Abstract
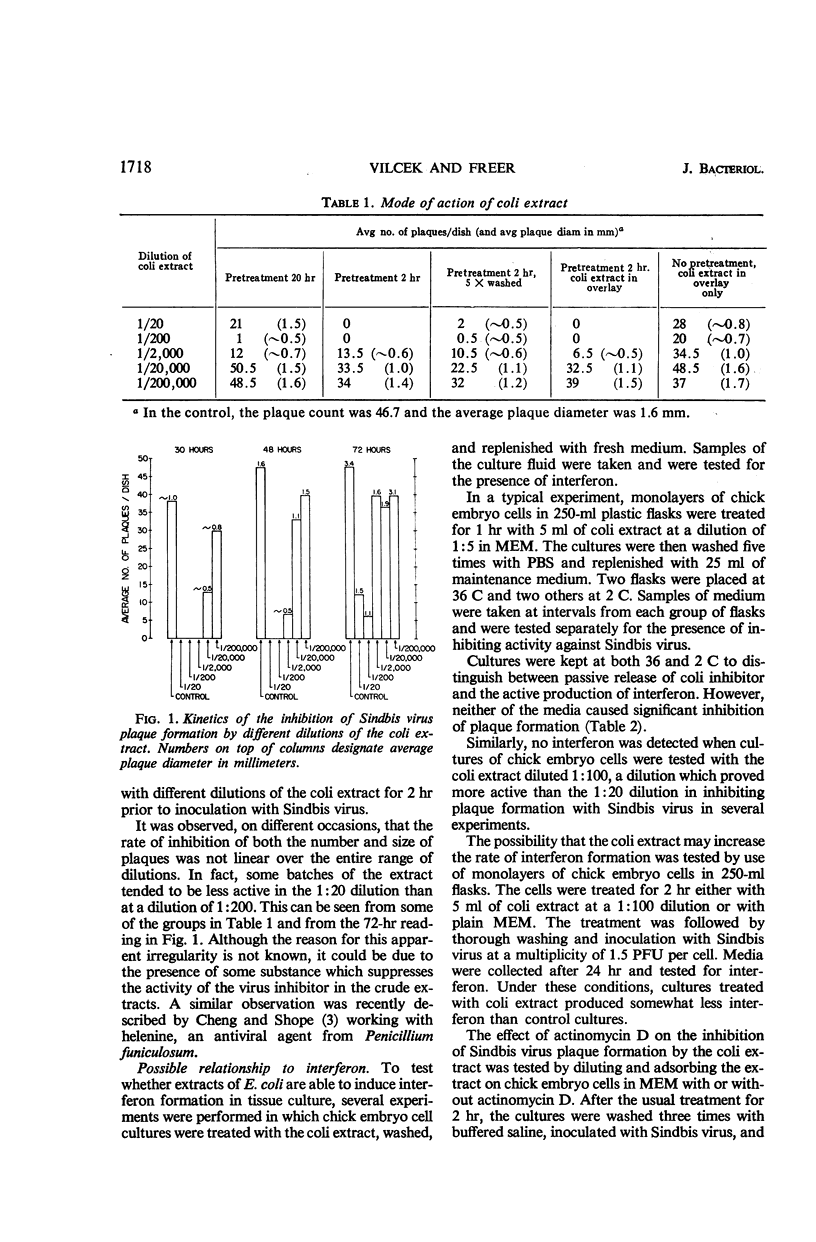
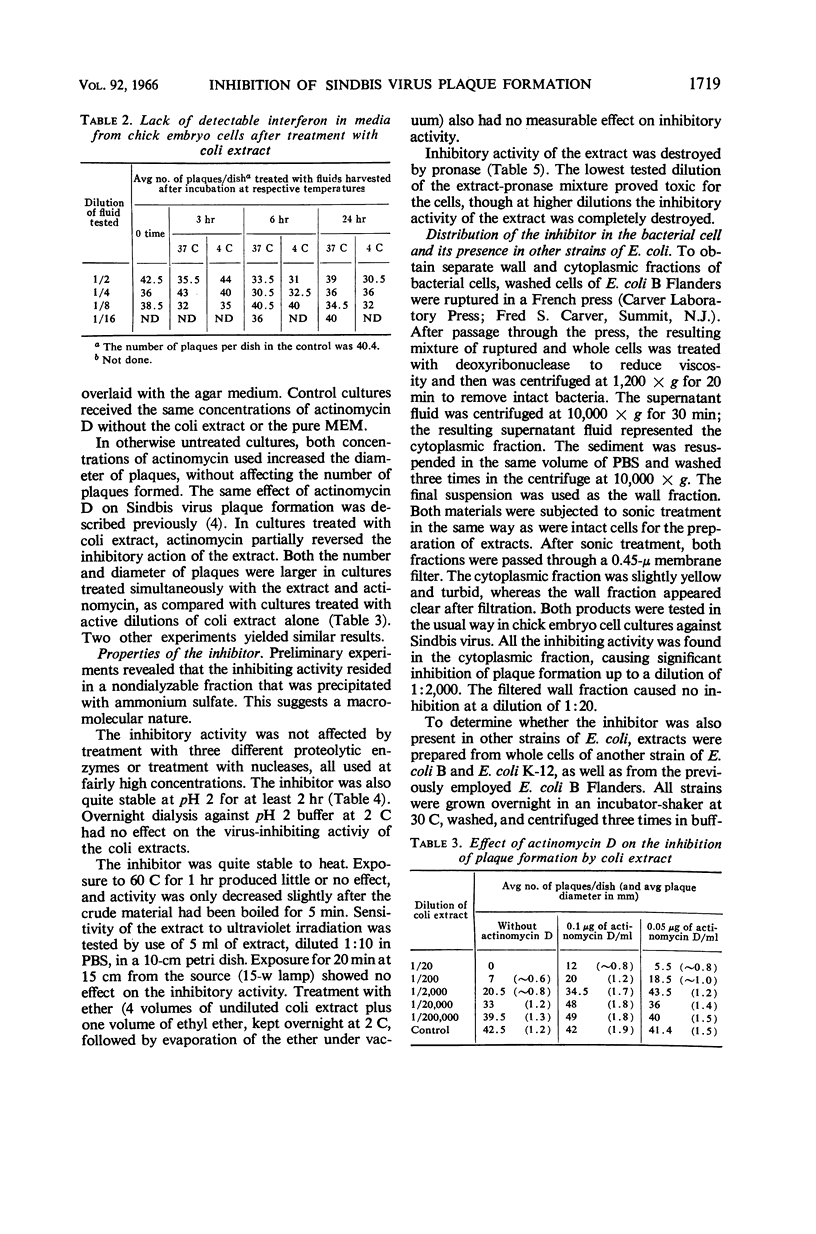
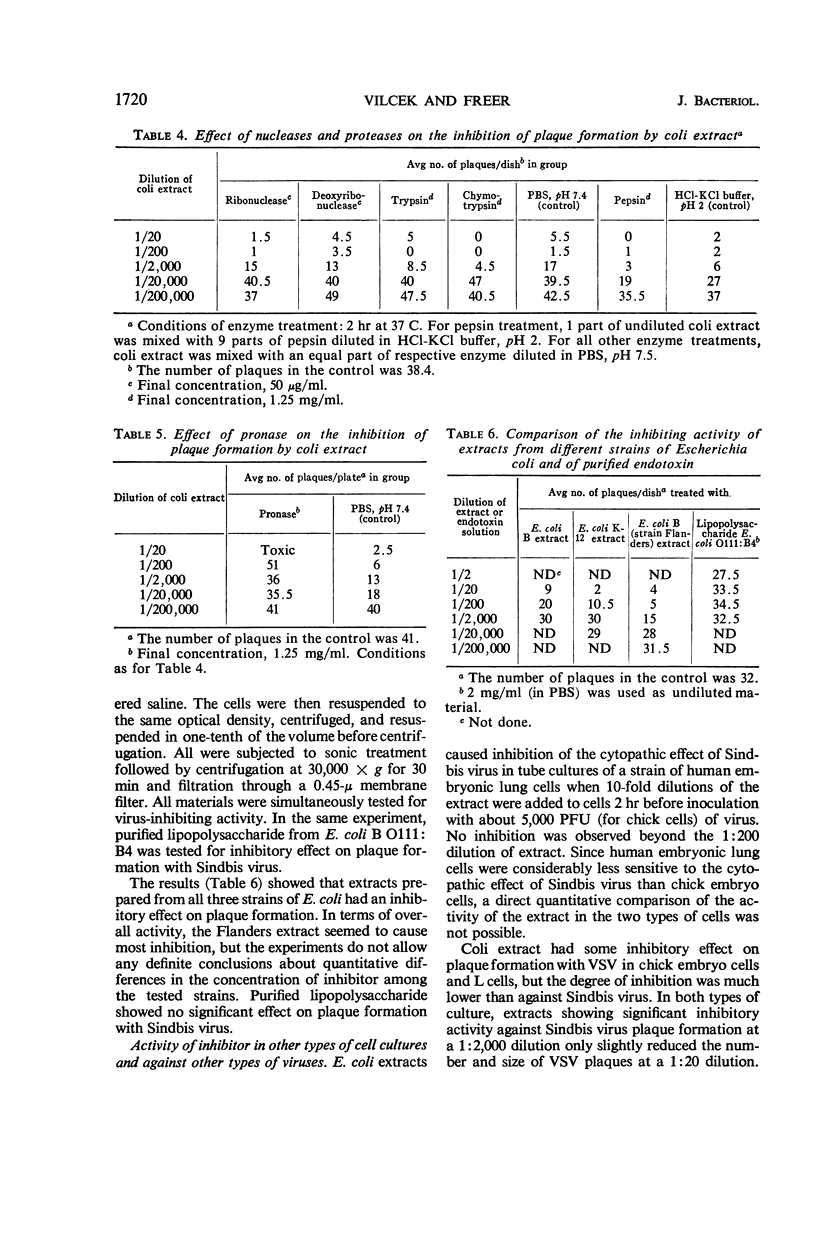
Vilček, Jan (New York University School of Medicine, New York, N.Y.), and John H. Freer. Inhibition of Sindbis virus plaque formation by extracts of Escherichia coli. J. Bacteriol. 92:1716–1722. 1966.—Extracts prepared from washed cells of Escherichia coli B by sonic treatment and subsequent filtration through a 0.45-μ membrane filter significantly inhibited plaque formation with Sindbis virus in cultures or primary chick embryo cells up to a dilution of 1:20,000. The inhibitor acted on the cells rather than directly on the virus. The inhibiting substance was nondialyzable. Treatment of crude extracts with nucleases, trypsin, chymotrypsin, pepsin, or ether had no effect on the activity. Treatment with pronase destroyed the virus-inhibiting effect. Extracts prepared from two strains of E. coli B and one strain of E. coli K-12 all showed inhibitory activity against Sindbis virus. The inhibitor was present in the cytoplasmic fraction of bacteria. It was also active against Sindbis virus in human cells and showed some activity against