Abstract
The white spotted tussock moth, Orgyia thyellina, is a typical insect that exhibits seasonal polyphenisms in morphological, physiological, and behavioral traits, including a life-history tradeoff known as oogenesis-flight syndrome. However, the developmental processes and molecular mechanisms that mediate developmental plasticity, including life-history tradeoff, remain largely unknown. To analyze the molecular mechanisms involved in reproductive polyphenism, including the diapause induction, we first cloned and characterized the diapause hormone-pheromone biosynthesis activating neuropeptide (DH-PBAN) cDNA encoding the five Phe-X-Pro-Arg-Leu-NH2 (FXPRLa) neuropeptides: DH, PBAN, and α-, β-, and γ-SGNPs (subesophageal ganglion neuropeptides). This gene is expressed in neurosecretory cells within the subesophageal ganglion whose axonal projections reach the neurohemal organ, the corpus cardiacum, suggesting that the DH neuroendocrine system is conserved in Lepidoptera. By injection of chemically synthetic DH and anti-FXPRLa antibody into female pupae, we revealed that not only does the Orgyia DH induce embryonic diapause, but also that this neuropeptide induces seasonal polyphenism, participating in the hypertrophy of follicles and ovaries. In addition, the other four FXPRLa also induced embryonic diapause in O. thyellina, but not in Bombyx mori. This is the first study showing that a neuropeptide has a pleiotropic effect in seasonal reproductive polyphenism to accomplish seasonal adaptation. We also show that a novel factor (i.e., the DH neuropeptide) acts as an important inducer of seasonal polyphenism underlying a life-history tradeoff. Furthermore, we speculate that there must be evolutionary conservation and diversification in the neuroendocrine systems of two lepidopteran genera, Orgyia and Bombyx, in order to facilitate the evolution of coregulated life-history traits and tradeoffs.
Introduction
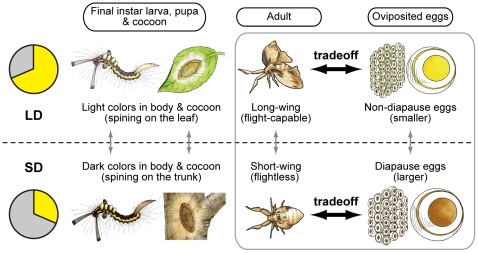
A seasonal polyphenism is a developmental phenotypic plasticity that has evolved for seasonal adaptation, and consists of the differential expression of alternative phenotypes from a single genotype depending on environmental conditions, including the photoperiod, temperature, and nutrition [1], [2]. The white-spotted tussock moth, Orgyia thyellina, which belongs to Lymantriidae, has two or three generation per year, and pass winter season as diapause egg. Further, only females of this moth, but not males, exhibits seasonal polyphenism with various phenotypes of morphological, physiological, and behavioral traits in response to photoperiod (Fig. 1) [3], [4]. This sexual difference in photoperiodic response is very unique among many moth species because the sexual dimorphism of other moths is genetically determined [5], [6], [7], [8]. In the adult stage, flight-capable, long-winged females of O. thyellina are produced from larvae reared at a long photoperiod, while flightless, short-winged females are formed at a short photoperiod. Females emerging in the summer are of the normal, long-winged morph, but those in the autumn are of the short-winged morph. The summer long-winged female lays non-diapause eggs, whereas the autumnal short-winged female lays diapause eggs, which arrest at early embryonic stage [9]. In addition, various reproductive polyphenisms are exhibited in egg size, eggshell thickness, ovarian weight, and egg number. The diapause eggs are heavier in weight, larger in size, and much thicker in the chorion than the non-diapause eggs, presumably providing richer reserves and a more protective chorion to enhance the adaptation to low temperatures in the overwintering state [3].
Figure 1. Seasonal polyphenism in the white spotted tussock moth, Orgyia thyellina.
