using the best-performing dyes. This work was conducted as part of a project focused on developing real-time PCR assays to detect shrimp, a crustacean food allergen.
Results and Discussion
Optimal concentration for each dye was determined to be that which gave the best combination of low Ct, high fluorescence, and low inhibition (Tables 1 and 2). Ct values were lower in general for the mitochondrial 16S target (Table 1) than for the nuclear tropomyosin target (Table 2) because mitochondrial targets are present in higher copy number in cells. Increasing Ct (or no Ct) values at higher dye concentrations indicate that the dye inhibits the PCR reaction. Fluorescent signals were normalized to that of SYBR Green. Effects of SYBR Green on amplicon Tm were determined using both fold (x) dye concentration--for comparison with EvaGreen data--and molar (μM) dye concentration--for comparison with SYTO dye data. In some cases, the dyes had very little effect on melting temperature. While they yielded low R2 values, absolute differences in Tm were small in these cases. This effect was more pronounced for the tropomyosin target (Table 2). No slope or R2 values are reported for effects of SYTO 24 on Tm because only the lowest concentration of dye yielded amplification. Effects of SYBR Green on Tm were determined using experiments conducted with dye concentrations below 2 μM (1×).
Table 1.
Reaction inhibition, fluorescent signal, and melting temperature data: 16S amplicon
| Dye | Optimal Concentration |
Ct: 0.64 μM (0.32×) |
Ct: 2 μM (1×) |
Ct: 10 μM (5×) |
Ct: 20 μM (10×) |
Maximum Fluorescence |
Tm shift slope (R2) |
|---|---|---|---|---|---|---|---|
| SYBR Green* | 0.64 μM (0.32×) | 27.4 ± 0.78 | no Ct | no Ct | no Ct | 1.00 | 5.89, × conc. 2.95, μM conc. (0.86) |
| EvaGreen* | 5× | 31.54 ± 0.21 | 29.98 ± 0.50 | 28.60 ± 0.50 | not determined | 1.03 | 0.50 (0.94) |
| Green* | |||||||
| SYTO 11 | 2 μM | 30.8 ± 1.26 | 29.9 ± 1.06 | 33.7 ± 2.58 | no Ct | 0.83 | 0.41 (0.96) |
| SYTO 13 | 10 μM | 31.8 ± 1.06 | 30.5 ± 1.16 | 29.1 ± 0.95 | 29.9 ± 1.11 | 0.85 | 0.15 (0.92) |
| SYTO 16 | 10 μM | 29.8 ± 0.95 | 28.5 ± 0.92 | 27.7 ± 1.04 | 30.3 ± 1.47 | 0.99 | 0.17 (0.93) |
| SYTO 21 | 0.64 μM | 28.5 ± 0.98 | 32.94 ± 3.7 | no Ct | no Ct | 0.79 | 1.73 (0.63) |
| SYTO 24 | 0.64 μM | 31.0 ± 0.0 | no Ct | no Ct | no Ct | 0.98 | not determined |
| Orange* | |||||||
| SYTO 80 | 20 μM | 33.9 ± 0.57 | 32.4 ± 0.09 | 30.27 ± 0.01 | 29.4 ± 0.09 | 0.54 | 0.018 (0.33) |
| SYTO 81 | 20 μM | 40.13 ± 0.0 | 35.7 ± 0.55 | 32.5 ± 0.10 | 32.0 ± 0.03 | 0.13 | -0.002 (0.003) |
| SYTO 82 | 20 μM | 31.8 ± 0.37 | 31.25 ± 0.06 | 29.4 ± 0.01 | 28.7 ± 0.08 | 0.77 | 0.040 (0.59) |
| SYTO 83 | 20 μM | 39.6 ± 2.09 | 35.2 ± 0.77 | 32.7 ± 0.41 | 32.4 ± 0.17 | 0.12 | 0.029 (0.38) |
| Red** | |||||||
| SYTO 17 | 20 μM | no Ct | no Ct | 32.9 | 30.9 | 0.05 | -0.001 (0.009) |
| SYTO 59 | 2 μM | 28.9 | 28.0 | 28.5 | 37.2 | 0.29 | 0.16 (0.96) |
| SYTO 60 | 2 μM | 29.5 | 29.37 | no Ct | no Ct | 0.16 | 0.24 (0.78) |
| SYTO 61 | 10 μM | 32.0 | 29.86 | 27.76 | 31.55 | 0.17 | 0.06 (0.91) |
| SYTO 62 | 0.64 μM | 28.8 | 30.75 | no Ct | no Ct | 0.18 | 0.26 (0.99) |
| SYTO 63 | 2 μM | 28.9 | 28.6 | 43.0 | no Ct | 0.20 | 0.12 (0.32) |
| SYTO 64 | 2 μM | 34.8 | 31.7 | 31.7 | 34.0 | 0.15 | 0.012 (0.27) |
No fluorescent signals were obtained for SYTO dyes 12, 14, 25, 84, or 85
*Ct values are average ± SD for at 2-3 independent experiments
**Ct values from one independent experiment, no SD
Table 2.