vesicular stomatitis and vaccinia viruses in different types of cells. Interferon was not shown to be involved in the inhibition, although actinomycin D partially reversed the inhibitory activity of the extracts.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CARVER D. H., NAFICY K. STUDIES ON VIRAL INHIBITORS OF BIOLOGICAL ORIGIN. I. INHIBITION OF VIRAL REPLICATION BY PROTEIN-LIKE CONSTITUENTS OF BACTERIA. Proc Soc Exp Biol Med. 1964 Jun;116:548–552. doi: 10.3181/00379727-116-29303. [DOI] [PubMed] [Google Scholar]
- Cheng P. Y., Shope R. E. The presence in Penicillium funiculosum of an inhibitor to the antiviral agent helenine. J Exp Med. 1966 Mar 1;123(3):505–508. doi: 10.1084/jem.123.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEMAEYER-GUIGNARD J., DEMAEYER E. EFFECT OF CARCINOGENIC AND NONCARCINOGENIC HYDROCARBONS ON INTERFERON SYNTHESIS AND VIRUS PLAQUE DEVELOPMENT. J Natl Cancer Inst. 1965 Feb;34:265–276. doi: 10.1093/jnci/34.2.265. [DOI] [PubMed] [Google Scholar]
- Friedman R. M., Sonnabend J. A. Inhibition of interferon action by puromycin. J Immunol. 1965 Oct;95(4):696–703. [PubMed] [Google Scholar]
