Abstract
Inositol stereoisomers, myo- and scyllo-inositol, are known to enter the brain and are significantly elevated following oral administration. Elevations in brain inositol levels occur across a concentration gradient as a result of active transport from the periphery. There are two sodium/myo-inositol transporters (SMIT1, SMIT2) that may be responsible for regulating brain inositol levels. The goals of this study were to determine the effects of aging and Alzheimer's disease (AD)-like amyloid pathology on transporter expression, to compare regional expression and to analyze substrate requirements of the inositol transporters. QPCR was used to examine expression of the two transporters in the cortex, hippocampus and cerebellum of TgCRND8 mice, a mouse model of amyloid pathology, in comparison to non-transgenic littermates. In addition, we examined the structural features of inositol required for active transport, utilizing a cell-based competitive uptake assay. Disease pathology did not alter transporter expression in the cortex or hippocampus (p>0.005), with only minimal effects of aging observed in the cerebellum (SMIT1: F2,26 = 12.62; p = 0.0002; SMIT2: F2,26 = 8.71; p = 0.0015). Overall, brain SMIT1 levels were higher than SMIT2, however, regional differences were observed. For SMIT1, at 4 and 6 months cerebellar SMIT1 levels were significantly higher than cortical and hippocampal levels (p<0.05). For SMIT2, at all three ages both cortical and cerebellar SMIT2 levels were significantly higher than hippocampal levels (p<0.05) and at 4 and 6 months of age, cerebellar SMIT2 levels were also significantly higher than cortical levels (p<0.05). Inositol transporter levels are stably expressed as a function of age, and expression is unaltered with disease pathology in the TgCRND8 mouse. Given the fact that scyllo-inositol is currently in clinical trials for the treatment of AD, the stable expression of inositol transporters regardless of disease pathology is an important finding.
Introduction
scyllo-Inositol is currently in human clinical trials for the treatment of patients with mild to moderate Alzheimer's disease (AD; www.clinicaltrials.gov). This stemmed from preclinical studies, in which scyllo-inositol was shown to be an effective treatment for AD-like amyloid pathology and cognitive deficits in TgCRND8 mice [1], [2]. TgCRND8 mice show many of the hallmark features of AD, including an increase in cerebral Aβ levels, Aβ aggregation and plaque deposition, along with cognitive deficits as the disease advances [3]. scyllo-Inositol treatment significantly improved spatial memory, synaptic function and survival rates in TgCRND8 mice [1]. These positive effects occurred both in animals given scyllo–inositol prophylactically, before the visible onset of symptoms, and therapeutically, once symptoms had fully developed [1], [2]. Gas chromatography/mass spectrometry results found ad libitum scyllo-inositol treatment increased scyllo-inositol levels within the brain 7-fold [2] and magnetic resonance spectroscopy also demonstrated a 2 to 3-fold increase in brain scyllo-inositol levels [4] suggesting that the beneficial effects are centrally induced.
scyllo-Inositol is found endogenously in the body and is the second most abundant inositol stereoisomer [5], [6]. The brain levels of myo- and scyllo-inositols are 100-fold greater than those found in the periphery [5], [7]. This indicates that active transport, in addition to simple diffusion, is required for the regulation of brain inositol levels. Active transport of inositol stereoisomers across cellular membranes including cells at the blood-brain and blood-CSF barriers, occurs via inositol transporters. Two transporters, SMIT1 and SMIT2, are important for regulating brain and peripheral inositol levels, by co-transporting two sodium ions along the concentration gradient, to generate enough energy to actively transport myo-inositol [8]–[12]. While both transporters are expressed in the brain [11], [13], [14], there is limited information on the regional expression of these transporters and no data on expression changes with various disease pathologies, including AD. In addition, there is little information on age-induced changes to transporter expression or comparative data on expression of SMIT1 and SMIT2. To answer these questions, transporter mRNA levels were examined in three brain regions: the cortex, hippocampus and cerebellum, as a function of age, genotype and Aβ/amyloid related pathology. These brain regions were chosen as the cortex and hippocampus are most affected in AD while the cerebellum is only affected very late in the disease. Expression was examined in both TgCRND8 mice and non-transgenic (Tg) littermates at 2, 4 and 6 months of age. These ages were selected because they correspond to pre-plaque deposition, mid-stage and advanced AD-like amyloid pathology in TgCRND8 mice [3]. Further, transport assays were conducted to determine the inositol structural features required to decrease myo or scyllo-inositol transport through SMIT1/2. The aims of this study were to extend our knowledge of SMIT1 and SMIT2 expression as a function of age and disease pathology and to better understand substrate transport through these transporters. Both of these experimental aims were achieved.
Results
Inositol transporter expression as a function of age and progressive amyloid deposition
scyllo- and myo-Inositol accumulate in the brain, at levels 100-fold higher than those in the periphery [5], [7], through active transport. There are two sodium myo-inositol transporters that have been reported in the literature, SMIT1 and SMIT2, both of which are expressed in the brain [11], [13], [14]. Normal brain aging has been linked with neuronal cell loss, an increase in gliosis and ultimately an increase in both myo- and scyllo-inositol as shown by magnetic resonance spectroscopy [15], [16]. This increased inositol signal may be due to increased transporter activity or expression. Therefore, one goal of these experiments was to examine whether the levels of these transporters change as a function of age. The mRNA expression of the two inositol transporters was examined at 2, 4 and 6 months of age in both TgCRND8 mice and non-Tg littermates in the cortex, hippocampus and cerebellum. Significant age effects on transporter expression were observed in the cerebellum of both groups (SMIT1: F2,26 = 12.62; p = 0.0002; SMIT2: F2,26 = 8.71; p = 0.0015), but not in the cortex or hippocampus (p>0.05). In both TgCRND8 mice (Figure 1) and non-Tg littermates (data not shown), 2 month SMIT1 and SMIT2 levels were significantly lower than 4 and 6 month levels (p<0.05). More specifically, in TgCRND8 mice, 2 month, cerebellar SMIT1 levels were significantly lower than 4 and 6 month levels (Figure 1A, C, E; p<0.05) and 2 month, cerebellar SMIT2 levels were significantly lower than 4 month levels (Figure 1B, D; p<0.05). In non-Tg littermates, 2 month, cerebellar SMIT1 and SMIT2 levels were significantly lower than 6 month levels (data not shown; p<0.05). These regional differences, for the effects of age on expression, may reflect the continual postnatal maturation of the cerebellum [17], which does not occur in the cortex or hippocampus.