Reaction inhibition, fluorescent signal, and melting temperature data: tropomyosin amplicon
| Dye | Optimal Concentration |
Ct: 0.64 μM (0.32×) |
Ct: 2 μM (1×) |
Ct: 10 μM (5×) |
Ct: 20 μM (10×) |
Maximum Fluorescence |
Tm shift slope (R2) |
|---|---|---|---|---|---|---|---|
| SYBR Green* | 0.64 μM | 33.00 ± 0.10 | 35.07 ± 1.10 | no Ct | no Ct | 1.00 | 3.99, × conc. 1.99, μM conc. (0.97) |
| EvaGreen* | 5× | 36.37 ± 0.48 | 35.04 ± 0.35 | 35.15 ± 1.13 | not determined | 0.91 | 0.36 (0.98) |
| Green* | |||||||
| SYTO 11 | 2 μM | 38.02 ± 0.97 | 36.42 ± 0.84 | 38.17 ± 1.03 | 42.27 ± 0 | 0.51 | -0.20 (0.12) |
| SYTO 13 | 10 μM | 37.58 ± 1.33 | 36.10 ± 1.32 | 36.06 ± 1.55 | 36.45 ± 2.06 | 0.87 | 0.001 (0.00) |
| SYTO 16 | 10 μM | 37.00 ± 1.86 | 34.20 ± 1.05 | 34.04 ± 0.96 | 34.97 ± 0.53 | 1.07 | 0.030 (0.004) |
| SYTO 21 | 0.64 μM | 34.55 ± 1.07 | 35.90 ± 0.83 | no Ct | no Ct | 0.82 | 0.62 (0.03) |
| SYTO 24 | 0.64 μM | 33.50 ± 0.01 | no Ct | no Ct | no Ct | 1.02 | not determined |
| Orange* | |||||||
| SYTO 80 | 20 μM | 39.16 ± 2.44 | 37.33 ± 1.86 | 35.84 ± 0.02 | 34.78 ± 0.08 | 0.40 | 0.048 (0.008) |
| SYTO 81 | 20 μM | 43.89 ± 0.10 | 40.62 ± 1.39 | 37.02 ± 0.39 | 36.45 ± 0.21 | 0.11 | 0.026 (0.002) |
| SYTO 82 | 20 μM | 37.18 ± 1.85 | 35.59 ± 0.95 | 33.50 ± 0.11 | 33.44 ± 0.51 | 0.61 | -0.17 (0.10) |
| SYTO 83 | 20 μM | 42.20 ± 0.01 | 39.28 ± 0.99 | 37.04 ± 0.13 | 35.92 ± 0.93 | 0.10 | 0.037 (0.0051) |
| Red* | |||||||
| SYTO 17 | 20 μM | no Ct | 42.26 ± 0.02 | 36.18 ± 0.14 | 35.32 ± 0.41 | 0.03 | 0.0009 (0.0005) |
| SYTO 59 | 10 μM | 35.63 ± 0.09 | 35.15 ± 1.50 | 33.70 ± 0.10 | 35.53 ± 0.53 | 0.20 | 0.11 (0.83) |
| SYTO 60 | 2 μM | 34.85 ± 0.41 | 34.29 ± 0.31 | 41.09 ± 1.97 | no Ct | 0.13 | 0.064 (0.10) |
| SYTO 61 | 10 μM | 37.45 ± 1.68 | 35.38 ± 0.57 | 34.14 ± 0.98 | 35.00 ± 1.19 | 0.14 | 0.043 (0.58) |
| SYTO 62 | 0.64 μM | 33.98 ± 0.42 | 33.69 ± 0.75 | no Ct | no Ct | 0.17 | 0.035 (0.008) |
| SYTO 63 | 2 μM | 34.78 ± 0.41 | 34.50 ± 0.31 | 37.07 ± 0.07 | no Ct | 0.12 | 0.24 (0.85) |
| SYTO 64 | 2 μM | 36.22 ± 0.10 | 36.68 ± 1.62 | 36.31 ± 1.05 | 36.48 ± 0.95 | 0.05 | -0.004 (0.013) |
No fluorescent signals were obtained for SYTO dyes 12, 14, 25, 84, or 85
*Ct values are average ± SD for at 2-3 independent experiments
There were significant differences in performance among the SYTO dyes. SYTO 21 and SYTO 24 were among the poorest-performing dyes in terms of reaction inhibition and effects on Tm, while SYTO 13 and SYTO 16 were among the best. These are all green dyes in the same family and yet they exhibited significantly different behavior; this emphasizes the need for empirical data. The results presented here are in general agreement with previous work showing that SYTO 13 and SYTO 16 have little effect on amplicon melting temperature and are less inhibitory