- Gresser I., Grogan E. A. Inhibition of arboviruses by a constituent of a staphylococcus. Proc Soc Exp Biol Med. 1965 Aug-Sep;119(4):1176–1181. doi: 10.3181/00379727-119-30407. [DOI] [PubMed] [Google Scholar]
- HELLER E. ENHANCEMENT OF CHIKUNGUNYA VIRUS REPLICATION AND INHIBITION OF INTERFERON PRODUCTION BY ACTINOMYCIN D. Virology. 1963 Dec;21:652–656. doi: 10.1016/0042-6822(63)90239-3. [DOI] [PubMed] [Google Scholar]
- HO M. INTERFERON-LIKE VIRAL INHIBITOR IN RABBITS AFTER INTRAVENOUS ADMINISTRATION OF ENDOTOXIN. Science. 1964 Dec 11;146(3650):1472–1474. doi: 10.1126/science.146.3650.1472. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT W. J., CLINE J. C., MURPHY E. B. INTERFERON PRODUCTION INDUCED BY STATOLON. Proc Natl Acad Sci U S A. 1964 Sep;52:741–744. doi: 10.1073/pnas.52.3.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy H. B., Buckler C. E., Baron S. Effect of interferon on early interferon production. Science. 1966 May 27;152(3726):1274–1276. doi: 10.1126/science.152.3726.1274. [DOI] [PubMed] [Google Scholar]
- NAFICY K., CARVER D. H. "CYCLOPIN": A TRYPSIN SENSITIVE CONSITUENT OF PENICILLIUM CYCLOPIUM WITH ANTIVIRAL PROPERTIES. Proc Soc Exp Biol Med. 1963 Oct;114:175–182. doi: 10.3181/00379727-114-28618. [DOI] [PubMed] [Google Scholar]