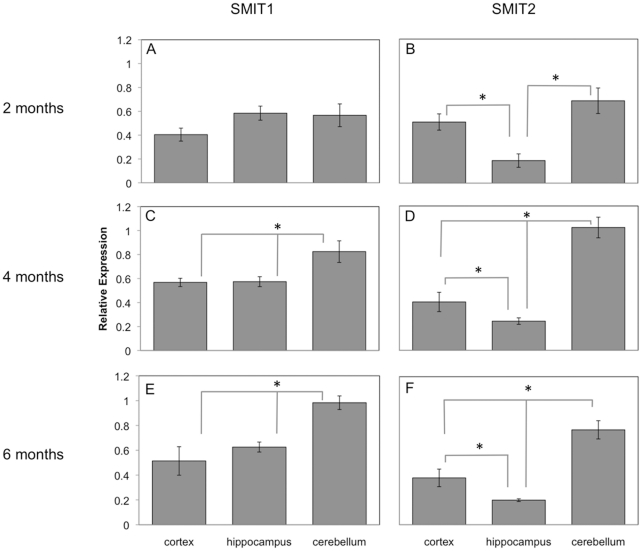
Figure 1. A comparison of regional expression for each of the inositol transporters.
A comparison of the regional expression patterns of SMIT1 (A, C, E) and SMIT2 (B, D, F) in the brain at 2 months (A, B), 4 months (C, D) and 6 months of age (E, F) showed a distinct pattern of expression. SMIT1 levels were significantly higher in the cerebellum than in the cortex or hippocampus at 4 and 6 months of age. SMIT2 levels were significantly higher in the cortex and cerebellum, compared to the hippocampus at all three time points and significantly higher in the cerebellum, compared to the cortex, at 4 and 6 months of age. (n = 10 animals/group; p<0.05).
A comparison of SMIT1 and SMIT2 expression between TgCRND8 mice and non-Tg littermate mice, at 2, 4 and 6 months of age, was used to determine the effects of AD-like amyloid pathology on transporter expression. No significant differences in SMIT1 or SMIT2 expression were observed between TgCRND8 mice and non-Tg littermates at all ages and in all regions, indicating that increasing amyloid pathology does not alter the expression of the transporters (p>0.05; data not shown).
Regional brain inositol transporter expression levels
The expression of SMIT1 and SMIT2 were quantified in the cortex, hippocampus and cerebellum (Figure 1). These three brain regions are affected to varying degrees by Aβ plaque deposition in AD [18]. The earliest plaque deposition is observed in the cortex and hippocampus, while deposition in the cerebellum occurs very late in the disease [18]. Both transporters were expressed in all three brain regions examined, however regional differences in expression for each transporter were observed. For both transporters, a significant correlation between brain region and transporter expression levels was found (Figure 1; SMIT1: F2,86 = 14.86, p<0.0001; SMIT2: F2,86 = 55.04, p<0.0001). For SMIT1, no significant differences in regional expression were observed at 2 months (Figure 1A), but at 4 and 6 months, cerebellar SMIT1 levels were significantly higher than cortical and hippocampal levels (Figure 1C, E; p<0.05). For SMIT2, at all three ages both cortical and cerebellar SMIT2 levels were significantly higher than hippocampal levels (Figure 1B, D, F; p<0.05) and at 4 and 6 months of age, cerebellar SMIT2 levels were also significantly higher than cortical levels (Figure 1D, F; p<0.05).
Structural requirements for inositol transport
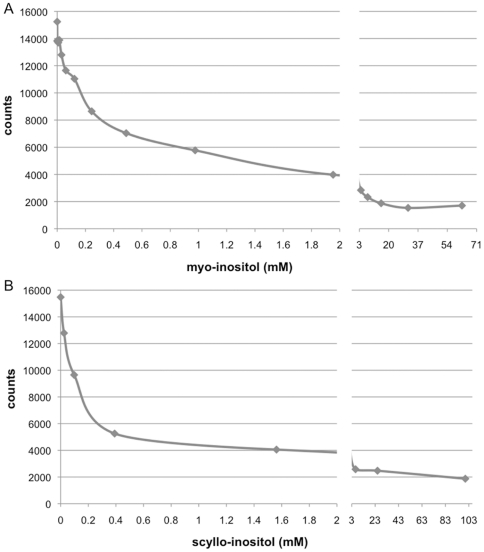
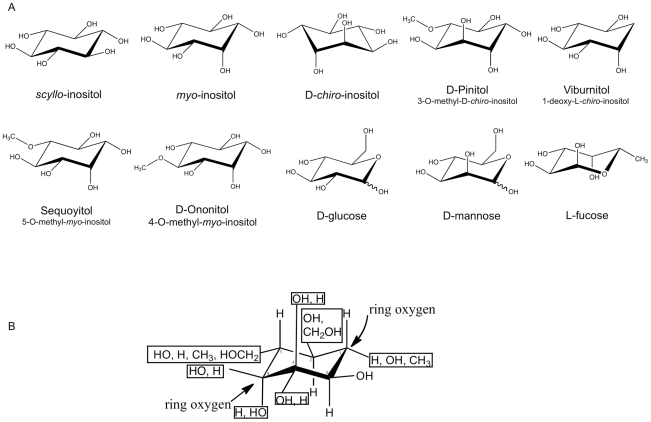
Although SMIT1/2 transport of various inositol stereoisomers has been reported, the structural requirements for transport have not been examined. To define these parameters, transport of myo- or scyllo-inositol-(2-3H) was examined in the presence of 15 inositol isomers, 6 hexoses and 2 pentose sugars which may serve as competive substrates, inhibitors or allosteric effectors on inositol transport (Figure 2). We first examined the myo- and scyllo-inositol transport kinetics of our cell system (Figure 3). The transport of myo- or scyllo-inositol-(2-3H) (100 µM) was assayed in the presence of increasing concentrations of cold myo- or scyllo-inositol. The concentration dependence of myo-inositol saturated near 10 mM of cold myo-inositol producing a near maximal inhibition of myo-inositol-(2-3H) transport, while half-maximal inhibition was observed at 200 µM of cold myo-inositol (Figure 3A). As was seen for myo-inositol, 10 mM of cold scyllo-inositol produced near maximal inhibition of scyllo-inositol-(2-3H) transport and half maximal inhibition was observed at 200 µM cold scyllo-inositol (Figure 3B). However, while the myo-inositol transport experiment was conducted using a 15 minute incubation window, the scyllo-inositol experiment required the incubation to be extended to 3 hours in order to produce similar uptake of scyllo-inositol. These results highlight an apparent slower uptake rate for scyllo-inositol by the transporters. Our half maximal inhibition values for myo-inositol agree well with the range previously reported in the literature; myo-inositol Km = 55–117 µM for SMIT1 and Km = 120–348 µM for SMIT2 [9], [10], [19]–[24].