to PCR than SYBR Green, though optimal concentrations were lower in the previous study [7]. This work was conducted using a Stratagene instrument equipped with halogen lamp excitation and photomultiplier tube (PMT) detection, while previous work was conducted using a BioRad instrument equipped with light-emitting-diode (LED) excitation and photodiode detection [7]. Thus, similar results have been obtained in independent laboratories employing instruments from different manufacturers and with fundamentally different optics. Fluorescent signal strength is in part a reflection of how well dye spectra match instrument detection channels. In the current study, green SYTO dyes had the greatest fluorescent signal strength. In general, the green dye spectra matched instrument detection channels better than the orange SYTO dyes, and the red SYTO dye spectra were the most divergent from available detection channels (Additional File 1: Table S1). Red dyes also had the lowest fluorescent signals.
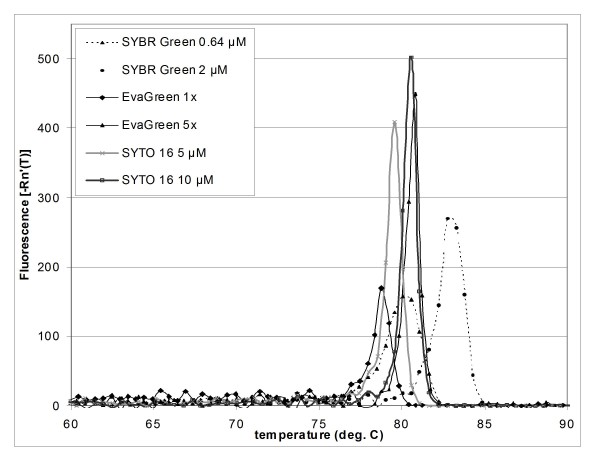
SYBR Green, EvaGreen and four SYTO dyes were used in tests of reaction efficiency for the 16S target. Two concentrations of each dye were tested (Table 3). Reaction efficiencies of 95%-101% and R2 values of 0.98-0.99 were obtained for SYBR Green at 0.64 μM and for the other dyes at both concentrations tested. At 2 μM (1×) SYBR Green, the R2 value was only 0.395, reflecting scatter and high variability in the data at this inhibitory dye concentration. Differences between reaction efficiency data (Table 3) and reaction inhibition data (Table 1) reported for SYBR Green are likely due to differences in thermal cycling, which was adjusted to optimize reaction efficiency. In order to achieve high reaction efficiencies, the annealing and extension step had to be carried out at a lower temperature for a longer time. This is in agreement with the findings of Hilscher et al. [15] who report that fast cycling times can result in lower reaction efficiencies. The work presented here demonstrates that high reaction efficiencies can be achieved using EvaGreen and the SYTO dyes 13, 16, 80, and 82 over a greater range of concentrations and at higher concentrations than SYBR Green. This is important not only for accurate and reliable quantitation, but also for melt curve analysis. Dyes which can be used at higher, saturating concentrations without adversely affecting the PCR reaction enable higher resolution melt curve analysis and even single-basepair mismatch discrimination [10,16]. SYTO 16 and EvaGreen gave higher, sharper peaks in melt curves than SYBR Green and the other dyes evaluated in this work (Figure 1). SYTO 16 displayed sharp melt curve peaks at both 5 μM and 10 μM, while peaks for EvaGreen were sharp at 5× but not at a 1× concentration.
