Abstract
Background
Adverse drug reactions (ADRs) contribute to ill-health or life-threatening outcomes of therapy during management of infectious diseases. The exposure to anti-malarial and use of mobile phone technology to report ADRs following drug exposures were investigated in Sagamu - a peri-urban community in Southwest Nigeria.
Methods
Purchase of medicines was actively monitored for 28 days in three Community Pharmacies (CP) and four Patent and Proprietary Medicine Stores (PPMS) in the community. Information on experience of ADRs was obtained by telephone from 100 volunteers who purchased anti-malarials during the 28-day period.
Results and Discussion
A total of 12,093 purchases were recorded during the period. Antibiotics, analgesics, vitamins and anti-malarials were the most frequently purchased medicines. A total of 1,500 complete courses of anti-malarials were purchased (12.4% of total purchases); of this number, purchases of sulphadoxine-pyrimethamine (SP) and chloroquine (CQ) were highest (39.3 and 25.2% respectiuvely). Other anti-malarials purchased were artesunate monotherapy (AS) - 16.1%, artemether-lumefantrine (AL) 10.0%, amodiaquine (AQ) - 6.6%, quinine (QNN) - 1.9%, halofantrine (HF) - 0.2% and proguanil (PR) - 0.2%. CQ was the cheapest (USD 0.3) and halofantrine the most expensive (USD 7.7). AL was 15.6 times ($4.68) more expensive than CQ. The response to mobile phone monitoring of ADRs was 57% in the first 24 hours (day 1) after purchase and decreased to 33% by day 4. Participants in this monitoring exercise were mostly with low level of education (54%).
Conclusion
The findings from this study indicate that ineffective anti-malaria medicines including monotherapies remain widely available and are frequently purchased in the study area. Cost may be a factor in the continued use of ineffective monotherapies. Availability of a toll-free telephone line may facilitate pharmacovigilance and follow up of response to medicines in a resource-poor setting.
Background
Infection with malaria parasites remains one of the leading causes of hospital visits in Nigeria. Half of the estimated population of 150 million experience at least one malarial attack each year [1]. Early diagnosis and treatment of malaria is well recognized as an effective means of reducing morbidity and mortality of the infection [2]. Chloroquine (CQ) and sulphadoxine-pyrimethamine were cheap and effective treatment for malaria introduced in the middle of the 20th century. Unfortunately emergence of Plasmodium falciparum resistance to these drugs in South East Asia and eventual spread to all malaria endemic areas compromised malaria treatment of falciparum malaria in several malaria endemic countries, including Nigeria [3] Artemisinin-based combination therapy (ACT) was subsequently recommended by the WHO and introduced as part of a multi-component strategy to mitigate the malaria burden [2-4]. As ACT become more widely available and accessible, there is a need to assess and document experience and occurrence of adverse drug reactions (ADRs) of these drugs when used in larger populations or communities [5]. In the industrialized economies of the northern hemisphere, relatively robust health systems with capability for systematic acquisition of post marketing data exist. However, in sub-Saharan Africa, the treatment-seeking pattern for maladies including malaria is complex and impacted by the local health system, culture and resources of the patient [6,7]. Anti-malarials are often purchased from private retail sector without a confirmed diagnosis of malaria. These vendors have been identified as an important source of medicines and health care close to people's homes [8] and thus complement formal health services. Information on the volume and types of medicine purchased from these vendors and specifically the level of exposure to anti-malarials is not available. Establishment of a simple practical system for surveillance from private drug outlets will be a useful tool for pharmacovigilance and tracking possible adverse drug reactions. With the recent introduction of ACT and distribution of millions of doses in sub-Saharan Africa, there is concern about adequately monitoring ADR in the population [9]. This study was designed to assess exposure to anti-malarial drugs in Sagamu community and to explore the use of widely available technology as a tool to monitor ADRs following exposure of malaria patients to antimalarial drugs.
Methods
Study setting
Sagamu, located 50 km north of Lagos is the seat of the Sagamu Local Government Area, Ogun State in south-west Nigeria. The town is spread over 614 Km2 (237/Sqm) with an estimated population of 228,382. A large proportion of the population commutes to the city of Lagos daily for work or other commercial activities. Malaria is highly endemic in the area, accounting for most outpatient visits in the health facilities. Transmission occurs all year around with an upsurge in the rainy season - June to September [10]. The community is served by several schools, hospitals (Primary Health Care Centers, Private and Tertiary Hospitals), banks and hotels.