Orgyia exhibits seasonal changes in various morphological, physiological, and behavioral traits, which are determined by the photoperiod during the late larval stages from 4th to 5th instar larvae. In the long-day condition (LD), the larval integument becomes light colored in the final instar larva (6th instar) as does the pupae of the females and their cocoons. The larval integuments and cocoons are darkly colored under short-day conditions (SD). In the adult stage, LD females are flight-capable long-winged morphs, but SD females are flightless short-winged morphs. Furthermore, LD female lay non-diapause eggs, whereas the SD female lay diapause eggs, which are arrested in early embryonic development. These diapause eggs are heavier in weight, larger in size, and much thicker in the chorion than non-diapause eggs [3], [4].
In general, long-winged and short-winged morphs have different energetic profiles reflecting their different propensities for migration versus reproduction [10]. The polyphenism consists of a flight-capable morph that delays reproduction, and a flightless morph that exhibits substantially elevated fecundity; this is a life-history tradeoff called the “oogenesis-flight syndrome” in various insects [10], [11]. These life-history traits alter the energy allocation of limited internal resources to maximize reproductive success [12], [13], [14]. However, the developmental processes and molecular mechanisms that are integrated with environmental stimuli for seasonal polyphenisms and tradeoff strategies are largely unknown, although presumably the developmental plasticity of a life-history trait has evolved to integrate signals from the environment into normal developmental processes through transcriptional and/or neuroendocrine regulators [2].
The silkworm, Bombyx mori (Bombycidae) is a typical insect entering diapause at an early embryonic stage, similar to Orgyia as described above [15]. Research on the diapause mechanisms of the silkworm has contributed to the understanding of insect neuroendocrinology and to the technical development of the sericultural industry [16]. Diapause hormone (DH) is a 24 aa peptide amide belonging the Phe-X-Pro-Arg-Leu-NH2 (FXPRL amide; FXPRLa) neuropeptide family, which is responsible for embryonic diapause, and functions by acting on a G protein-coupled receptor in the developing ovaries during pupal-adult development in females [16], [17]. The DH-pheromone biosynthesis activating neuropeptide (DH-PBAN) gene encodes a polypeptide precursor consisting of five FXPRLa neuropeptides: DH, PBAN, and α-, β-, and γ-SGNPs in various insects, including B. mori [18], [19], which is exclusively expressed in eight pairs of neurosecretory cells (DH-PBAN-producing neurosecretory cells; DHPCs) located within the subesophageal ganglion (SG) [18], [20], [21].
In this study, we cloned and characterized the Orgyia DH-PBAN cDNA, and showed that Orgyia DH has a pleiotropic effect in the seasonal reproductive polyphenism, including diapause induction, which may be orchestrated via several signaling pathways to integrate various traits to accomplish the seasonal adaptation. These are the first results to show that a novel factor (i.e., the DH neuropeptide) acts as an important inducer of seasonal polyphenism underlying a life-history tradeoff. Furthermore, we speculate that there must be evolutionary conservation and diversification in the neuroendocrine systems of two lepidopteran genera, Orgyia and Bombyx, in order to facilitate the evolution of coregulated life-history traits and tradeoffs.
Results
Characterization of the Orgyia thyellina DH-PBAN cDNA
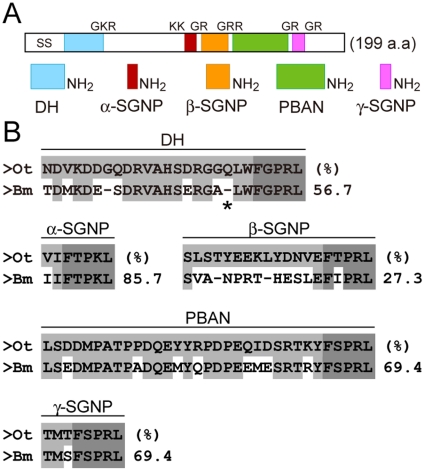
The DH-PBAN cDNA had already been cloned in various insect species, including the Lepidoptera [19], [22]. We cloned the Orgyia thyellina DH-PBAN (OtDH-PBAN) cDNA from brain-SG complex mRNA using a PCR-based strategy. By aligning the deduced amino acid sequence with the DH-PBAN precursor polyprotein from various insects, we found highly similar characteristics of those precursor polyproteins, especially in the Lepidoptera (data not shown). The DH-PBAN cDNAs were highly conserved in all five encoded FXPRLa neuropeptides, including those of Bombyx (Fig. 2) [19]. These seemed to include processing sites for the molecular maturation of DH and other FXPRLa neuropeptides through tryptic cleavage and amidation of the GKR, KK, GRR, and 3 GR sequences, as well as a signal peptide cleavage site (Fig. 2A) [18]. Compared with that in Bombyx, the C-terminal amino acid sequence, FXPRLa, was completely conserved in each peptide (Fig. 2B). In addition, a glutamine residue at position 19 in DH was inserted in Orgyia, although this insertion was not found in other insect species (Fig. 2B). Thus, we first identified the DH-PBAN cDNA in Lymantriidae insects, and found that it was highly conserved in other species.