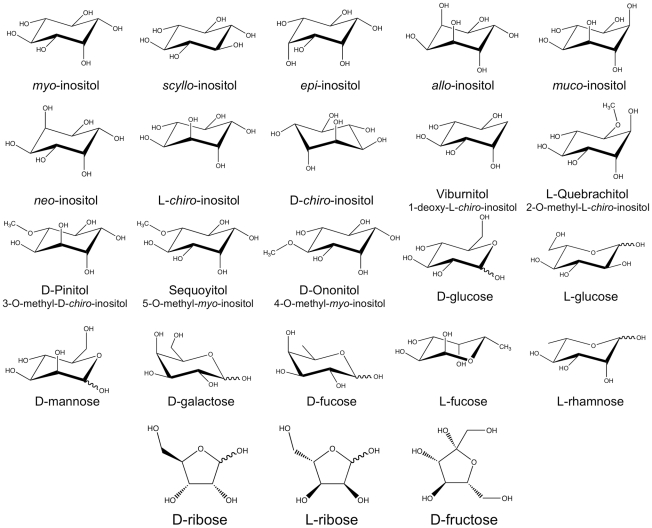
Figure 2. Structures of the inositol stereoisomers, derivatives and related compounds.
The structures of the inositol stereoisomers, derivatives and related compounds used for the initial competitive transport assays.
Figure 3. myo-Inositol and scyllo-inositol transport in HEK293 cells.
myo-Inositol testing was conducted in HEK293 cells, by incubating cells for 15 minutes in medium containing 100 µM of myo-inositol-(2-3H) in the presence of increasing concentrations of cold myo-inositol (A). Addition of 10 mM of cold myo-inositol, the concentration used in the competitive transport assays, resulted in a near maximal inhibition of myo-inositol-(2-3H) transport in these cells. scyllo-Inositol testing was conducted over a period of 3 hours in these cells, due to slower uptake rates (B). Once again, 10 mM of cold scyllo-inositol produced near maximal inhibition of scyllo-inositol-(2-3H). (n = 3 wells per concentration).
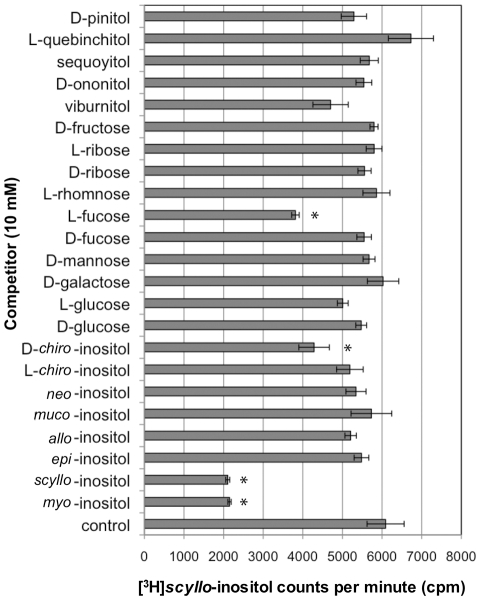
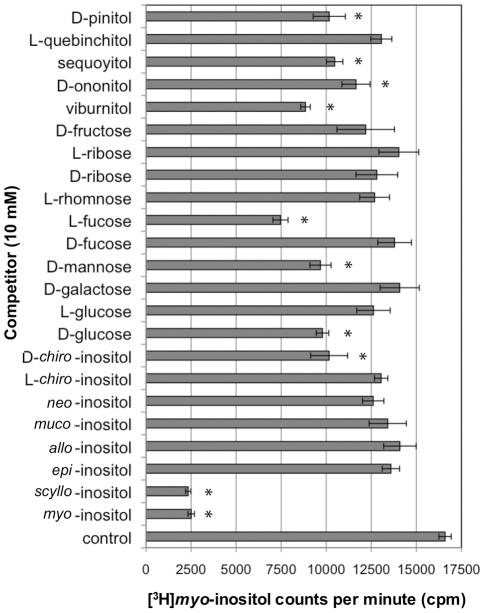
An examination of scyllo-inositol-(2-3H) transport, in the presence or absence of the 23 polyols, showed myo-, scyllo-, D-chiro-inositol and L-fucose to significantly depress scyllo-inositol-(2-3H) transport (Figure 4; F23,48 = 14.47, p<0.05). In contrast, when myo-inositol-(2-3H) transport was tested in the presence or absence of the 23 polyols, in addition to those identified for scyllo-inositol, D-glucose, D-mannose, viburnitol, sequoyitol, D-ononitol and D-pinitol also significantly depressed myo-inositol-(2-3H) transport (Figure 5; F23,48 = 18.46, p<0.05). This difference in experimental findings likely stems from differences in the relative transport rates of myo- and scyllo-inositol by SMIT1/2 and differences in specificity of the transporters, which would result in different observed inhibition profiles. Overall, our results are in agreement with previous studies that identified myo-, scyllo-, D-chiro-inositol, D-glucose and D-pinitol as substrate competitors [9], [10], [19], [22], [24].