Table 3.
Reaction efficiency data (16S amplicon)
| Dye | Concentration | R2 | Slope | Efficiency |
|---|---|---|---|---|
| SYBR Green | 0.32 × (0.64 μM) | 0.985 | -3.40 | 97% |
| SYBR Green | 1× (2 μM) | 0.395 | -2.13 | 195% |
| EvaGreen | 1× | 0.995 | -3.30 | 101% |
| EvaGreen | 5× | 0.999 | -3.46 | 95% |
| SYTO 13 | 5 μM | 0.993 | -3.42 | 96% |
| SYTO 13 | 10 μM | 0.993 | -3.44 | 95% |
| SYTO 16 | 5 μM | 0.983 | -3.30 | 101% |
| SYTO 16 | 10 μM | 0.992 | -3.43 | 96% |
| SYTO 80 | 10 μM | 0.992 | -3.42 | 96% |
| SYTO 80 | 20 μM | 0.986 | -3.41 | 96% |
| SYTO 82 | 10 μM | 0.998 | -3.41 | 96% |
| SYTO 82 | 20 μM | 0.996 | -3.37 | 98% |
Figure 1.
Melt curves for SYBR Green, EvaGreen, and SYTO 16. All reactions contained 1000 pg shrimp genomic DNA amplified using the 16S primer set. Similar data were obtained for all experiments and amounts of template DNA tested.
Conclusions
Real-time PCR data were generated using 21 SYTO dyes, EvaGreen and SYBR Green. Wide variation in performance within the same dye family emphasizes the need for empirical data of the type presented here. EvaGreen and the SYTO dyes 13, 16, 80, and 82 performed better than other dyes; EvaGreen and SYTO 16 produced the sharpest peaks in melt curve analysis.