Selection of study centers
Study centers were identified and selected from the list of registered pharmacies and Patent and Proprietary Medicine Stores (PPMS) compiled by the Department of Pharmaceutical Services, Ministry of Health, Ogun State. There are 128 PPMS and 13 pharmacies listed in the state's official records. The patient load in these facilities record average of 225 per day; (pharmacies, range 150-400 per day; PPMS, 35-50 per day).
From the 141 drug outlets a sample size of seven (three pharmacies and four PPMS) was determined at confidence interval of 50% and confidence level of 95% using Survey System sample size calculator http://www.surveysystem.com/sscalc.htm. The specific facilities were chosen using computer generated simple randomization procedure.
The study was descriptive and prospective, and involved active follow-up of sales of drugs in the selected centres for a period of four weeks (28 days) to determine the exposure to anti-malarial drug, measured by the amount of drugs purchased within the period. A drug-data chart was designed to capture information on category of drugs purchased. The proportion of anti-malarial of the different drugs purchased at the drug retail outlets, which at the time of study were not tracked by any active surveillance method, and sales per week of the medicines were determined. The anti-malarial drugs were classified to assess the drug use pattern for the different anti-malarial drugs and the rate of purchase between PPMS and the community pharmacies. The retail prices of the different anti-malarial drugs in a complete dosage pack were recorded.
The willingness of the buyers of the anti-malarial drugs in this community to participate in pharmacovigilance (PV), through a toll free mobile phone monitoring of adverse drug reactions, was evaluated in a total of 100 subjects who presented at the study sites to purchase anti-malarial drugs, volunteered to participate and provided information via mobile phones over a period of two weeks following purchase of medicine. The mobile phones were used to provide information on any ADRs experienced following ingestion of the anti-malarial drug, particularly ACT.
Prior to enrolment in the mobile phone ADR monitoring, the objective was explained to each of subjects after drug purchase was completed by a member of the study team and informed consent obtained.
The mobile phone number of the participant was obtained on permission and entered into a registration form. One member of the study team called the mobile number to validate correctness of entry and connectivity. A series of questions were prepared to inquire about any complaint or reactions observed by the subjects to whom the drug is administered, if adult, or a caregiver when subject is a child. Three of the investigators (SB, TA, AM) were involved in the documentation of any complaints or observations following drug use. Each participant is called from mobile phone designated for the exercise by a member of the study team. The participants mobile phones were called at specified times to record time of use of drug and 6-hourly for the first three days and 24 hourly from day 3 to day 14. All complaints or observations were recorded.
Data collection
The pharmacies and medicine stores operate on an average of 13 hours daily (09.00 hr - 22.00 hr). Drugs purchased are recorded daily to monitor sales and to prevent stock-outs. The data on anti-malaria drug sales was collected daily from the outlets in the 28 days and transferred into a computer database. Information provided during mobile phone calls were transferred to forms designed for the purpose.
Following compilation of mobile phone interaction with patients or caregivers, each complaint or observation was examined and determined qualified for ADR. A complaint or response to inquiries by one of the research team member on phone is categorized as ADR from a patient if it qualifies to be 'any response to the drug which is noxious and unintended, and that occurs at doses normally used in man for prophylaxis, diagnosis, or therapy of diseases or modification of physiological function'; and an adverse event if 'any untoward medical occurrence present in a patient administered the medicine and which does not necessarily have to have causal relation with the treatment'.
Data entry and analysis
Generic names, brand names (if possible), pharmacological classifications, collection centre, date of collection and day of study (0-27 days) were entered into a Database. The data generated were analysed using SPSS version 10 for Windows (SPSS Inc, Chicago USA). Chi-square analysis was used to compare proportions and linear regression analysis was used to obtain relationship between responses and duration of follow up for the monitoring aspect of this study.
Ethics
Ethical approval for the study was obtained from the Joint Ethics Committee of the Olabisi Onabanjo University Teaching Hospital and Obafemi Awolowo College of Health Science, Olabisi Onabanjo University, Sagamu.
Results
The study was carried out between May-September 2010. In the seven drug retail outlets, the distribution of drugs purchased in this community in the period of the study is shown in Table 1. Analgesics (15.8%). antibiotics (15.2%), vitamin supplements (13.3%), and anti-malarials (12.4%), respectively, had the highest frequencies of purchased drugs at these retail outlets. A total of 10,581 (87.5%) complete treatment dosages were sold at the pharmacies, while 1,512 (12.5%) complete treatment dosages were sold at the PPMS.