Figure 4. scyllo-Inositol-(2-3H) transport in HEK 293 cells.
scyllo-Inositol transport in the presence of inositol and sugar isomers was examined by measuring scyllo-inositol-(2-3H) transport in the presence of each of those potential substrates. For the control experiment, scyllo-inositol-(2-3H) transport was quantified in the absence of any added compounds. Cells were incubated for 3 hours in medium containing 100 µM of scyllo-inositol-(2-3H) in the presence or absence of 10 mM of potential inositol or sugar isomer transport competitors and the resulting radioactivity in the cells was measured (n = 3 wells per variable; * = p<0.05).
Figure 5. myo-Inositol-(2-3H) transport in HEK 293 cells.
myo-inositol transport was examined by measuring uptake of myo-inositol-(2-3H) (100 µM) in the presence or absence of 23 different inositols or sugars (10 mM) over a 3 hour incubation period, after which radioactivity was measured. For the control experiment, myo-inositol-(2-3H) transport was quantified in the absence of any competitive substrates. (n = 3 wells per variable; * = p<0.05).
A comparison of the structural features of the compounds, which depressed myo-inositol, allowed the design of a model of the structural requirements for inhibition of transport through SMIT1/2 (Figure 6). With the panel of potential substrates tested, this model is best used to identify compounds that will not be recognized by the transporters, as additive effects between the substituents cannot be determined. All of the active compounds identified have equatorially positioned side chains at carbons 1, 2, 3 and 6, while the substituents at carbons 4 and 5 can be in either orientation. In addition, a comparison of the substrate competitors shows that the side group at position 1 can be either a hydroxyl, hydrogen, or methyl group and the carbon can be exchanged for a ring oxygen atom and maintain activity in the transport assay. At position 2 either a hydroxyl or a methoxy side chain are tolerated. At position 3, the hydroxyl group can be exchanged for a hydrogen, methyl or methoxyl group, while at position 6, the side chain must remain a hydroxyl group. At positions 4 and 5, either a hydroxyl or a hydrogen group can be placed in either orientation without disrupting the substrates competitive nature and the carbon at position 4 can be exchanged for a ring oxygen atom.
Figure 6. Basic structural model for SMIT1/2 substrate transport competitors.
The structures of the substrates which competed for SMIT1/2 myo-inositol-(2-3H) transport (A), along with the structures of substrates that did not compete, were used to design a basic structural model for SMIT1/2 substrate transport competitors (B).
Substrate recognition
We examined the recognition of the enantiomers D-chiro-inositol, which depressed scyllo/myo-inositol transport through SMIT1/2, and L-chiro-inositol, which had no effect, in greater detail. To determine whether this enantiomeric change in structure results in a complete lack of recognition by the transporter or decreased transporter affinity, the effect of increasing concentrations of each enanitomer on transport was examined. L-chiro-inositol competed for myo-inositol-(2-3H) transport only at the highest concentrations assayed (160 mM), consistent with our model and the previous assay carried out using 10 mM L-chiro-inositol. In contrast, D-chiro-inositol demonstrated a dose-dependent inhibition of myo-inositol transport (Figure S1). These results confirm the transporters' selectivity in the enantiomer of chiro-inositol recognized.
Based on our initial substrate transport studies L-fucose decreases the rate of myo-inositol transport. Further analysis confirmed this result showing that L-fucose reduced myo-inositol-(2-3H) transport in a concentration-dependent manner (Figure S2A). To determine if L-Fucose was a competitive substrate for SMIT or simply an inhibitor of the transporters the uptake of L-fucose-(5,6-3H) was measured. In these experiments no reduction in cell associated L-fucose-(5,6-3H) uptake was observed with any of the inositol substrates tested (Figure S2B) and no changes in radioactivity were observed in the presence of increasing concentrations of cold L-fucose (Figure S2C). Therefore, we propose that L-fucose is a SMIT1/2 transport inhibitor as has been previously proposed [9], [25].
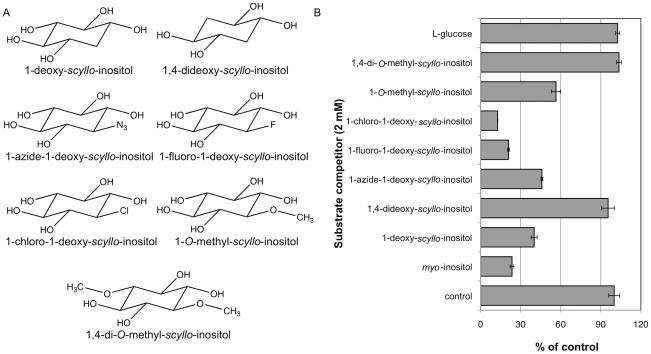
The SMIT1/2 basic recognition model was further tested and refined using a set of scyllo-inositol derivatives initially designed for examination of the effects of scyllo-inositol derivatives on Aβ fibrillogenesis [26], [27]. The use of these compounds allowed us to address further the hydrogen-bonding requirements and steric interactions between a given compound and the transporters. Seven compounds were tested, in which either: one or two hydroxyl groups were substituted with hydrogen or methoxy, or one hydroxyl group was replaced with a fluorine, azide, or chlorine (Figure 7A). Transporter recognition was observed following the substitution of one hydroxyl group on scyllo-inositol with a hydrogen atom or a hydroxy methyl group, however, the substitution of two hydroxyl groups removed any recognition of the compound by the transporters (Figure 7B). The finding that 1,4-di-O-methyl-scyllo-inositol was not transported is in agreement with the SMIT1/2 model and suggests steric interference of the bulky hydroxy methyl groups. Surprisingly, 1,4-dideoxy-scyllo-inositol was also not recognized by the transporters, suggesting that five hydroxyl groups or hydrogen bonding pairs are necessary for transporter interaction. When the effects of substituting one hydroxyl group were further examined, both 1-chloro- and 1-fluoro-1-deoxy-scyllo-inositol competed as readily as myo-inositol for active transport, while the hydrogen, azide and hydroxy methyl substitutions were less effective (Figure 7B). These results suggest that single small polar or non-polar substitutions on the scyllo-inositol scaffold are tolerated but larger substitutions such as methoxy or azide compromise transporter recognition.