Figure 2. Schematic drawing of the DH-PBAN precursor polyprotein in Orgyia.
A; DH-PBAN cDNA encoding pre-prohormone consisting of 199 amino acids. It seems to undergo post-translational processing via a series of enzymatic steps that cleave and further modify by amidation the GKR, KK, GRR, and 3 GR sequences at the C-terminal amino acid of the intermediate peptide substrates to yield the signal sequence (SS), DH, α-, β-, and γ-SGNP, and PBAN, similar to other Lepidopteran DH-PBAN precursor polyproteins. B; Alignment of Orygia DH-PBAN with Bombyx DH-PBAN. Conserved amino acids are indicated with shadow boxes; highly conserved amino acids in FXPRLa sequences are indicated with dark shadow boxes. The percentages of identical amino acids is represented on the right side of the peptide sequences. A glutamine residue at position 19 in Orygia DH is shown by an asterisk (*).
Developmental expression profiles of DH-PBAN
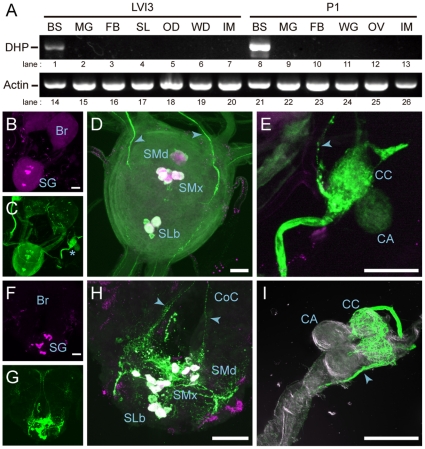
We looked at the expression of OtDH-PBAN in various tissues during larval-pupal and pupal-adult development using RT-PCR analysis. OtDH-PBAN mRNA was exclusively expressed in the brain-SG complex during post-embryonic development (Fig. 3A, lanes 1 and 8), although actin mRNA was expressed ubiquitously throughout post-embryonic development (Fig. 3A, lanes 14–26). Next, we examined the localization of OtDH-PBAN in the central nervous system by whole-mount in situ hybridization and immunohistochemistry (Figs. 3B–I). The intensity signal was detected in large somata along the ventral midline within the larval and pupal SG, respectively, in in situ hybridization (Fig. 3B and F). Furthermore, immunohistochemical staining with an anti-FXPRLa antibody identified somata in the SGs of both larval and pupal stages (Fig. 3C and G) whose immunofluorescence overlapped with the HNPP/FastRed TR fluorescences of the DH-PBAN mRNA probe (Fig. 3D and H). We found that the arrangements of these neuromeres were similar to DHPCs conserved among insect species, which contain three neuromeres, four mandibular cells (SMd), six maxillary cells (SMx), and two labial cells (SLb) located along the ventral midline [23], [24], [25]. Using an anti-FXPRLa antibody, the axons projecting from the DHPCs extended to the circumesophageal connective (Fig. 3H), and their axonal projections reached the neurohemal organ corpus cardiacum (CC) in the larval and pupal SG, respectively (Fig. 3E and I). Therefore, we concluded that OtDH-PBAN is expressed in the DHPCs of SG, and the FXPRLa peptides are transported into the CC where they are released into the hemolymph, and that these neuroendocrine processes conserved in other lepidopteran insects.
Figure 3. Developmental expression profiles of OtDH-PBAN.
