Abstract
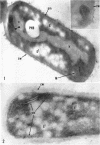
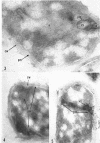
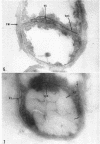
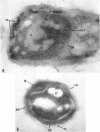
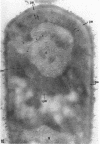
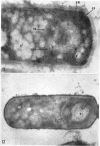
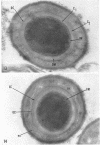
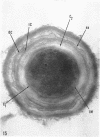
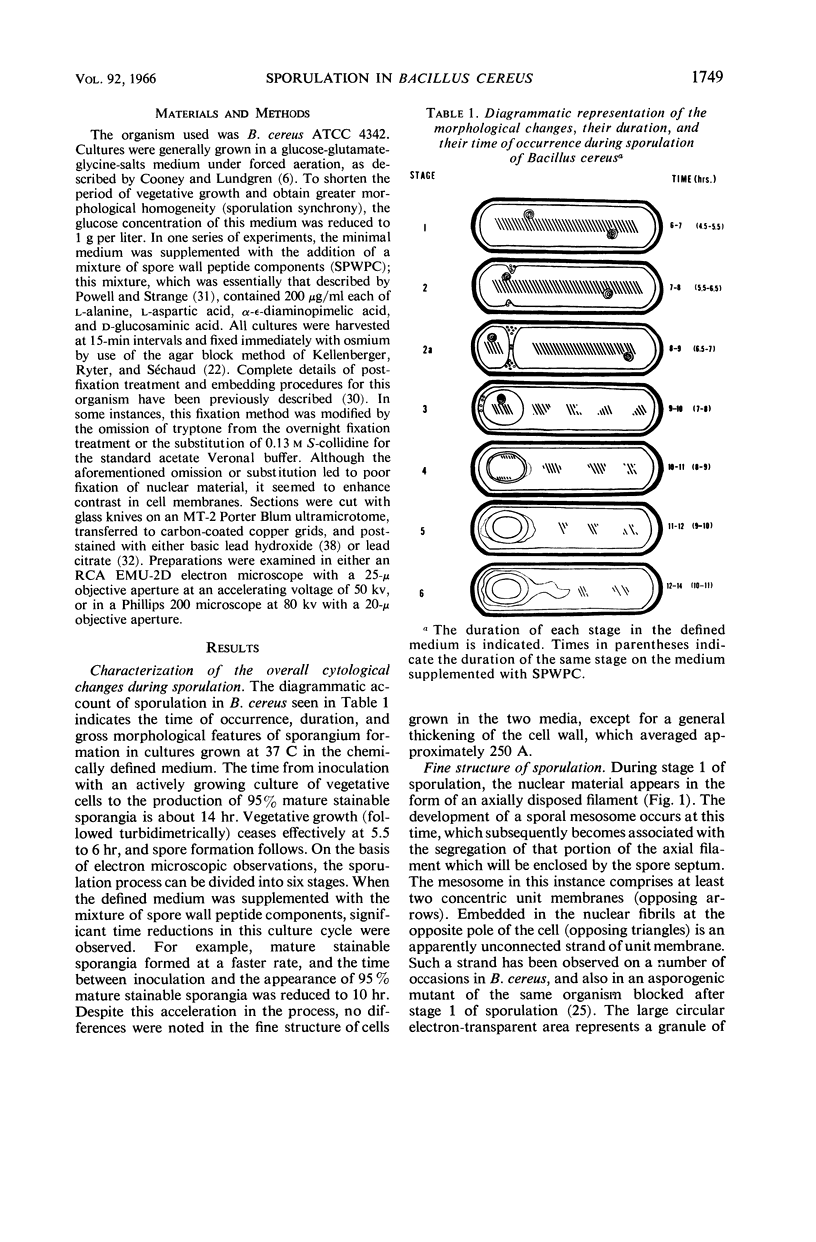
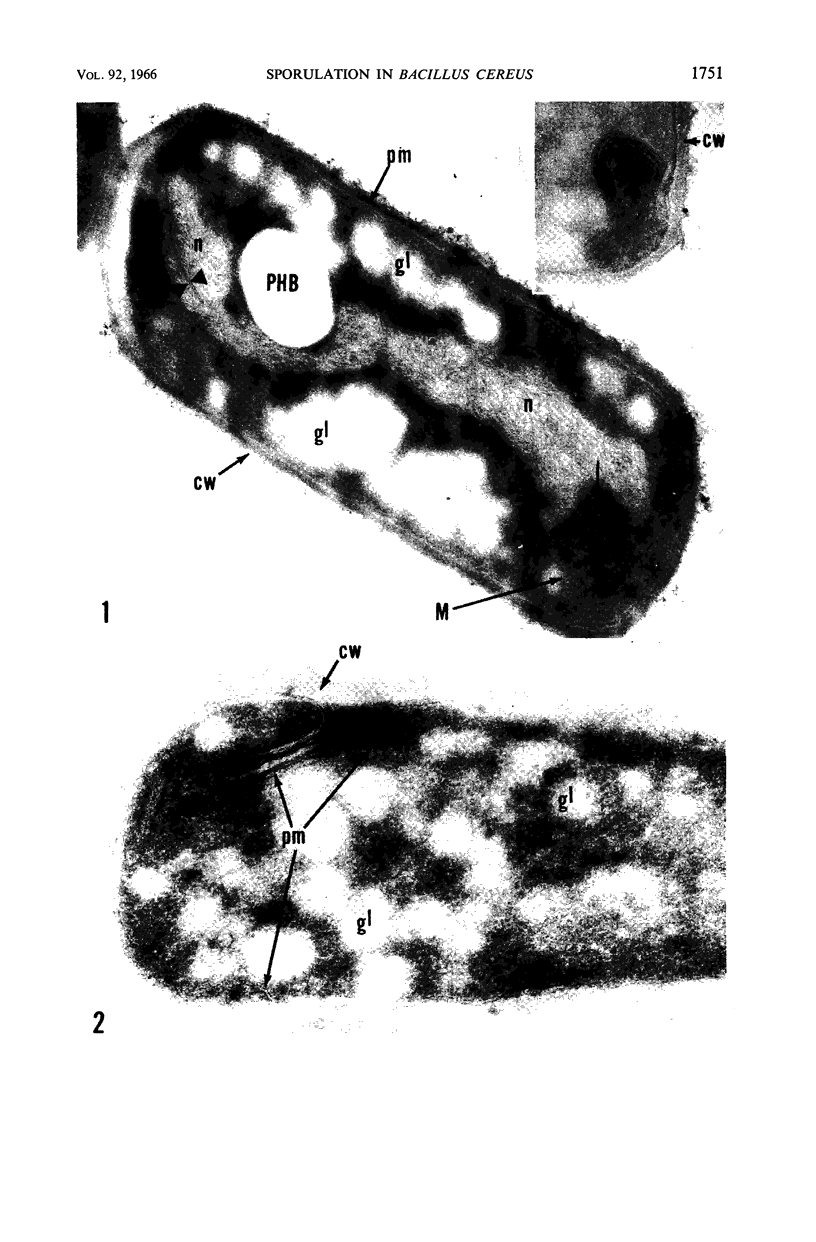
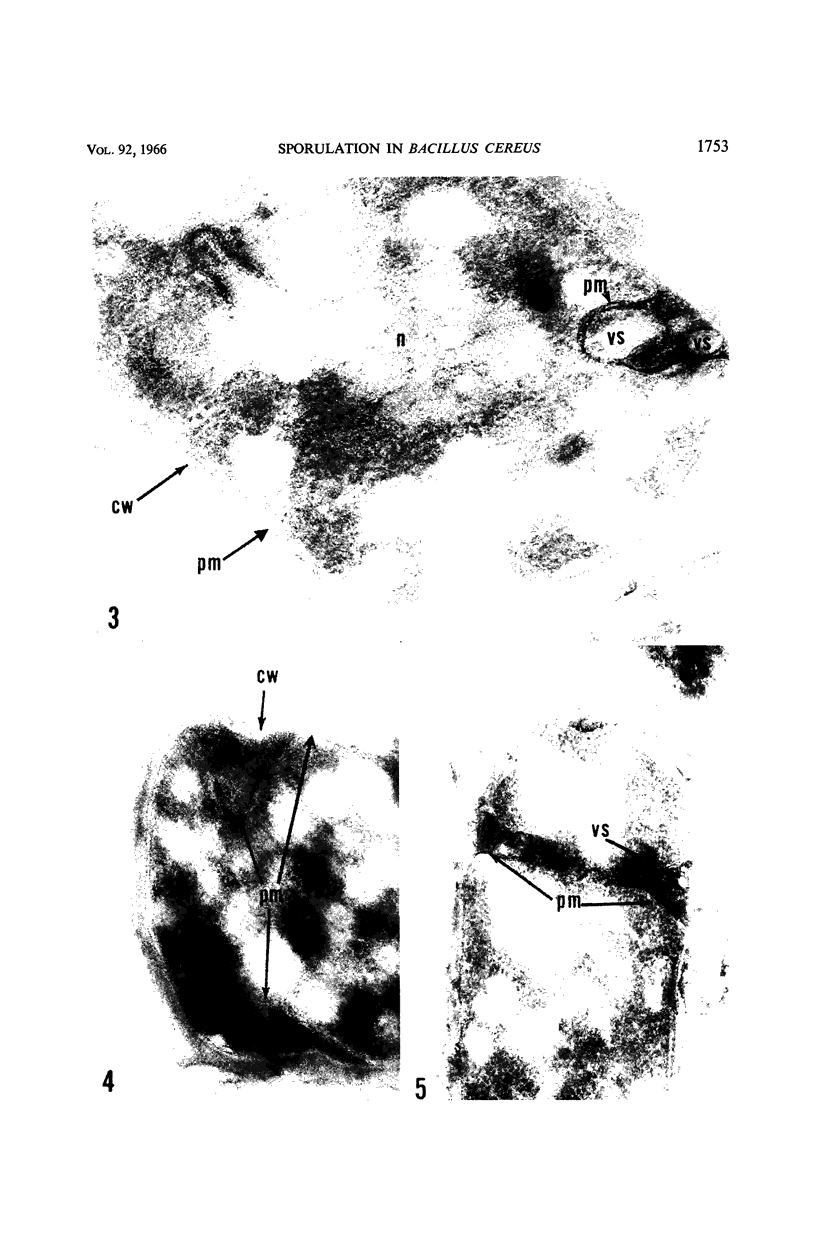
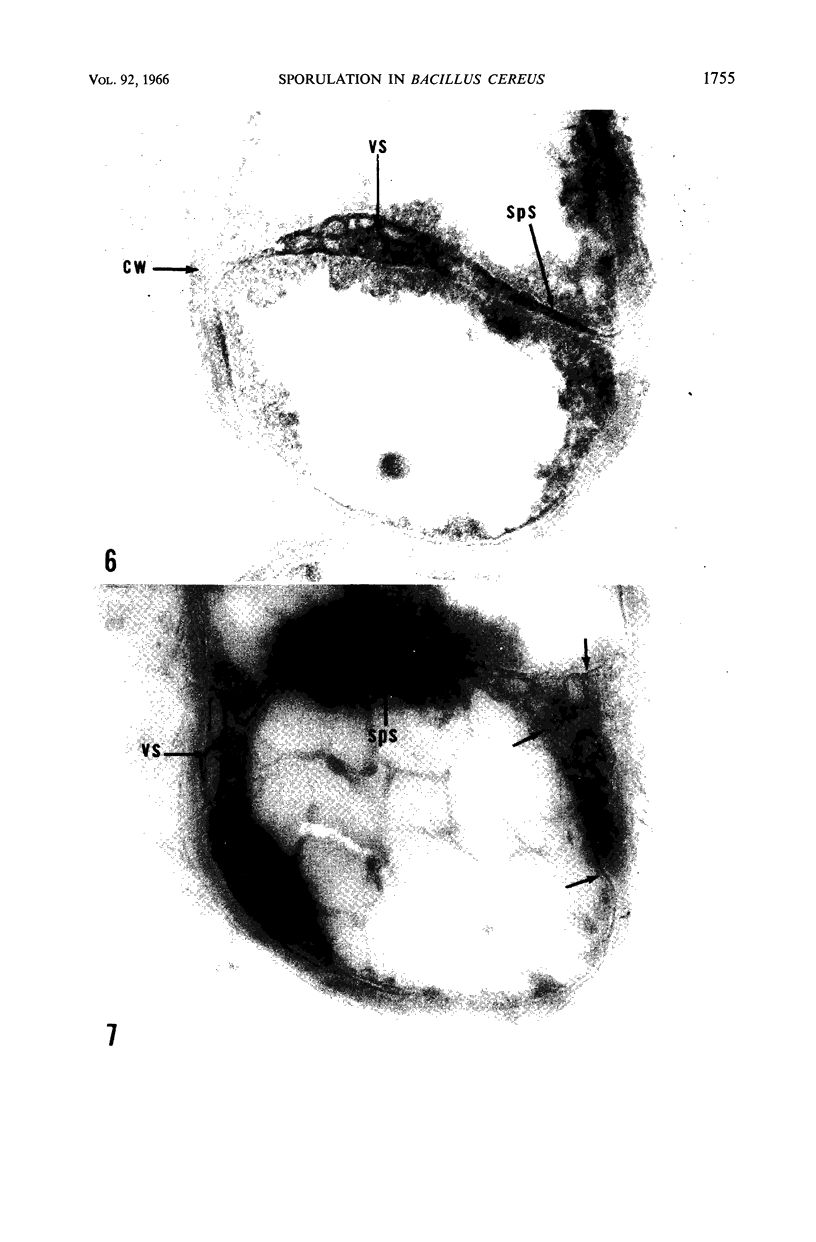
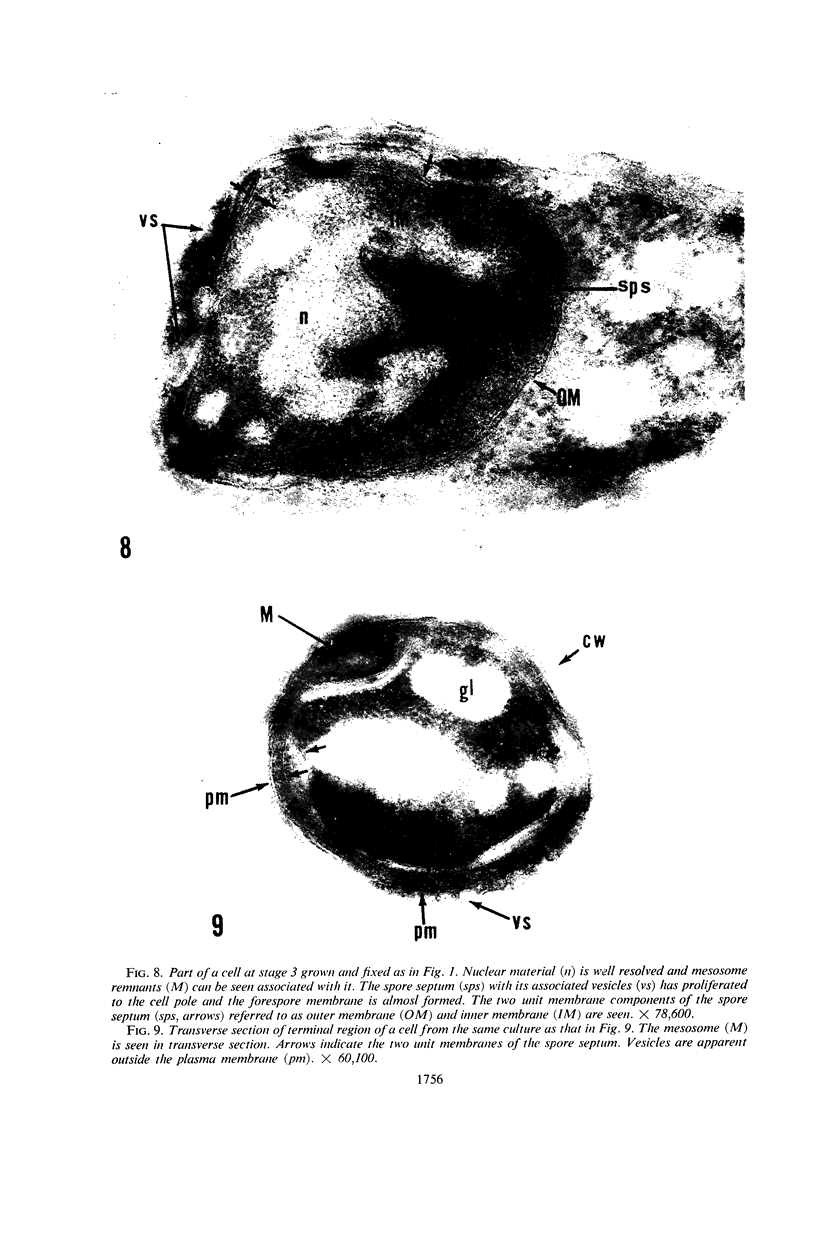
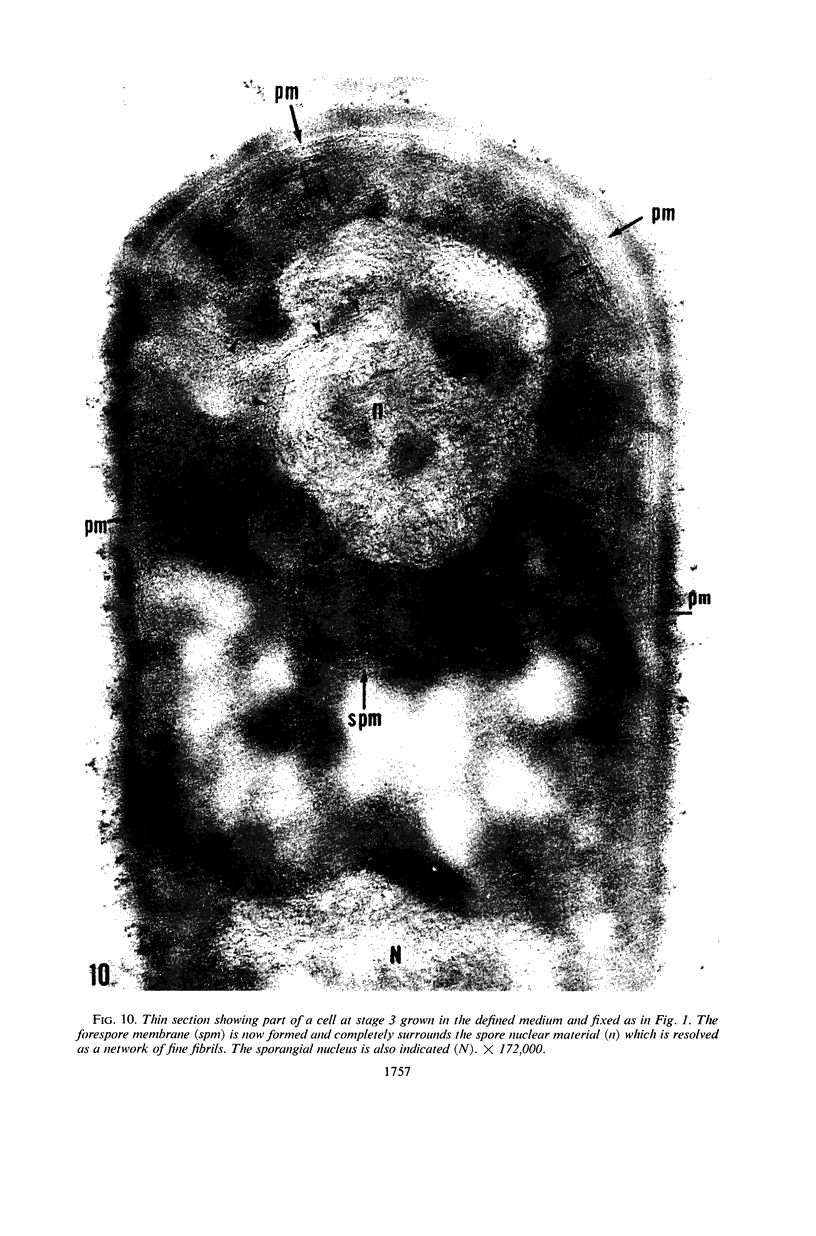
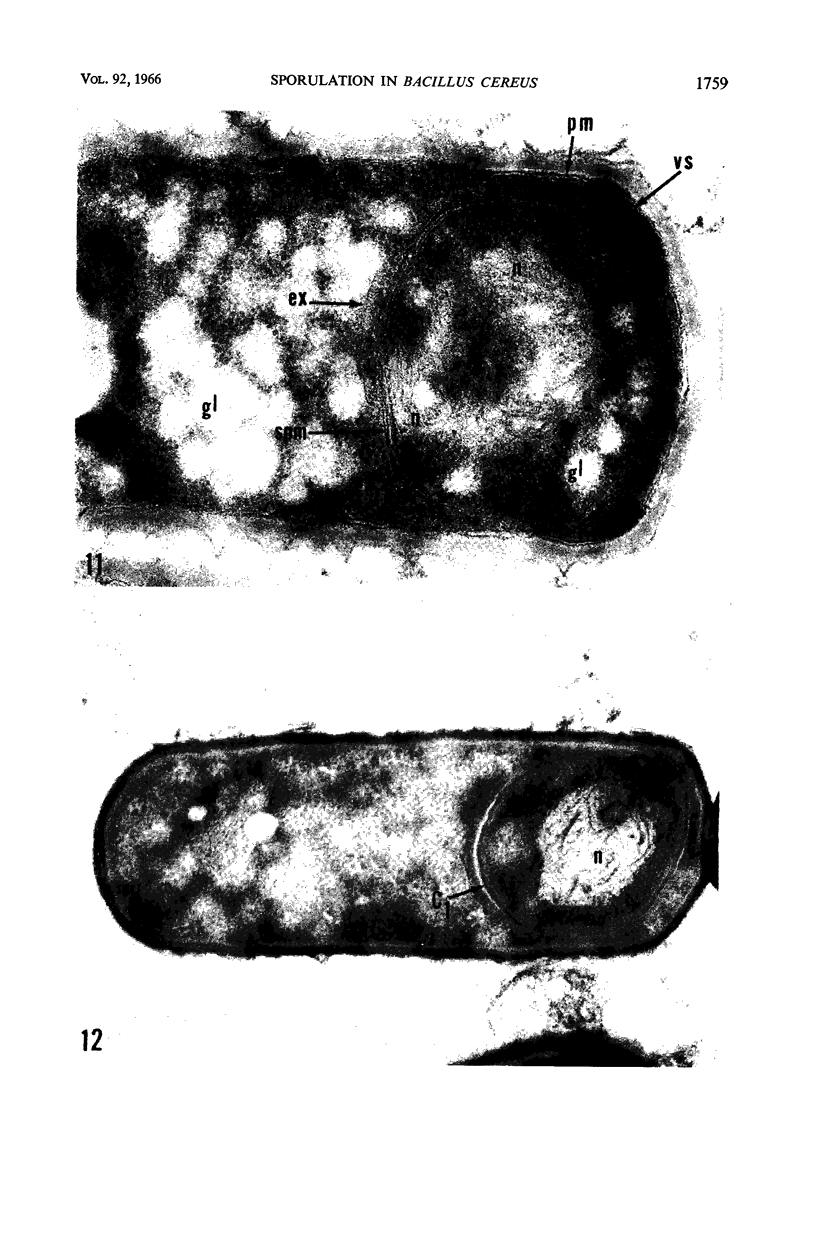
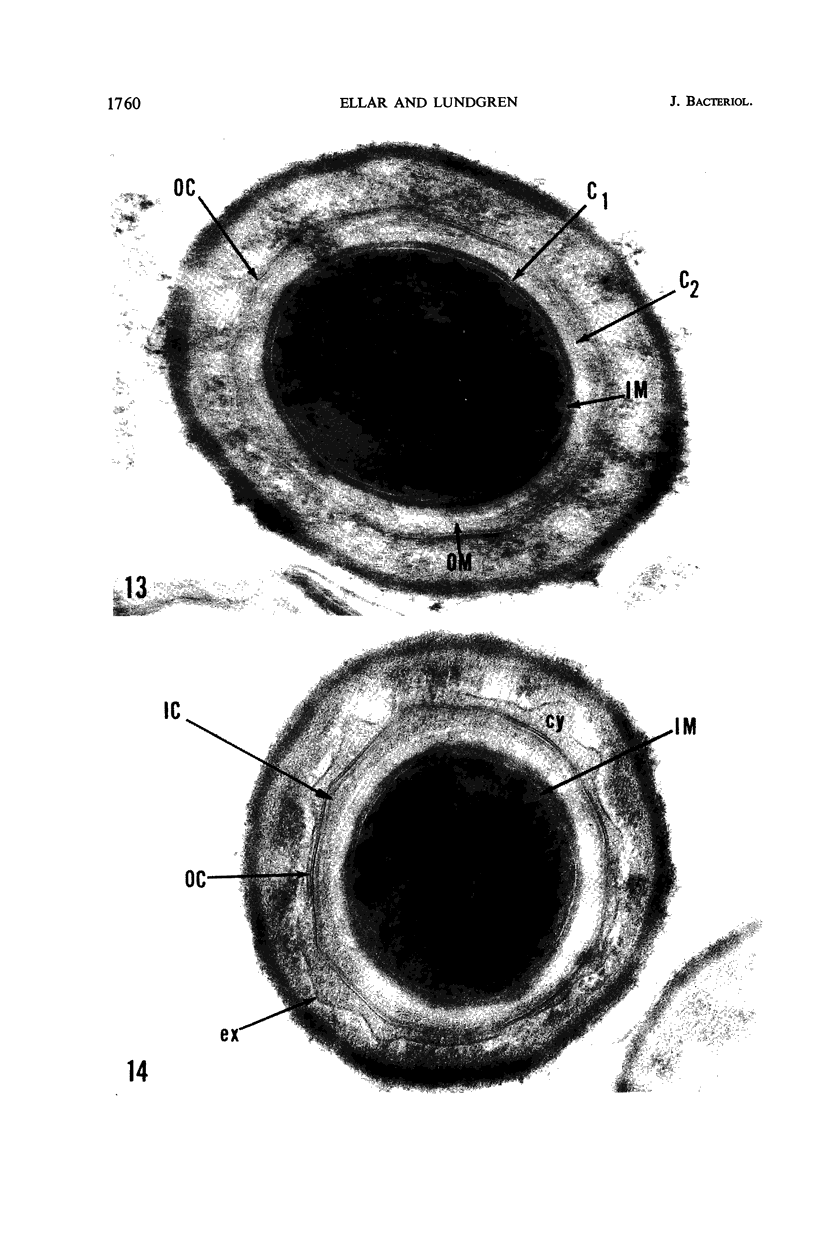
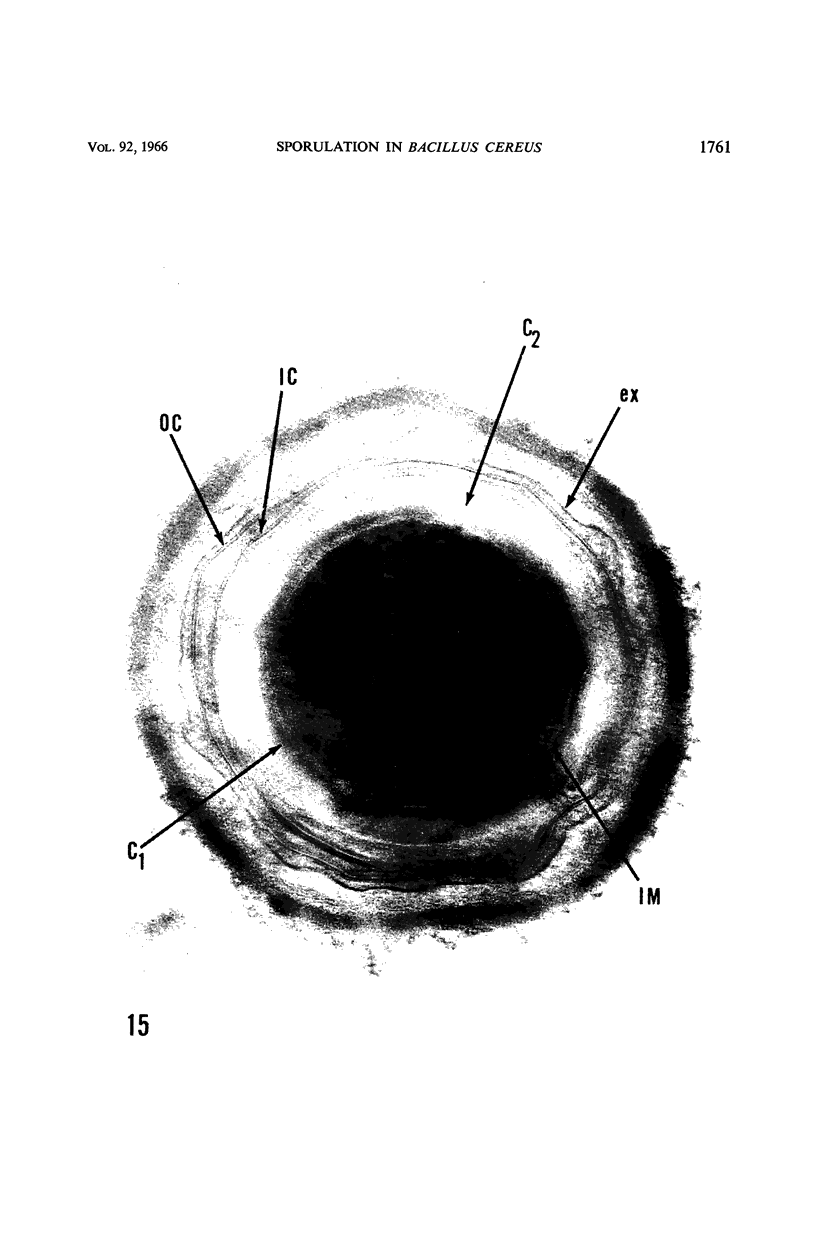
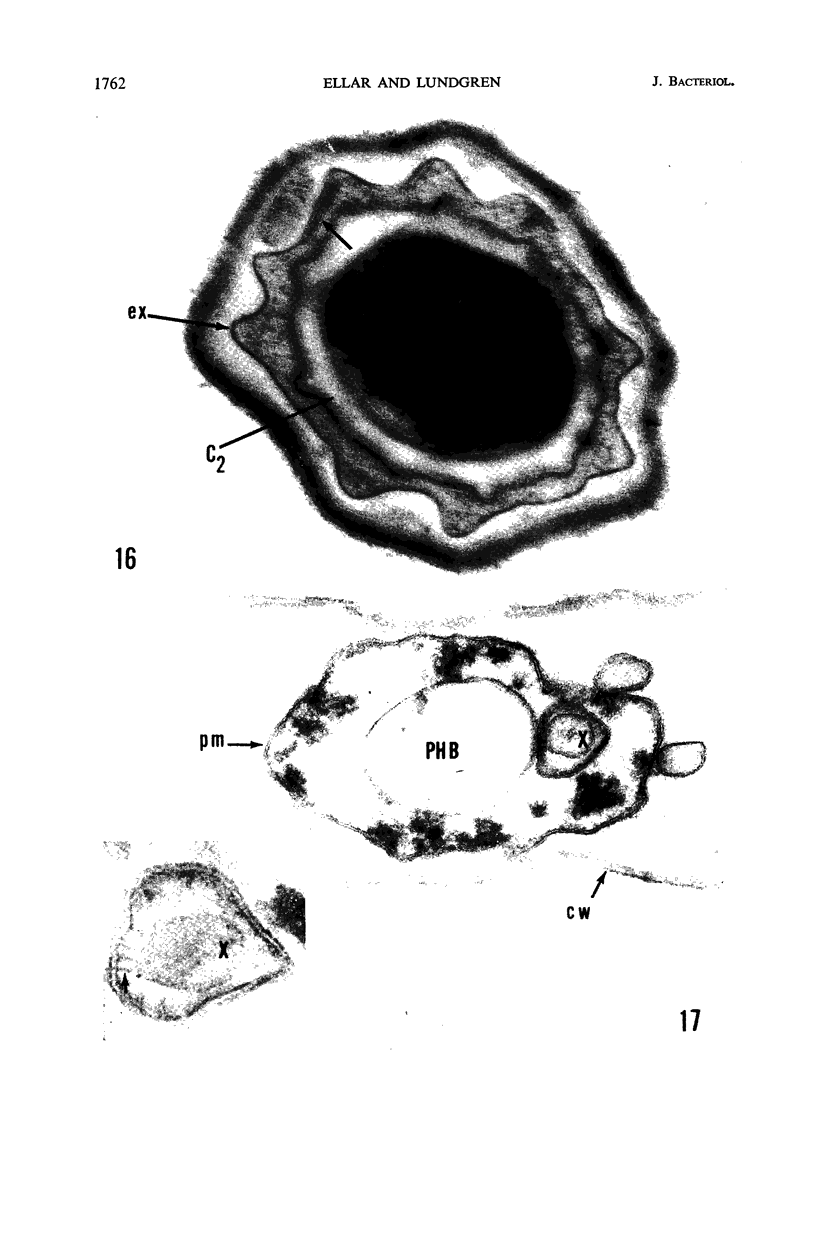
Ellar, D. J. (Syracuse University, Syracuse, N.Y.), and D. G. Lundgren. Fine structure of sporulation in Bacillus cereus grown in a chemically defined medium. J. Bacteriol. 92:1748–1764. 1966.