Figure 7. Transport of scyllo-inositol derivatives by SMIT1/2.
Further testing of SMIT1/2 myo-inositol transport inhibition was conducted using derivatives of scyllo-inositol, in which one or two hydroxyl groups were removed or substituted (A). For the control experiment, myo-inositol-(2-3H) transport was quantified in the absence of any competitors. An analysis of myo-inositol-(2-3H) transport in the presence of each of the compounds, found monodeoxy- but not dideoxy-scyllo-inositol competed for myo-inositol-(2-3H) transport (B). Single hydroxyl group substitutions were tolerated in the order chlorine>fluorine>nitrate>hydroxy methyl. (n = 3 wells per variable).
Discussion
scyllo-Inositol is present endogenously in both the body and the brain [5], [6], with brain levels 100-fold higher than those in the periphery [5], [7]. This points to the importance of active transport for scyllo-inositol accumulation within the brain. Oral administration of ad libitum myo- or scyllo-inositol to mice increased the corresponding levels in the brain [2]. Both TgCRND8 mice and their non-Tg littermates showed a significant increase in scyllo-inositol levels in the brain following ad libitum administration, with no significant differences observed in baseline or treatment-induced brain inositol levels between the two groups [2]. In the present study, no significant differences in SMIT1 or SMIT2 expression profiles were found between TgCRND8 mice and their non-Tg littermates at any of the ages examined. Since the three time points chosen correspond to pre-plaque deposition, early plaque deposition and an advanced stage AD-like amyloid phenotype [3], we can conclude that neither age nor amyloid pathology alters SMIT1 or SMIT2 expression. A stable expression pattern, irrespective of pathology, is an important factor for successful scyllo-inositol treatment.
A comparison of SMIT1 versus SMIT2 expression in subregions of the brain was also conducted. Although it is known that both inositol transporters are expressed in the brain [11], [13], [14], our study found differences in the relative expression levels of the two transporters across brain regions that are affected in AD. Overall, expression in the brain of SMIT1 was higher than SMIT2. SMIT1 expression was observed in the order cerebellum>cortex = hippocampus while SMIT2 expression was also highest in the cerebellum>cortex>hippocampus. Both transporters were expressed in all the brain regions examined.
The specificity and structural requirements for transport by SMIT1/2 has not been previously investigated. To investigate the recognition of inositol and sugars by the transporters a substrate transport study was conducted. Substrate transport of labeled myo- and scyllo-inositol showed transport of scyllo-inositol to be substantially slower despite the recognition of both substrates with similar affinity (IC50 ∼200 µM). An analysis of 23 different inositols and sugars was conducted. Our findings indicate that a number of substrates inhibit scyllo- and myo-inositol-(2-3H) transport. This inhibition could be due to direct competition for transport, active inhibition of the transporters or through allosteric inhibition. Specifically the SMIT1/2 transporters were found to recognize D-chiro-inositol, viburnitol, D-glucose, D-mannose, sequoyitol, D-ononitol and D-pinitol all to lesser extents than myo- or scyllo-inositol. Previously an equal preference for myo- and scyllo-inositol was shown in Xenopus oocytes transfected with canine SMIT1 [9]. SMIT1/2 transport of D-chiro-inositol [10], [19], [22], [24] and SMIT2 transport of D-glucose and D-pinitol have been previously reported [24]. We confirm previous observations of SMIT2 preference of these stereochemistries by the transporters [19]. The HepG2 human liver cell-line was shown to preferentially transport D-chiro-inositol and D-glucose over L-stereoisomers [19]. An additional substrate, L-fucose, has been previously identified as a competitor for myo-inositol transport [9], [25]. The present observations that myo-inositol and increasing concentrations of cold L-fucose did not reduce cell associated L-fucose-(5,6-3H) levels, allows us to conclude that L-fucose is a SMIT1/2 transport inhibitor.
To further define transporter selectivity, novel derivatives of scyllo-inositol, containing single or double hydroxyl group substitutions were examined for transport. One or two hydroxyl groups on scyllo-inositol were substituted with chlorine, fluorine, hydrogen, azide or hydroxy methyl groups and transport competition in relation to tritiated myo-inositol was examined. Single substitutions competed against tritiated myo-inositol transport, with chlorine and fluorine substitutions competing against tritiated myo-inositol transport as efficiently as myo- or scyllo-inositol. However, double substitutions were not recognized as efficiently, highlighting the apparent specificity of the transporters.
In conclusion, inositol transporter expression in the brain was unaltered by amyloid disease pathology, as determined by comparing SMIT1 versus SMIT2 expression profiles in TgCRND8 mice. Overall, SMIT1 expression in the brain was higher than SMIT2, with equivalent expression in the cortex and hippocampus. Expression of both transporters was observed in all the brain regions examined and regional expression profiles were unique. When the inhibition of substrate transport was examined, SMIT1/2 transported scyllo-inositol as expected, although at a slower uptake rate than myo-inositol. The findings of this study suggest that the beneficial effects seen in the preclinical studies on the efficacy of scyllo-inositol in a Tg mouse model of AD can be attributed to the expression of the SMIT1/2 transporters in brain regions susceptible to AD pathology and the steady transport of scyllo-inositol to those regions to target AD pathology. These results are particularly important as the intent to advance scyllo-inositol to phase III trials was announced after the release of the Topline summary results of the phase II study [28], [29].
Materials and Methods
Ethics Statement
All experiments were performed according to the Canadian Council on Animal Care guidelines (Protocol #: 20008707).