A; RT-PCR analysis was performed on day 3 in sixth instar larvae (LVI3) as well as on day 1 in pupae (P1). Levels of DH-PBAN (DHP, lanes 1–13) and actin (lanes 14–26) mRNAs were examined. BS, brain-subesophageal ganglion complex; MG, midgut; FB, fat body; SL, silk gland; OD, ovarian disc; WD, wing disc; IM, integument and muscle; WG, wing; OV, ovary. Whole-mount in situ hybridization was performed in larval (B) and pupal (F) brain-SG complexes by using antisense RNA of OtDH-PBAN as a probe. Using anti-FXPRLa antibody, immunohistochemistry was performed in larvae (C) and pupae (G) stages. Magnified double stained images are shown in magenta (DH-PBAN RNA) and green (anti-FXPRLa) in larval (D) and pupal (H) SG, and larval corpus cardiacum, CC (E). Immunostaining was performed in pupal CC (I). FXPRLa immunoreactive somata were detected in three neuromeres, mandibular cells (SMd), maxillary cells (SMx), and labial cells (SLb), located along the ventral midline. The projective axons (arrowhead) from these somata run into the CC via the circumesophageal connective (CoC). Scale bar = 10 µm.
Effects of Orgyia DH on diapause induction and its metabolism
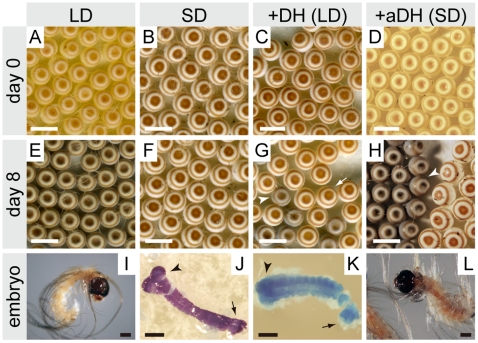
To determine whether Orgyia DH induces embryonic diapause, we injected chemically synthetic DH into pupae kept under long-day condition (LD; non-diapause type) (Fig. 4). When DH was injected into female pupae at 333 pmol/pupa, oviposited eggs took on a dark brown color (Fig. 4C), similar to diapause eggs induced by short-day condition (SD) (Fig. 4B), and were bigger than non-diapause eggs induced by LD (Fig. 4A). At 8 days after oviposition, non-diapause eggs took on pigmentation in the head and body of developing embryos (Fig. 4E and I). In other words, some of the eggs oviposited from DH-injected pupae had the appearance of diapause eggs (Fig. 4G, arrow) obtained by SD (Fig. 4F), although some of the eggs took on pigmentation in the head and body of developing embryos similar to non-diapause eggs (Fig. 4G, arrowhead). In SD animals, we observed that the embryos were arrested and entered diapause in the early embryonic stage, with cephalic lobe and talson formation (Fig. 4J). We also observed variation in diapause stages in DH-injected animals, not only in cephalic lobe and talson formation similar to SD animals, but also in segmentation of the mesoderm in early embryonic development in Orgyia (Fig. 4K). These diapause eggs never hatched, even after a year kept at 25°C. However, when the eggs were kept at 5°C for 3 months starting from 7 days after oviposition, and then returned to 25°C, larvae hatched within 10 days (data not shown). Therefore, we categorized these eggs injected with DH as diapause the same as those induced by SD. Next, we injected various amounts of Orgyia DH into LD-types. DH caused a dose-dependent increase in the percentage of diapause eggs in a range of 12–333 pmol/pupa (Fig. 5A). The activity was saturated at more than 333 pmol/pupa, and the half-maximal dose was estimated to be 111 pmol/pupa.
Figure 4. Morphology of Orgyia eggs.
Eggs laid just after oviposition (A–D), 8 days after oviposition (E–H), and embryos (I–L). LD and SD show eggs (A, E and B, F) and embryos (I and J) oviposited from long-day and short-day conditions, respectively. +DH (LD) and +aDH (SD) show eggs (C, G and D, H) and embryos (K and L) oviposited from pupae injected with DH and anti-FXPRLa, respectively. The white arrow and arrowhead indicate diapause and non-diapause eggs, respectively. The black arrow and arrowheads indicate talson and cephalic lobes, respectively. Scale bar = 5 mm.