—A study was made of the fine structure of sporulating cells of Bacillus cereus grown in a chemically defined medium. The developmental stages of sporulation occurred in a fairly synchronous manner and were complete by 14 hr. This time period was shortened when spore wall peptide components were added to the medium, but the addition had no effect upon fine structure except to thicken the cell wall. Sporulation could be separated into six morphological stages which generally agreed with those published for other sporulating bacteria. The initiation of the spore (forespore) septum takes the form of an inward folding of the cytoplasmic membrane toward the pole of the cell. The inward folding forms a characteristic Y-shaped membrane structure enclosing an area within which vesicles are found. These vesicles comprise the perisporal mesosome of the cell. The membranes on opposite sides of the cell progress toward the cell center where they fuse to form the double unit membrane of the spore septum. As the proliferation of the spore septum continues, the vesicular areas move towards the pole. The end result is a double forespore membrane which completely encloses a part of the vegetative cell's chromatin. Sporal mesosomes, as well as membrane vesicles, are involved in the proliferation of the forespore. Vesicles are generally bounded by a single unit membrane, whereas in the sporal mesosomes several unit membranes are arranged concentrically. The latter become associated with the segregation of a portion of the nuclear material into the forespore region of the cell.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAM D. ELECTRON MICROSCOPE OBSERVATIONS ON INTACT CELLS, PROTOPLASTS, AND THE CYTOPLASMIC MEMBRANE OF BACILLUS STEAROTHERMOPHILUS. J Bacteriol. 1965 Mar;89:855–873. doi: 10.1128/jb.89.3.855-873.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono F., Testa R., Lundgren D. G. Physiology of growth and sporulation in Bacillus cereus. I. Effect of glutamic and other amino acids. J Bacteriol. 1966 Jun;91(6):2291–2299. doi: 10.1128/jb.91.6.2291-2299.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPMAN G. B., KROLL A. J. Electron microscopy of ultrathin sections of Spirillum serpens. J Bacteriol. 1957 Jan;73(1):63–71. doi: 10.1128/jb.73.1.63-71.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONTI S. F., HIRSCH P. BIOLOGY OF BUDDING BACTERIA. 