Table 1.
Distribution of drugs purchased in the 4 weeks of drug surveillance in the retail outlets studied
| Classes | Complete treatment dosages | Percentage (%) |
|---|---|---|
| Analgesics | 1916 | 15.8 |
| Antibiotics | 1837 | 15.2 |
| Anti-malarials | 1500 | 12.4 |
| Haematinics | 870 | 7.2 |
| Herbal preparations | 71 | 0.6 |
| NSAIDs | 1273 | 10.5 |
| Vitamins | 1612 | 13.3 |
| Others | (1-269)* | 0.0-2.2 |
* = Purchase of other classes of drugs (e.g antidiabetics, haematinics and hormonal drugs) from these drug outlets ranges from 1 to 269 sales.
Drug sales
Of 10,581 complete treatment dosage drugs purchased at the pharmacies, 10.7% were anti-malarials. Sales of anti-malarials at the PPMS were 23.7% of complete treatment dosages (1,521) sold. The weekly sales of anti-malarial and other categories of drugs from these outlets are shown in Table 2. On average, 375 complete treatment dosages of anti-malarial drugs were purchased weekly.
Table 2.
Complete treatment dosages of anti-malarial drugs purchased per week during the active drug surveillance in the retail outlets
| Weeks | Anti-malarials | Antibiotics | Analgesics | Vitamins | Total drug dosage consumed |
Percentage (%) |
|---|---|---|---|---|---|---|
| 1 | 434 | 443 | 476 | 455 | 3088 | 25.5 |
| 2 | 468 | 454 | 530 | 427 | 3208 | 26.6 |
| 3 | 365 | 556 | 432 | 431 | 3356 | 27.8 |
| 4 | 233 | 384 | 478 | 299 | 2441 | 20.2 |
Types of anti-malarial and frequency of purchase
The various anti-malarial drugs that were available and frequently purchased from the study drug outlets were sulphadoxine-pyrimethamine (SP), chloroquine (CQ), artesunate (ART), artemether-lumefantrine (AL), amodiaquine (AQ), quinine (QN), herbal preparations (HP), halofantrine (HF), and proguanil (PR). Table 3 shows the distribution of anti-malarial drugs purchased as complete treatment dosage from the private retail drug outlets studied. The PPMS recorded higher purchases of SP (44.5%) and CQ (29.8%) compared to community pharmacies which had 37.7% and 23.7% for SP and CQ, respectively. However, artesunate (monotherapy) purchase was higher at community pharmacies compared with PPMS (18.1% vs. 9.7%, χ2 = 13.6, P = 0.0002).
Table 3.
The distribution of anti-malarial drugs purchased (in packs of a complete treatment dosage) from the private retail drug outlets studied
| Anti-malarial drugs | Community Pharmacies (%) N = 1141 |
PPMS (%) N = 359 |
|---|---|---|
| Sulphadoxine-pyrimethamine | 430 (37.7) | 160 (44.6) |
| Chloroquine | 271(23.7) | 107 (29.8) |
| Artesunate | 207(18.1) | 35 (9.7) |
| Artemether-Lumefantrine | 117(10.3) | 33 (9.2) |
| Amodiaquine | 75 (6.6) | 24 (6.7) |
| Quinine | 28 (2.4) | 0 (0) |
| Herbal preparations | 7 (0.6) | 0 (0) |
| Halofantrine | 2 (0.1) | 1 (0.2) |
| Proguanil | 3 (0.2) | 0 (0) |
PPMS- Patent and Proprietary Medicine Stores
On average, artesunate was the most purchased anti-malarial at the pharmacies each week of the study period, but the least purchased at the PPMS. CQ was however the least purchased at the community pharmacies while it was the most purchased at the PPMS. The retail price per dose of anti-malarial drug (Table 4) varied with CQ being the cheapest and halofantrine, the most expensive.
Table 4.