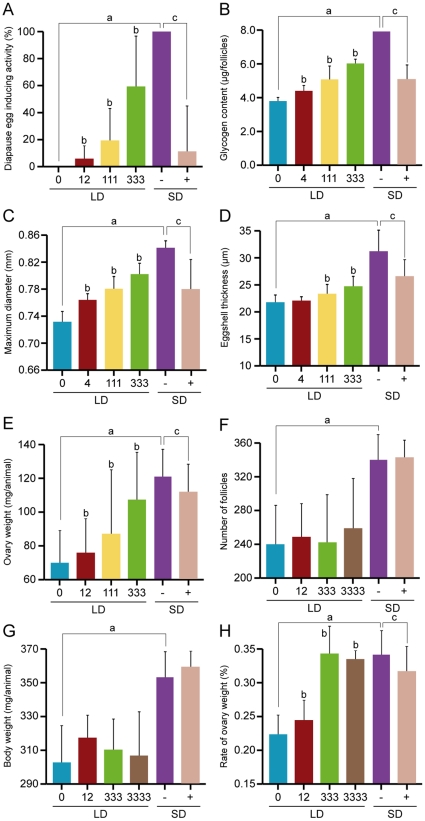
Figure 5. Effects of Orgyia DH on diapause and oogenesis involved in seasonal polyphenism.
The Orgyia DH was injected into LD-pupae at various doses, and analyzed for diapause egg inducing activity (A), glycogen content (B), maximum diameter of follicles (C), eggshell thickness (D), ovary weight (E), number of follicles (F), body weight (G), and rate of ovarian weight (H). Furthermore, anti-FXPRLa serum (+) and pre-immuno serum (−) were injected into SD-pupae to examine the DH functions as described in the text. Each bar represents the mean value of 10 samples ± SD. Lower-case letters indicate statistically significant differences at the 5% level in DH-injected animals at 0 pmol in LD vs. pre-immuno serum-injected animals in SD (a), DH-injected animals at 0 pmol in LD vs. DH-injected animals at various doses (b), and pre-immuno serum-injected animals in SD vs. anti-FXPRLa-injected animals (c).
In addition to DH injection, we examined whether the injection of anti-FXPRLa antibody affects diapause induction. We injected anti-FXPRLa serum 1 day after pupation in SD-pupae, and observed the diapause nature of the eggs (Fig. 4D, H, and L). Although pre-immune serum had no effect on the diapause status, so that all eggs entered diapause as did the non-treated controls (Fig. 5A, −), injection of anti-FXPRLa caused non-diapause eggs to become light yellow and smaller than diapause eggs in most of the egg batch just after oviposition (Fig. 4D and 5A, +). With advancing embryogenesis, non-diapause eggs took on pigmentation in the head and body of developing embryos (Fig. 4H). The larvae were hatched within 10 days (Fig. 4L). Consequently, these results suggest that the anti-FXPRLa serum acts in the hemolymph to inactivate DH through immunoneutralization.
In general, insects accumulate reserves prior to diapause. Sufficient reserves must be sequestered to both survive the diapause period and enable postdiapause development [26]. In Bombyx, diapause eggs are associated with qualitative and quantitative shifts in glycogen metabolism, which accumulate larger glycogen reserves than their nondiapausing counterparts [15]. Therefore, we investigated whether the Orgyia DH affects glycogen content in the ovary. The Orgyia DH caused an increase in glycogen content in ovaries in a dose-dependent manner, and the injection of anti-FXPRLa suppressed that accumulation (Fig. 5B). Collectively, we demonstrated that the neuropeptide DH induced not only embryonic diapause, but also shifts to diapause metabolism in Orgyia.