Materials
All reagents were purchased from Sigma (St. Louis, MO, USA) unless otherwise noted. epi-Inositol, allo-inositol and cold scyllo–inositol were acquired from Transition Therapeutics Inc. (Toronto, Ontario, Canada). Viburnitol (1-D-3-deoxy-myo-inositol; Cat #: FC-041), D-Ononitol (1-D-4-O-methyl-myo-inositol; Cat #: FC-040), Sequoyitol (5-O-methyl-myo-inositol; Cat #: FC-047) and D-Pinitol (3-O-methyl-D-chiro-inositol; Cat #: FC-026) were purchased from Industrial Research Ltd. (Lower Hutt, New Zealand). myo-Inositol-(2-3H) (3 µCi/mL; Cat #: ART 0116), scyllo-inositol-(2-3H) (3 µCi/mL; Cat #: ART 0264) and L-fucose-(5,6-3H) (3 µCi/mL; Cat #: ART 0106A) were purchased from American Radiolabeled Chemicals Inc. (St. Louis, MO, USA).
Mice
TgCRND8 mice were maintained on an outbred C3H/C57Bl6 background. These mice over express the human amyloid precursor protein gene containing both the Swedish (KM670/671NL) and Indiana (V717F) mutations under control of the Syrian hamster prion gene promoter [3]. Mice were kept on a 12-hour light/dark cycle and given water and standard rodent chow ad libitum. To quantify inositol transporter expression, TgCRND8 mice and non-Tg littermates at 2, 4 and 6 months of age were anesthetized with pentobarbital, transcardially perfused with cold PBS-heparin, after which, the brains were removed and the cortex, hippocampus and cerebellum dissected for RNA isolation.
Quantification of inositol transporter expression
QPCR was performed using an Applied Biosystems 7500 Real-Time PCR system and a SYBR® GreenER™ qPCR SuperMix Universal kit (Invitrogen, Cat #: 11762). Primers were designed using the Beacon Designer 7.5 software program for Mac OS X and are listed in Table 1. Tissue RNA was isolated using phenol/chloroform extraction and the sample concentrations determined using a NanoDrop™ Spectrometer (Thermo Scientific, Wilmington, USA). RNA samples were treated with DNase I (Fermentas, Cat #: EN0521) to remove any genomic DNA. RNase inhibitor (Fermentas, Cat #: EO0381) was added to the reaction mixture to prevent RNA degradation during DNase I treatment. The RNA concentration was determined and for each sample, 3 cDNA reactions were performed using a SuperScript III First-Strand Synthesis SuperMix for qRT-PCR kit (Invitrogen, Cat #: 11752). Following reverse transcription, residual RNA was removed through E. coli RNase H treatment and the cDNA concentrations normalized. For the QPCR reaction, 10 ng of cDNA was used per well of a 96-well plate. A 2 month, non-tg, kidney, with ideal 260/280 and 260/230 ratios was selected for use as a between plate control. The genes TATA-box binding protein (Tbp) and Glyceraldehyde 3-phosphate dehydrogenase (Gapdh) were selected as control genes, following a scan of the National Center for Biotechnology Information (NCBI), Gene Expression Omnibus website to confirm stable expression in all three of the brain regions examined in this study. For analysis, relative quantification values were normalized to the control genes, inter-plate calibrators and variations in primer efficiencies, then adjusted to an average expression level of 1 using the MultiD, GenEx software program (genex.gene-quantification.info/) for analysis.
Table 1. QPCR primers.
| Protein Name | Gene | Accession # | Primers |
| TATA-box Binding Protein | Tbp | NM_013684 | Forward: GCC TTC CAC CTT ATG CTC AG Reverse: GAG TAA GTC CTG TGC CGT AAG |
| Glyceraldehyde 3-phosphate dehydrogenase | Gapdh | XM_001473623 | Forward: AAG AAG GTG GTG AAG CAG GCA TC Reverse: CGA AGG TGG AAG AGT GGG AGT TG |
| Sodium/myo-inositol transporter 1 | Slc5a3 | NM_017391 | Forward: CTG TGG TGC TGT GGG ATG ATG Reverse: CCT GCT GGG TCT GAA CTT TGC |
| Sodium/myo-inositol transporter 2 | Slc5a11 | NM_146198 | Forward: CAA GGT GGT GAG GGC TAT CC Reverse: CTA TGA CAG GTT CCG CTT TGC |
Substrate selectivity analysis
Human epithelial kidney (HEK293; ATCC, Cat #: CRL-1573) cells were cultured in DMEM medium containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were plated onto 24-well plates and grown to 90% confluency. Once 90% confluent, cells were washed in PBS (pH 7.0), then incubated in PBS containing: scyllo-inositol-(2-3H), myo-inositol-(2-3H) or L-fucose-(5,6-3H) (3 µCi/mL, 100 µM with 0.1% w/v BSA), with or without a substrate competitor. The substrate competitors (Figure 2) consisted of either inositol stereoisomers (myo-, scyllo-, epi-, allo-, muco-, neo-, L-chiro-, or D-chiro-inositol), inositol derivatives (viburnitol, D-ononitol, sequoyitol, L-quebinchitol or D-pinitol) or structurally similar sugar substrates (D-glucose, L-glucose, D-galactose, D-mannose, D-fucose, L-fucose, L-rhamnose, D-ribose, L-ribose or D-fructose). One well was dedicated to testing each competitor and triplicate plates were run for each experiment. Following incubation (3 h, 37°C), cells were washed twice with PBS containing 1 mM cold myo-inositol, to stop transporter activity, following which the cells were dissolved using 2% SDS and the radioactivity measured using a scintillation counter. Competitors were tested either at a fixed concentration (10 mM) or in a dose range (10–400 mM). Novel scyllo-inositol derivatives were used to test myo-inositol-(2-3H) transport requirements at a fixed concentration of 2 mM.
Statistical analysis
Statistical analysis was conducted using the Statistical Analysis System (SAS) and the Graphpad Prism programs. Groups were compared using a one-way ANOVA. If a significant F score was observed (p<0.05), a Bonferroni post hoc test was used to compare groups with the statistical significance, p<0.05.