Cost of anti-malarial drugs at the time of the present study in the retail outlets
| S/N | DRUGS | PRICES | |
|---|---|---|---|
| NAIRA (N) | DOLLAR ($) | ||
| 1 | Sulphadoxine-pyrimethamine | 140 | 0.9 |
| 2 | Chloroquine | 50 | 0.3 |
| 3 | Artesunate | 280 | 1.9 |
| 4 | Artemether-Lumefantrine | 700 | 4.7 |
| 5 | Amodiaquine | 150 | 1 |
| 6 | Quinine | 90 | 0.6 |
| 7 | Herbal preparations | 450 | 3 |
| 8 | Halofantrine | 1,150 | 7.7 |
| 9 | Proguanil | 500 | 3.3 |
Mobile phone for monitoring of adverse drug reaction
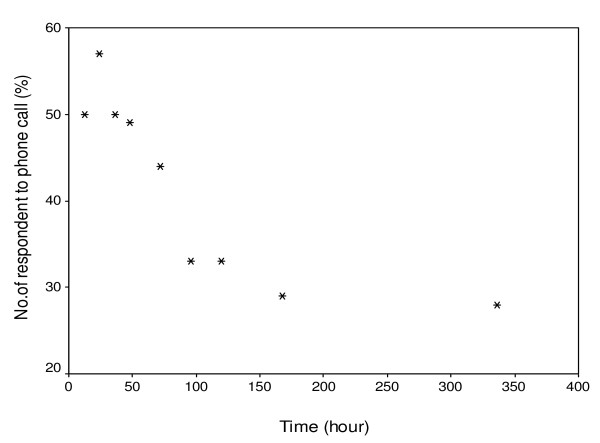
100 volunteers (84 women and 26 men) participated in the toll free mobile phone monitoring of adverse drug reactions. 54% of the participants had between 6 and 12 years of formal education and 46% of the subjects had more than 12 years of formal education (post-secondary school), 5% of them were healthcare providers (nurses or doctors). The trend of the responses received showed that most people responded within the first twenty-four hours of follow-up. By 96 hours, the level of responses began to decline (Figure 1) with only few respondents completing the entire follow up period. No incidence of unknown ADRs was recorded and neither was there any increase in the frequency of known ADRs during the period of monitoring.
Figure 1.
Relationship between responses to toll-free mobile phone calls and time of call during monitoring of adverse drug reactions to anti-malarial drugs purchased in the retail outlets (r = 0.81, F = 13.7, P = 0.008).
Discussion
Exposure to anti-malarial drugs as a result of chemoprophylaxis or treatment of the infection and choice of drug depend on the clinical judgment of the physician, treatment policy, availability or cost. The adoption of ACT for malaria treatment and increasing access to this class of anti-malarial drugs offer an opportunity to evaluate occurrence of ADRs to these medicines in the larger population or community [5], Post-marketing surveillance offers assessment of drug released to the market in different categories of people, other than those in whom the drug was tested. ACT is 'new' to anti-malarial therapy in Nigeria and this is additional reason this study was carried to actively monitor possible ADRs over an extended period.
Data from this study indicate that medicines in general are purchased more from pharmacies compared to PPMS. However, a high volume of anti-malarial drugs (23.7%) is purchased at the PPMS. This implies that people in the study area, prefer PPMS as the first point of contact for anti-malarials than the pharmacies. Since the Alma Ata Declaration recognized the Primary Health Care (PHC) centres as positioned to be the first contact to individual, family and community, the frequency of visit of the people studied on PPMS for anti-malarials may suggest that the primary function of the PHC is not optimized in the study area. The centres are either underutilized or not easily accessible. The number of PPMS (128) and community pharmacies (13) in the community is a strong indicator of the challenges of PHC programme. PPMS appear more accessible given the high purchase rate for anti-malarial drugs recorded in this study and may be considered viable units for intervention programmes for malaria control in endemic areas, where PPMS exist. Negligence of these drug retail outlets may in turn, increase the risk of incorrect dosing, inappropriate treatment, impacting negatively on drug safety [11].
The large volume of analgesics may suggest the probability that most people at the point of purchasing anti-malarials also buy analgesics to relief the symptoms of malaria, which include fever and arthralgia. The observation during the study showed that over 50% of those purchasing anti-malarial drugs also bought analgesics.
The frequency of purchase of anti-malarials in communities will differ greatly and may influence serious ADRs, such as allergic and alveolitis. Despite continuing education by the Federal Ministry of Health, SP and CQ use still remains high despite widespread resistance reported in the area of study [12]; indicating that although ACT is the recommended first line treatment for malaria, the official malaria treatment policy is yet to be fully practiced in the study community, Similar studies conducted in other malaria endemic areas in Africa also show that continued use of ineffective anti-malarial drugs, especially due to poverty, remain a challenge [13,14]. It will, therefore, be necessary to improve strategies for the implementation of the policy on treatment of malaria and encourage the inclusion of community drug outlets level for policy compliance.