Effects of Orgyia DH on oogenesis involved in seasonal polyphenism
We further examined whether DH induces various traits in seasonal polyphenism in oogenesis, although none of those changes are observed in Bombyx. First, we observed various traits of oogenesis in LD- and SD-pupae. The eggs of SD-animals were larger, heavier, had a thicker chorion, had a greater number of follicles, and were heavier in the whole body compared to those of LD-animals (Fig. 5C–G). Next, we investigated whether the Orgyia DH affected those phenotypes. Orgyia DH caused an increase in the maximum diameter, eggshell thickness, and ovary weight in a dose-dependent manner (Fig. 5C–E), although there were differences in response for each trait (Fig. 5D vs. A–C and E). In addition, injection of the anti-FXPRLa into SD animals induced dwarfness in eggs including smaller size, thinner maximum eggshell thickness, and lighter ovaries similar to non-diapause eggs (Fig. 5C–E). However, no changes were observed in the number of follicles and body weight by Orgyia DH and antiserum injections (Fig. 5F and G).
Consequently, Orgyia DH acts on the ovarian mass (Fig. 5E and H). Interestingly, although the ovarian masses of LD- and SD-pupae were 20% and 35%, respectively (Fig. 5H), DH-injected animals in LD-pupae were similar to SD animals in terms of ovary mass and whole body weight irrespective of the injection of excess amounts of DH. Thus, Orgyia DH induced various traits involved in the seasonal reproductive polyphenism underlying the life-history tradeoff known as oogenesis-flight syndrome.
Effects of other Orgyia FXPRLa on diapause induction in both Orgyia and Bombyx
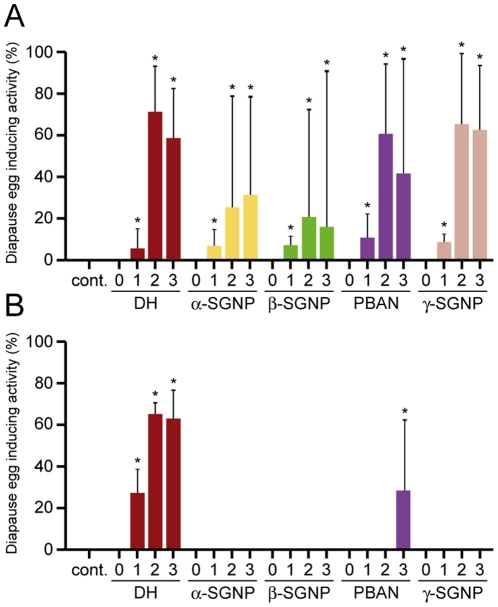
Next, we examined whether other FXPRLa (α-, β-, γ-SGNPs, and PBAN) induced embryonic diapause with a similar dose-response as DH (Fig. 6). We found that all FXPRLa had diapause egg inducing activity, which increased dose-dependently in a range of 33–333 pmol/pupa similar to Orgyia DH in Orgyia (Fig. 6A). These diapause eggs inducing activities were saturated at more than 333 pmol/pupa. Furthermore, we tested whether each Orgyia FXPRLa also induced embryonic diapause in Bombyx (Fig. 6B). Interestingly, only Orgyia DH had a similar effect with the dose used in Orgyia, but PBAN showed diapause egg inducing activity only at 10 times amount of DH. No activity was found in α-, β-, or γ-SGNPs in this experiment (Fig. 6B). These results showed that the diapause inducing activity of Orgyia FXPRLa is different between Orgyia and Bombyx, suggesting that the mechanism of hormonal reception is different in the two species, given that DH and the other FXPRLa have similar structures in both species.
Figure 6. Effects of other Orgyia FXPRLa on diapause induction in both Orgyia and Bombyx.
The Orgyia DH, α-, β-, and γ-SGNPs, and PBAN were injected into LD-pupae of Orgyia (A) and the non-diapause type of Bombyx (B) at various doses [3 (0), 33 (1), 333 (2), and 3333 (3) pmol/pupa], and subjected to analysis of diapause egg inducing activity. Each bar represents the mean value of 20 samples ± SD. Asterisks indicate statistically significant differences at the 5% level.