Methods
Farm-raised shrimp were obtained from a local market and DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen, Valencia, CA). Primers targeting the shrimp 16S rRNA gene (forward primer: TTGCGACCTCGATGTTGAATTAAGG, reverse primer: CCGGTCTGAACTCAGATCATGTAAGG, amplicon: TTGCGACCTCGATGTTGAATTAAGGGTTCCTTATAATGCAGCAGTTATAAAGGAGGGTCTGTTCGACCTTTAAATCCTTACATGATCTGAGTTCAGACCGG) were designed from conserved regions of the alignment provided in Khamnamtong et al. [17] and used to amplify a 101 bp fragment from the 3' end of this alignment. Primers targeting the shrimp tropomyosin gene (forward primer: TGCAGCAACTTGAGAACGACCTTG, reverse primer: TGTCCTTCTCCACAAGCTGGATGT, amplicon: TGCAGCAACTTGAGAACGACCTTGACCAGGTGCAGGAATCCTTGCTGAAGGCTA ACATCCAGCTTGTGGAGAAGGACA) were designed from Genbank accession number AY827100 using PrimerQuest (Integrated DNA Technologies, Coralville, IA). All primers were purchased from Integrated DNA Technologies. SYTO dyes were obtained from Invitrogen/Molecular Probes (Carlsbad, CA) and EvaGreen was obtained from Biotium (Hayward, CA). Molar concentrations of SYBR Green were calculated using the estimation provided by Zipper et al. [18]. PCR was carried out using the Brilliant SYBR Green QPCR Core Reagent Kit on an Mx3005P qPCR system supplied by Agilent Technologies (Santa Clara, CA). Reactions contained 1× PCR buffer, 3 mM MgCl2, 1.2 mM dNTP mix, 200 nM each primer, 3% DMSO, 4% glycerol, and 1.25 units Taq polymerase in 25 μl total volume. Data on reaction inhibition, fluorescent signal strength, and melting temperature for both the 16S target (Table 1) and the tropomyosin target (Table 2) were generated using 10 pg of shrimp genomic DNA per reaction. Thermal cycling for these data consisted of an initial step at 95°C for 10 minutes, and 45 cycles of denaturation at 95°C for 30 seconds plus annealing/extension at 65°C for 1 minute. Effects on amplicon melting temperature (Tm) were determined by plotting Tm vs. dye concentration. Slopes and R2 values for Tm data are reported for each dye (Tables 1 and 2). Reaction efficiency tests were carried out using the 16S gene target. Thermal cycling for reaction efficiency data (Table 3) consisted of an initial step at 95°C for 10 minutes, and 45 cycles of denaturation at 95°C for 30 seconds plus annealing/extension at 60°C for 2 minutes. Reaction efficiency data were generated by determining Ct values using 10-fold dilutions of template DNA ranging from 0.1 to 1000 pg of shrimp genomic DNA per reaction. A linear standard curve of Ct vs. log DNA concentration was plotted. Reaction efficiencies were calculated using the equation E = 10(-1/m) -1, where E = reaction efficiency and m = slope of the linear standard curve [14].
Competing interests
The author declares that they have no competing interests.
Authors' contributions
AE conceived, designed, and carried out all experiments reported here and wrote the manuscript.
Author Details
AE has over 14 years of molecular biology research experience and has conducted molecular biology and PCR work in both the life science and environmental engineering fields. She completed her Ph.D. at Duke University in 2009, and currently develops and validates real-time PCR assays for detection of food allergens at the U. S. Food and Drug Administration.
Supplementary Material
Table S1. Excitation and emission maxima of dyes and Mx3005P detection channels.
Acknowledgements and Funding
The author wishes to thank the following for critical readings of the manuscript: Haile Yancy, Ph.D. (US FDA, Center for Veterinary Medicine, Laurel, MD), and Sara Handy, Ph.D. (US FDA, Center for Food Safety and Applied Nutrition, College Park, MD). This work was funded by the U.S. Food and Drug Administration.