Resistance to CQ and failure of mono therapy in many endemic countries led to a widespread promotion of ACT [15]. Nevertheless, its adoption appears compromised within the study area with only 10% purchase rate for ACT. This may be due to the cost of the artemisinins being more expensive than the older anti-malarials, either as artesunate monotherapy or as a combination [15]. Thus the cost of ACT may compromise effective treatment of malaria in poor countries with high malaria intensity. The relatively high cost of ACT in spite of huge financial and logistic commitments to intervention programmes from donor agencies remains an enigma.
The monitoring of adverse reactions to these drugs should be an important component of the health care system. The challenges of pharmacovigilance in Africa are not unconnected to the poor reporting structures and lack of motivation to report. The present study evaluated the use of mobile phone technology for monitoring ADRs, especially knowing that the exposure to antimalarial is relatively high in the community. Finding from the study showed that mobile phones offer a practical means of reporting adverse reaction to anti-malarial drugs and may be a model of choice in Africa where mobile telephony penetration and coverage is already driving innovation in agriculture and commerce [16]. The enthusiasm to report ADRs in the volunteers, who participated in this study, was highest within 24 hours and dwindled after 96 h. Improved education or enlightenment may improve this pattern over time.
The aim of this research is to provide "proof of principle" of an innovative approach to support pharmacovigilance in Africa. With over 60% of Africans, in the cities and small villages owning mobile phones,[16] it is expected that response to monitoring ADRs in this part of the world can be improved.
It is noteworthy that retail sectors do serve as source of malaria treatment and care, complementary to health facility [17], however, a major concern in this study is the high exposure to ineffective anti-malarial drugs at these drug outlets. Education and monitoring of community drug outlets by the health authority for compliance may provide a pragmatic approach. Training on the use of anti-malarials and concept of its PV for these shop keepers may be an option for improving safety of anti-malarial drugs and the quality of services rendered at PPMS. Coupling of the training with appropriate rewards for good practice may improve their performances [18].
Although, there was neither increase in the frequency of known ADRs or new ADRs detected during the study, it is important to ensure continuing monitoring of anti-malarial drugs safety and scale up use of mobile phone technology to support pharmacovigilance of anti-malarial drugs.
List of abbreviations
ADRs: Adverse drug reactions; AL: Artemether-lumefantrine; AQ: Amodiaquine; AS: Artesunate monotherapy; CP: Community Pharmacies; CQ: Chloroquine; HF: Halofantrine; NSAIDs: Non Steroidal Anti-inflammatory Drugs; PHC: Primary Health Care; PPMS: Patent and Proprietary Medicine Stores; PR: Proguanil; QN: Quinine; SP-Sulphadoxine-pyrimethamine; USD: United State Dollars
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AAA participated in the conception of the study, field collection of data, analysis and writing of the manuscript. BS, AT, MA, OO, MOA, and OAT participated in planning the study, field collection of data, entering of data and analysis, and writing of the manuscript. IAO- participated in the conception of the study, planning of field work and monitoring. OAO- participated in the conception of the study, planning of field work and monitoring. OOA - participated in the conception of the study, planning of field work and monitoring. TTA- participated in the conception of the study, technical analysis, evaluation and writing of script. FAF - participated in the conception of the study, technical analysis, evaluation and writing of script. OATO- participated in the conception of the study, technical analysis, evaluation and writing of script. All authors have read and approved the final manuscript.
Contributor Information
Ahmed A Adedeji, Email: ahmedade1@yahoo.co.uk.
Bilqees Sanusi, Email: rxcist_sabsworld@yahoo.com.
Azeez Tella, Email: tellaazeez@gmail.com.
Motunrayo Akinsanya, Email: akinsanya_motunrayo@yahoo.com.
Olubusola Ojo, Email: ojoolubusola@yahoo.com.
Mufliat O Akinwunmi, Email: abejetope@yahoo.com.
Olubukola A Tikare, Email: bukitiko@yahoo.co.uk.
Isiaka A Ogunwande, Email: abu2900@yahoo.com.
Omobola A Ogundahunsi, Email: ogundagunsibola@yahoo.com.
Olajide O Ayilara, Email: ayilaraolu@yahoo.com.
Taofeeqah T Ademola, Email: tundeosundina@yahoo.com.
Fatai A Fehintola, Email: fentolamine@yahoo.com.
Olumide AT Ogundahunsi, Email: ogundahunsio@who.int.
Acknowledgements
The authors acknowledge support of colleagues from Communicable Disease Research Unit Olabisi Onabanjo University Teaching Hospital Sagamu, Nigeria; the access to sales records provided by the management of Adun-Ade, Mex and Royal Pharmacies and the National Association of Patient and Proprietary Medicine Stores Sagamu, Ogun State Nigeria.