Discussion
Previously, Bombyx DH was known as the only hormone that induces diapause among known FXPRLa neuropeptides [16]. Furthermore, Bombyx DH stimulates the transcription of the trehalase gene in ovaries, and thereby increases trehalase activity which facilitates higher accumulation of glycogen in eggs, a prerequisite for diapause initiation [27], [28] that sequesters sufficient reserves for survival of the diapause period and postdiapause development [26]. We found that Orgyia DH also acts as a diapause inducer during embryogenesis causing development to be arrested, and enhances the glycogen content, which seems to affect the conserved neuroendocrine system in both Bombyx and Orgyia. We think it is most likely that in SD-type morphs, transportation of DH from the DHPCs somata is actively modified in response to the photoperiod during late larval stages and results in active release into the hemolymph during the pupal stages, likely as in Bombyx [29]. Thus, we showed that the DH acts as a diapause inducer beyond the families Bombycidae and Lymantriidae in lepidopteran insects.
DH and other FXPRLa are multifunctional peptides that play a conserved role in the coordination of life-cycles among and within species. DH acts not only during diapause induction in Bombyx [16], but is also thought to function during ecdysteroidgenesis [30]. Furthermore, the neuropeptides of this family are involved in pheromone biosynthesis, cuticular tanning, myostimulation, pupariation behavior, and the termination of pupal diapause in several lepidopteran insects [31], [32], [33], [34], [35]. In addition to diapause induction, Orgyia DH affects various traits in ovarian growth and maturation such as follicle size, eggshell thickness, and ovarian mass in different dose-dependent manner, although none of these changes are observed in Bombyx. These results suggest that there are several signaling pathways involved in the induction of these various traits. In general, the developing follicle processes the transition among broad developmental periods, which is mediated by hormones such as ecdysteroid and a sesquiterpenoid lipid-like hormone, JH, in insects [36]. In Bombyx, ecdysteroid was shown to be essential and sufficient for ovarian development through the activation of the ecdysone regulatory cascade [37], [38]. Furthermore, the injection of large amounts of 20-hydroxyecdysone (20E) into normal female pupae of Bombyx alters egg production, so that these eggs are larger and heavier than normal eggs [39], suggesting that the ecdysteroid may induce an increase in the number of oocytes by functional activation of nurse cells, to accelerate the growth of oocytes accompanied by enhanced accumulation of yolk proteins and accelerated choriogenesis. Taken together, this suggests that Orgyia DH may induce egg polyphenism directly and/or indirectly via several signaling pathways, including 20E signaling, which may function via ecdysteroidgenesis as in Bombyx [30]. Thus, this is the first evidence suggesting that a neuropeptide (i.e., Orgyia DH) alone has a pleiotropic effect in seasonal reproductive polyphenims, which may be orchestrated via several signaling pathways to integrate various traits, since these polyphenisms are considered to provide richer reserves and more protective chorion to enhance adaptation to low temperatures.
Neuroendocrine systems are known to coordinate the integrated expression of multiple life-history traits across environmental conditions as developmental phenotypic plasticity, which mediates pleiotropy, life-history correlations, and tradeoffs in organisms as diverse as lizards, birds, insects, and echinoderms [13], [40]. In insects, JH and ecdysteroid are well known to be important mediators of life-history tradeoffs [13], [14], [41]. Indeed, it has been suggested that JH and ecdysteroid are involved not only in the tradeoff between flight capability and reproduction in wing-dimorphic crickets, Gryllus firmus [42], but also in tradeoffs in various insects including Drosophila, butterflies, and beetles [14], [43]. Furthermore, it is known that JH alters lipid metabolism that contributes significantly to the tradeoff in G. firmus [44]. We have also confirmed that various traits with respect to ovarian and embryonic development change between long-winged and short-winged morphs in Orgyia (Fig. 1) [3], [4]. Furthermore, it has been observed that the flight muscles become extended and increase in thickness in all long-winged morphs, while the flight muscles remain thin in short-winged morphs (Y. K, unpublished results). Thus, Orgyia exhibits an extreme seasonal polyphenism resulting in a tradeoff between flight capability and reproduction, resulting in differential allocation of internal reserves to ovarian growth versus somatic growth, maintenance, or storage. These are the first results to show that a factor (i.e., DH neuropeptide) other than a sesquiterpenoid lipid-like hormone or steroid hormone (i.e., JH and ecdysteroid) can act as an important inducer of developmental phenotypic plasticity underlying a life-history tradeoff, although it is still unknown what the mechanism of energy allocation is and its involvement in wing development.