3. FINE STRUCTURE OF RHODOMICROBIUM AND HYPHOMICROBIUM SPP. J Bacteriol. 1965 Feb;89:503–512. doi: 10.1128/jb.89.2.503-512.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DE PETRIS S., KARLSBAD G., KESSEL R. W. THE ULTRASTRUCTURE OF S AND R VARIANTS OF BRUCELLA ABORTUS GROWN ON A LIFELESS MEDIUM. J Gen Microbiol. 1964 Jun;35:373–382. doi: 10.1099/00221287-35-3-373. [DOI] [PubMed] [Google Scholar]
- DE ROBERTIS E. D., BENNETT H. S. A submicroscopic vesicular component of Schwann cells and nerve satellite cells. Exp Cell Res. 1954 May;6(2):543–545. doi: 10.1016/0014-4827(54)90209-8. [DOI] [PubMed] [Google Scholar]
- EDWARDS M. R., STEVENS R. W. FINE STRUCTURE OF LISTERIA MONOCYTOGENES. J Bacteriol. 1963 Sep;86:414–428. doi: 10.1128/jb.86.3.414-428.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. C. Participation of the cytoplasmic membrane in the growth and spore fromation of bacilli. J Biophys Biochem Cytol. 1960 Oct;8:507–528. doi: 10.1083/jcb.8.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. FATE OF THE MESOSOMES OF BACILLUS MEGATERIUM DURING PROTOPLASTING. J Bacteriol. 