Supporting Information
A comparison of D- and L- chiro -inositol inhibition of myo-inositol transport in HEK293 cells. Based on reduction in myo and scyllo-inositol transport by, D-chiro-inositol it is recognized by SMIT1/2, while L-chiro-inositol is not. This finding was examined more closely in HEK293 cells, by comparing the transport of myo-inositol-(2-3H) in the presence of increasing concentrations of D-chiro-inositol (A), to transport observed in the presence of increasing concentrations of L-chiro-inositol (B). As expected myo-inositol-(2-3H) transport was inhibited by D-chiro-inositol in a concentration dependent manner. In contrast, L-chiro-inositol did not inhibit myo-inositol-(2-3H) transport, except at the highest concentration, 160 mM. (n = 3 wells per variable).
(TIF)
L-fucose-(5,6-3H) transport. L-fucose was found to be a competitive inhibitor of myo- and scyllo-inositol-(2-3H) transport through SMIT1/2. This finding was further examined by: (A) examining myo-inositol-(2-3H) transport in the presence of increasing concentrations of L-fucose. A concentration-dependent reduction of myo-inositol-(2-3H) transport was observed. (B) An examination of L-fucose-(5,6-3H) transport in these cells, in the presence or absence of potential competitive substrates. Only background radioactivity was observed, with not active transport. (C) L-fucose-(5,6-3H) transport was examined in the presence or absence of D-fucose and increasing concentrations of cold L-fucose and again only background radioactivity was observed. (n = 3 wells per variable).
(TIF)
Footnotes
Competing Interests: The authors have read the journal's policy and have the following conflicts: named inventor on patent and patent applications relating to scyllo-inositol. Patent #s: US 7521481, WO 2004/075882 (issued April 21, 2009); US 2006-0189582, WO 2006/053428 (published March 5, 2009); PCT/CA2007/000395, WO 2007/101353 (filed 03/09/2007). This does not alter the authors' adherence to all the PLoS ONE policies on sharing data and materials.
Funding: The authors acknowledge support from the Canadian Institutes of Health Research (#37857, #55042, #93603), the Natural Science and Engineering Research Council of Canada (#227923-05, #312961-05), the Ontario Alzheimer's Society, the Scottish Rite Charitable Foundation of Canada, and the Ontario Graduate Scholarship program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.McLaurin J, Kierstead ME, Brown ME, Hawkes CA, Lambermon MH, et al. Cyclohexanehexol inhibitors of Abeta aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat Med. 2006;12:801–808. doi: 10.1038/nm1423. [DOI] [PubMed] [Google Scholar]
- 2.Fenili D, Brown M, Rappaport R, McLaurin J. Properties of scyllo-inositol as a therapeutic treatment of AD-like pathology. J Mol Med. 2007;85:603–611. doi: 10.1007/s00109-007-0156-7. [DOI] [PubMed] [Google Scholar]
- 3.Chishti MA, Yang DS, Janus C, Phinney AL, Horne P, et al. Early-onset amyloid deposition and cognitive deficits in transgenic mice expressing a double mutant form of amyloid precursor protein 695. J Biol Chem. 2001;276:21562–21570. doi: 10.1074/jbc.M100710200. [DOI] [PubMed] [Google Scholar]
- 4.Choi JK, Carreras I, Dedeoglu A, Jenkins BG. Detection of increased scyllo-inositol in brain with magnetic resonance spectroscopy after dietary supplementation in Alzheimer's disease mouse models. Neuropharmacology. 2010;59:353–357. doi: 10.1016/j.neuropharm.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Michaelis T, Helms G, Merboldt KD, Hänicke W, Bruhn H, et al. Identification of Scyllo-inositol in proton NMR spectra of human brain in vivo. N M R Biomed. 1993;6:105–109. doi: 10.1002/nbm.1940060116. [DOI] [PubMed] [Google Scholar]
- 6.Seaquist ER, Gruetter R. Identification of a high concentration of scyllo-inositol in the brain of a healthy human subject using 1H- and 13C-NMR. Magn Reson Med. 1998;39:313–316. doi: 10.1002/mrm.1910390220. [DOI] [PubMed] [Google Scholar]
- 7.Palmano KP, Whiting PH, Hawthorne JN. Free and lipid myo-inositol in tissues from rats with acute and less severe streptozotocin-induced diabetes. Biochem J. 1977;167:229–235. doi: 10.1042/bj1670229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwon HM, Yamauchi A, Uchida S, Preston AS, Garcia-Perez A, et al. Cloning of the cDNa for a Na+/myo-inositol cotransporter, a hypertonicity stress protein. J Biol Chem. 1992;267:6297–6301. [PubMed] [Google Scholar]
- 9.Hager K, Hazama A, Kwon HM, Loo DD, Handler JS, et al. Kinetics and specificity of the renal Na+/myo-inositol cotransporter expressed in Xenopus oocytes. J Membr Biol. 1995;143:103–113. doi: 10.1007/BF00234656. [DOI] [PubMed] [Google Scholar]
- 10.Coady MJ, Wallendorff B, Gagnon DG, Lapointe JY. Identification of a novel Na+/myo-inositol cotransporter. J Biol Chem. 2002;277:35219–35224. doi: 10.1074/jbc.M204321200. [DOI] [PubMed] [Google Scholar]
- 11.Roll P, Massacrier A, Pereira S, Robaglia-Schlupp A, Cau P, et al. New human sodium/glucose cotransporter gene (KST1): identification, characterization, and mutation analysis in ICCA (infantile convulsions and choreoathetosis) and BFIC (benign familial infantile convulsions) families. Gene. 2002;285:141–148. doi: 10.1016/s0378-1119(02)00416-x. [DOI] [PubMed] [Google Scholar]