- Oh J. O., Gill E. J. Role of interferon-like viral inhibitor in endotoxin-induced corneal resistance to Newcastle disease virus. J Bacteriol. 1966 Jan;91(1):251–256. doi: 10.1128/jb.91.1.251-256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rytel M. W., Shope R. E., Kilbourne E. D. An antiviral substance from Penicillium funiculosum. V. Induction of interferon by helenine. J Exp Med. 1966 Apr 1;123(4):577–584. doi: 10.1084/jem.123.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STINEBRING W. R., YOUNGNER J. S. PATTERNS OF INTERFERON APPEARANCE IN MICE INFECTED WITH BACTERIA OR BACTERIAL ENDOTOXIN. Nature. 1964 Nov 14;204:712–712. doi: 10.1038/204712a0. [DOI] [PubMed] [Google Scholar]
- Taylor J. Inhibition of interferon action by actinomycin. Biochem Biophys Res Commun. 1964;14:447–451. doi: 10.1016/0006-291x(64)90084-1. [DOI] [PubMed] [Google Scholar]
- WAGNER R. R., SNYDER R. M., HOOK E. W., LUTTRELL C. N. Effect of bacterial endotoxin on resistance of mice to viral encephalitides; including comparative studies of the interference phenomenon. J Immunol. 1959 Jul;83(1):87–98. [PubMed] [Google Scholar]