1964 Jun;87:1483–1491. doi: 10.1128/jb.87.6.1483-1491.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P. C. DISCUSSION. Bacteriol Rev. 1965 Sep;29(3):293–298. doi: 10.1128/br.29.3.293-298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitz-James P., Hancock R. The initial structural lesion of penicillin action in Bacillus megaterium. J Cell Biol. 1965 Aug;26(2):657–667. doi: 10.1083/jcb.26.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERHARDT P., RIBI E. ULTRASTRUCTURE OF THE EXOSPORIUM ENVELOPING SPORES OF BACILLUS CEREUS. J Bacteriol. 1964 Dec;88:1774–1789. doi: 10.1128/jb.88.6.1774-1789.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLAUERT A. M., BRIEGER E. M., ALLEN J. M. The fine structure of vegetative cells of Bacillus subtilis. Exp Cell Res. 1961 Jan;22:73–85. doi: 10.1016/0014-4827(61)90087-8. [DOI] [PubMed] [Google Scholar]
- Gibson K. D. Electron microscopy of chromatophores of Rhodopseudomonas spheroides. J Bacteriol. 1965 Oct;90(4):1059–1072. doi: 10.1128/jb.90.4.1059-1072.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLME T., CEDERGREN B. Demonstration of intracellular polysaccharide in Escherichia coli by electron microscopy and by cytochemical methods. Acta Pathol Microbiol Scand. 1961;51:179–186. doi: 10.1111/j.1699-0463.1961.tb00357.x. [DOI] [PubMed] [Google Scholar]
- IMAEDA T., OGURA M. Formation of intracytoplasmic membrane system of mycobacteria related to cell division. J Bacteriol. 1963 Jan;85:150–163. doi: 10.1128/jb.85.1.150-163.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KESSEL R. G. Electron microscope observations on the submicroscopic vesicular component of the subesophageal body and pericardial cells of the grasshopper, Melanoplus differentialis differentialis (Thomas). Exp Cell Res. 1961 Jan;22:108–119. doi: 10.1016/0014-4827(61)90090-8. [DOI] [PubMed] [Google Scholar]
- KUSHNAREV V. M., PEREVERZEV N. A. THE MEMBRANES IN ESCHERICHIA COLI CELLS. J Ultrastruct Res. 1964 Jun;10:610–614. doi: 10.1016/s0022-5320(64)80034-4. [DOI] [PubMed] [Google Scholar]