- 12.Bourgeois F, Coady MJ, Lapointe JY. Determination of transport stoichiometry for two cation-coupled myo-inositol cotransporters: SMIT2 and HMIT. J Physiol. 2005;563:333–343. doi: 10.1113/jphysiol.2004.076679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berry GT, Mallee JJ, Kwon HM, Rim JS, Mulla WR, et al. The human osmoregulatory Na+/myo-inositol cotransporter gene (SLC5A3): molecular cloning and localization to chromosome 21. Genomics. 1995;25:507–513. doi: 10.1016/0888-7543(95)80052-n. [DOI] [PubMed] [Google Scholar]
- 14.Inoue K, Shimada S, Minami Y, Morimura H, Miyai A, et al. Cellular localization of Na+/MYO-inositol co-transporter mRNA in the rat brain. Neuroreport. 1996;7:1195–1198. doi: 10.1097/00001756-199604260-00020. [DOI] [PubMed] [Google Scholar]
- 15.Liu X, Erikson C, Brun A. Cortical synaptic changes and gliosis in normal aging, Alzheimer's disease and frontal lobe degeneration. Dementia. 1996;7:128–134. doi: 10.1159/000106867. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser LG, Schuff N, Cashdollar N, Weiner MW. Scyllo-inositol in normal aging human brain: 1H magnetic resonance spectroscopy study at 4 Tesla. N M R Biomed. 2005;18:51–55. doi: 10.1002/nbm.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang VY, Zoghbi HY. Genetic regulation of cerebellar development. Nat Rev Neurosci. 2001;2:484–491. doi: 10.1038/35081558. [DOI] [PubMed] [Google Scholar]
- 18.Thal DR, Rüb U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/wnl.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 19.Ostlund RE, Jr, Seemayer R, Gupta S, Kimmel R, Ostlund EL, et al. A stereospecific myo-inositol/D-chiro-inositol transporter in HepG2 liver cells. Identification with D-chiro-[3-3H]inositol. J Biol Chem. 1996;271:10073–10078. doi: 10.1074/jbc.271.17.10073. [DOI] [PubMed] [Google Scholar]
- 20.Hakvoort A, Haselbach M, Galla HJ. Active transport properties of porcine choroid plexus cells in culture. Brain Res. 1998;795:247–256. doi: 10.1016/s0006-8993(98)00284-4. [DOI] [PubMed] [Google Scholar]
- 21.Bissonnette P, Coady MJ, Lapointe JY. Expression of the sodium-myo-inositol cotransporter SMIT2 at the apical membrane of Madin-Darby canine kidney cells. J Physiol. 2004;558:759–768. doi: 10.1113/jphysiol.2004.064311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aouameur R, Da Cal S, Bissonnette P, Coady MJ, Lapointe JY. SMIT2 mediates all myo-inositol uptake in apical membranes of rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1300–G1307. doi: 10.1152/ajpgi.00422.2007. [DOI] [PubMed] [Google Scholar]
- 23.Klaus F, Palmada M, Lindner R, Laufer J, Jeyaraj S, et al. Up-regulation of hypertonicity-activated myo-inositol transporter SMIT1 by the cell volume-sensitive protein kinase SGK1. J Physiol. 2008;586:1539–1547. doi: 10.1113/jphysiol.2007.146191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin X, Ma L, Fitzgerald RL, Ostlund RE., Jr Human sodium/inositol cotransporter 2 (SMIT2) transports inositols but not glucose in L6 cells. Arch Biochem Biophys. 2009;481:197–201. doi: 10.1016/j.abb.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubin LJ, Hale CC. Characterization of a Mg-dependent, Na-inositol co-transport process in cardiac sarcolemmal vesicles. J Mol Cell Cardiol. 1993;25:721–731. doi: 10.1006/jmcc.1993.1084. [DOI] [PubMed] [Google Scholar]
- 26.Sun Y, Zhang G, Hawkes CA, Shaw JE, McLaurin J, et al. Synthesis of scyllo-inositol derivatives and their effects on amyloid beta peptide aggregation. Bioorg Med Chem. 2008;16:7177–7184. doi: 10.1016/j.bmc.2008.06.045. [DOI] [PubMed] [Google Scholar]
- 27.Hawkes CA, Deng LH, Shaw JE, Nitz M, McLaurin J. Small molecule beta-amyloid inhibitors that stabilize protofibrillar structures in vitro improve cognition and pathology in a mouse model of Alzheimer's disease. Eur J Neurosci. 2010;31:203–213. doi: 10.1111/j.1460-9568.2009.07052.x. [DOI] [PubMed] [Google Scholar]
- 28.Transition Therapeutics Inc website. Available: http://www.transitiontherapeutics.com/media/archive.php. Accessed 2011 August 5.
- 29.Elan Corporation Website. Available: http://newsroom.elan.com/phoenix.zhtml?c=88326&p=irol-newsArticle&ID=1458241. Accessed 2011, August 5.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A comparison of D- and L- chiro -inositol inhibition of myo-inositol transport in HEK293 cells. Based on reduction in myo and scyllo-inositol transport by, D-chiro-inositol it is recognized by SMIT1/2, while L-chiro-inositol is not. This finding was examined more closely in HEK293 cells, by comparing the transport of myo-inositol-(2-3H) in the presence of increasing concentrations of D-chiro-inositol (A), to transport observed in the presence of increasing concentrations of L-chiro-inositol (B). As expected myo-inositol-(2-3H) transport was inhibited by D-chiro-inositol in a concentration dependent manner. In contrast, L-chiro-inositol did not inhibit myo-inositol-(2-3H) transport, except at the highest concentration, 160 mM. (n = 3 wells per variable).
(TIF)
L-fucose-(5,6-3H) transport. L-fucose was found to be a competitive inhibitor of myo- and scyllo-inositol-(2-3H) transport through SMIT1/2. This finding was further examined by: (A) examining myo-inositol-(2-3H) transport in the presence of increasing concentrations of L-fucose. A concentration-dependent reduction of myo-inositol-(2-3H) transport was observed. (B) An examination of L-fucose-(5,6-3H) transport in these cells, in the presence or absence of potential competitive substrates. Only background radioactivity was observed, with not active transport. (C) L-fucose-(5,6-3H) transport was examined in the presence or absence of D-fucose and increasing concentrations of cold L-fucose and again only background radioactivity was observed. (n = 3 wells per variable).
(TIF)