References
- Federal Ministry of Health. National Antimalarial Treatment Policy. Abuja, Nigeria: Federal Ministry of Health; 2004. [Google Scholar]
- World Health Organization. World Malaria Day April 25th 2010. http://www.who.int/malaria/en/
- Mokuolu OA, Okoro EO, Ayetoro SO, Adewara AA. Effect of artemisinin-based treatment policy on consumption pattern of antimalarials. Am J Trop Med Hyg. 2007;76:7–11. [PubMed] [Google Scholar]
- Lyda O, Iveth G, Piero O, Walt RJ. Artemisinin-based combination therapy for uncomplicated Plasmodium falciparum malaria in Colombia. Malar J. 2007;6:25. doi: 10.1186/1475-2875-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adisa R, Fakeye TO, Dike D. Evaluation of adverse drug reactions to artemisinin-based combination therapy in a Nigeria university community. Trop J Pharm Res. 2008;7:937–944. [Google Scholar]
- Orbit B, Iteba N, Lengler C, Makemba A, Mshana C, Mshinda H, Nathan R, Alba S, Dillip A, Hetzel MW, Mayumana I, Schulze A. Exploring and improving access to health care in contexts of livelihood in security. Towards a framework for analysis and action. PLos Med. 2007;4:e308. doi: 10.1371/journal.pmed.0040308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCombie SC. Self-treatment for malaria: the evidence and methodological issues. Health Policy Plan. 2002;17:333–344. doi: 10.1093/heapol/17.4.333. [DOI] [PubMed] [Google Scholar]
- Goodman C, Kachur SP, Abdulla S, Mwageni E, Nyoni J, Schellenberg JA, Mills A, Bloland P. Retail supply of malaria-related drugs in rural Tanzania: risk and opportunities. Trop Med Int Health. 2004;9:655–633. doi: 10.1111/j.1365-3156.2004.01245.x. [DOI] [PubMed] [Google Scholar]
- Taylor WR, White NJ. Antimalarial drug toxicity: a review. Drug Saf. 2004;27:25–61. doi: 10.2165/00002018-200427010-00003. [DOI] [PubMed] [Google Scholar]
- Salako LAS, Ajayi FO, Sowunmi A, Walker O. Malaria in Nigeria. A revisit. Ann Trop Med Parasitol. 1990;84:435–445. doi: 10.1080/00034983.1990.11812493. [DOI] [PubMed] [Google Scholar]
- Talisuna AO, Staedke SG, D'Alessandro U. Pharmacovigilance of anti-malarial treatment in Africa: is it possible? Malar J. 2006;5:50. doi: 10.1186/1475-2875-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowunmi A, Fehintola FA, Adedeji AA, Gbotosho GO, Falade CO, Tambo E, Fateye BA, Happi TC, Oduola AMJ. Open randomized study of pyrimethamine sulphadoxine vs. pyrimethamine-sulphadoxine plus probenecid for the treatment of uncomplicated Plasmodium falciparum malaria in children. Trop Med Int Health. 2004;9:606–19. doi: 10.1111/j.1365-3156.2004.01233.x. [DOI] [PubMed] [Google Scholar]
- Talisuna AO, Bloland P, D'Alessandro U. History, dynamics, and public health importance of malaria parasite resistance. Clin Microbiol Rev. 2004;17:235–254. doi: 10.1128/CMR.17.1.235-254.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood B. Anti-malarial drugs and the prevention of malaria in the population of malaria endemic areas. Malar J. 2010;9(Suppl 3):S2. doi: 10.1186/1475-2875-9-S3-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachur SP, Black C, Abdulla S, Goodman C. Putting the genie back in the bottle? Availability and presentation of oral artemisinin compounds at retail pharmacies in urban Dar-es-Salaam. Malar J. 2006;5:25. doi: 10.1186/1475-2875-5-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker JC, Mbiti IM. Mobile phones and economic development. J Economic Perspectives. 2010;24:207–232. [Google Scholar]
- World Health Organization. Partnership for malaria control: engaging the formal and informal private sectors. Geneva, World Health Organization/TDR; 2006. [Google Scholar]
- Brugha R, Zwi A. Improving the quality of private sector delivery of public health services: challenges and strategies. Health Policy Plan. 1998;13:107–120. doi: 10.1093/heapol/13.2.107. [DOI] [PubMed] [Google Scholar]