- Lundgren D. G., Remsen C. C. Fine structure of an asporogenic mutant of Bacillus cereus. J Bacteriol. 1966 May;91(5):2096–2098. doi: 10.1128/jb.91.5.2096-2098.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. H. Bacterial protoplasts--a review. J Theor Biol. 1963 Jul;5(1):1–34. doi: 10.1016/0022-5193(63)90034-1. [DOI] [PubMed] [Google Scholar]
- ODOR D. L. Uptake and transfer of particulate matter from the peritoneal cavity of the rat. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):105–108. doi: 10.1083/jcb.2.4.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHYE D. F., MURRELL W. G. Formation and structure of the spore of Bacillus coagulans. J Cell Biol. 1962 Jul;14:111–123. doi: 10.1083/jcb.14.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFISTER R. M., LUNDGREN D. G. ELECTRON MICROSCOPY OF POLYRIBOSOMES WITHIN BACILLUS CEREUS. J Bacteriol. 1964 Oct;88:1119–1129. doi: 10.1128/jb.88.4.1119-1129.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWELL J. F., STRANGE R. E. Alpha-Epsilon-Diaminopimelic acid metabolism and sporulation in Bacillus sphaericus. Biochem J. 1957 Apr;65(4):700–708. doi: 10.1042/bj0650700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A. ETUDE MORPHOLOGIQUE DE LA SPORULATION DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1965 Jan;108:40–60. [PubMed] [Google Scholar]
- RYTER A., LANDMAN O. E. ELECTRON MICROSCOPE STUDY OF THE RELATIONSHIP BETWEEN MESOSOME LOSS AND THE STABLE L STATE (OR PROTOPLAST STATE) IN BACILLUS SUBTILIS. J Bacteriol. 1964 Aug;88:457–467. doi: 10.1128/jb.88.2.457-467.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTON M. R., CHAPMAN J. A. Isolation of the membranemesosome structures from Micrococcus lysodeikticus. J Ultrastruct Res. 1962 Jun;6:489–498. doi: 10.1016/s0022-5320(62)80004-5. [DOI] [PubMed] [Google Scholar]
- VAN ITERSON, LEENE W. A CYTOCHEMICAL LOCALIZATION OF REDUCTIVE SITES IN A GRAM-POSITIVE BACTERIUM. TELLURITE REDUCTION IN BACILLUS SUBTILIS. J Cell Biol. 1964 Mar;20:361–375. doi: 10.1083/jcb.20.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOUNG I. E., JAMES P. C. Chemical and morphological studies of bacterial spore formation. IV. The development of spore refractility. J Cell Biol. 1962 Jan;12:115–133. doi: 10.1083/jcb.12.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]