References
- Higuchi R, Fockler C, Dollinger G, Watson R. Kinetic PCR analysis: real-time monitoring of DNA amplification reactions. Bio/Technology. 1993;11:1026–1030. doi: 10.1038/nbt0993-1026. [DOI] [PubMed] [Google Scholar]
- Wilhelm J, Pingoud A. Real-time polymerase chain reaction. ChemBioChem. 2003;4:1120–1128. doi: 10.1002/cbic.200300662. [DOI] [PubMed] [Google Scholar]
- Gasparic MB, Tengs T, La Paz JL, Holst-Jensen A, Pla M, Esteve T, Zel J, Gruden K. Comparison of nine different real-time PCR detection chemistries for qualitative and quantitative applications in GMO detection. Anal Bioanal Chem. 2010;396:2023–2029. doi: 10.1007/s00216-009-3418-0. [DOI] [PubMed] [Google Scholar]
- Ririe KM, Rasmussen RP, Wittwer CT. Product differentiation by analysis of DNA melting curves during the polymerase chain reaction. Anal Biochem. 1997;245:154–160. doi: 10.1006/abio.1996.9916. [DOI] [PubMed] [Google Scholar]
- Anderson TP, Werno AM, Beynon KA, Murdoch DR. Failure to genotype herpes simplex virus by real-time PCR assay and melting curve analysis due to sequence variation within probe binding sites. J Clin Microbiol. 2003;41(5):2135–2137. doi: 10.1128/JCM.41.5.2135-2137.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papin JF, Vahrson W, Dittmer D. SYBR Green-based real-time quantitative PCR assay for detection of West Nile virus circumvents false-negative results due to strain variability. J Clin Microbiol. 2004;42(4):1511–1518. doi: 10.1128/JCM.42.4.1511-1518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudnason H, Dufva M, Bang DD, Wolff A. Comparison of multiple dyes for real-time PCR: effects of dye concentration and sequence composition on DNA amplification and melting temperature. Nucleic Acids Res. 2007;35(19):e127. doi: 10.1093/nar/gkm671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglio S, Monis PT, Saint CP. Demonstration of preferential binding of SYBR Green I to specific DNA fragments in real-time multiplex PCR. Nucleic Acids Res. 2003;31(22):e136. doi: 10.1093/nar/gng135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga A, James D. Real-time PCR and SYBR Green I melting curve analysis for the identification of Plum pox virus strains C, EA, and W: effect of amplicon size, melt rate, and dye translocation. J Virol Meth. 2006;132:146–153. doi: 10.1016/j.jviromet.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Wittwer CT, Reed GH, Gundry CN, Vandersteen JG, Pryor RJ. High-resolution genotyping by amplicon melting analysis using LCGreen. Clin Chem. 2003;49(6):853–860. doi: 10.1373/49.6.853. [DOI] [PubMed] [Google Scholar]
- Hannah KC, Armitage BA. M DNA-templated assembly of helical cyanine dye aggregates: a supramolecular chain polymerization. Acct Chem Res. 2004;37:845–853. doi: 10.1021/ar030257c. [DOI] [PubMed] [Google Scholar]
- Mao F, Leung W-Y, Xin X. Characterization of EvaGreen and the implication of its physicochemical properties for qPCR applications. BMC Biotechnol. 2007;7:76. doi: 10.1186/1472-6750-7-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monis PT, Giglio S, Saint CP. Comparison of SYTO 9 and SYBR Green I for real-time polymerase chain reaction and investigation of the effect of dye concentration on amplification and DNA melting curve analysis. Anal Biochem. 2005;340:24–34. doi: 10.1016/j.ab.2005.01.046. [DOI] [PubMed] [Google Scholar]
- Wong ML, Medrano JF. Real-time PCR for mRNA quantitation. BioTechniques. 2005;39(1):75–85. doi: 10.2144/05391RV01. [DOI] [PubMed] [Google Scholar]
- Hilscher C, Vahrson W, Dittmer D. Faster quantitative real-time PCR protocols may lose sensitivity and show increased variability. Nucleic Acids Res. 2005;33(21):e182. doi: 10.1093/nar/gni181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed GH, Kent JO, Wittwer CT. High-resolution DNA melting analysis for simple and efficient molecular diagnostics. Pharmacogenomics. 2007;8(6):597–608. doi: 10.2217/14622416.8.6.597. [DOI] [PubMed] [Google Scholar]
- Khamnamtong B, Klinbunga S, Menasveta P. Species identification of five penaeid shrimps using PCR-RFLP and SSCP analyses of 16S ribosomal DNA. J Biochem Molec Biol. 2005;38(4):491–499. doi: 10.5483/BMBRep.2005.38.4.491. [DOI] [PubMed] [Google Scholar]
- Zipper H, Brunner H, Bernhagen J, Vitzthum F. Investigations on DNA intercalation and surface binding by SYBR Green I, its structure determination and methodological implications. Nucleic Acids Res. 2004;32(12):e103. doi: 10.1093/nar/gnh101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Excitation and emission maxima of dyes and Mx3005P detection channels.