Tight control is necessary to produce an animal in which each organ is of an appropriate size relative to the whole body. Different organs typically grow at different rates in developing animals, a phenomenon called “allometry” [45], [46]. The flexibility of allometry is necessary to produce an animal in which the whole body is of an appropriate size relative to environmental conditions such as nutrition, temperature, and population density [47], [48]. Recent studies have indicated that the insulin-signaling pathway controls body and organ size in Drosophila and most metazoans by signaling nutritional conditions to the growing organs [49], [50]. In this study, the rates of ovarian mass to body increased to accompany the increase in ovarian weight by DH injection, so that LD animals injected with DH, even in excess doses, shifted to become similar to SD animals. Therefore, we speculate that there is inherent allometry in ovarian mass in both LD- and SD-types, which determine the photoperiodism. Additionally, the DH-signaling pathway might be involved in the regulation of ovary-body allometry, although it is unknown whether other signals are involved, such as the insulin-signaling pathway.
It has been suggested that variations in endocrine mechanisms may be an important substrate for the evolution of coregulated life-history traits and tradeoffs [14]. Evolutionary modifications of the hormone response may be facilitated by its modular structure [40]. In a previous study, Bombyx DH was found to be solely responsible for embryonic diapause and functions by acting on a specific G protein-coupled receptor in developing ovaries, as other FXPRLa never act at similar doses to DH [16], [17]. Likewise, Orgyia FXPRLa, except for DH, never induce embryonic diapause at similar doses of DH in Bombyx. Interestingly, in the present study we found that Orgyia FXPRLa functions in diapause induction in a similar dose-dependent manner to DH in Orgyia. This result suggests that the structure and/or densities of receptor protein(s) may be different with evolutionary diversification in the two species. Variation in the expression of the receptor protein(s) of the Orgyia FXPRLa family may occur in the two species, so that those differences may promote the hormonal pleiotropy in the polyphenism. Therefore, the present study may provide not only clues for solving the molecular mechanisms of life-history evolution, but also provide new insights into comparative neuroendocrinology in insects.
Materials and Methods
Insects
Eggs of the white spotted tussock moth, Orgyia thyellina, were collected at an apple orchard in the Nagano Fruit Tree Experiment Station (Suzaka, Japan). The hatched larvae were reared on an artificial diet (Silkmate-2S, Nosan Co., Yokohama, Japan) from first instar to third instar, and fresh apple leaves from fourth instar to final instar (sixth instar in females and fifth instar in males) at 22°C under LD (16-h light/8-h dark cycle; 16L:8D) or SD (8L:16D). Pupae were collected within the day just after pupation (referred to as day 0) to synchronize their subsequent development. Pupae were kept at 25°C to allow adult development.
The polyvoltine (N4) strain of Bombyx mori was used in this study. Eggs were incubated at 25°C under continuous darkness. Larvae were reared on an artificial diet (Kuwano-hana, JA Zennoh Gunma, Gunma, Japan). During larval stages, silkworms were reared at 25 to 27°C under a 12L:12D. Pupae were kept at 25°C to allow adult development. N4 moths laid only non-diapause eggs genetically, so that eggs never entered diapause.
cDNA cloning
Poly (A)+ RNA was directly purified from the brain-SG complexes of day 0 pupae of Orgyia using Dynabeads Oligo (dT)25 (Dynal Biotech LLC., Brown Deer, WI, USA). To amplify the DH-PBAN cDNA, RT-PCR was performed using degenerate primers based on sequences of the DH-PBAN cDNA cloned from various insects: 5′-TGGTTCGGYCCCMGRCT-3′ (forward) and 5′-GGHGTRGCWGGCAT-3′ (reverse). The full-length sequence (Accession no. AB259122) was determined using a SMART RACE cDNA amplification kit (Clontech, Mountain View, CA, USA). The location of signal peptide cleavage sites in amino acid sequences were predicted by the SignalP 3.0 server (http://www.cbs.dtu.dk/services/SignalP/). Amino acid alignment of FXPRLa neuropeptides was performed by CLUSTALW (1.83) (http://clustalw.ddbj.nig.ac.jp/top-j.html).
The ORF sequence of the actin cDNA was amplified using primers based on sequences of the actin cDNA cloned from various insects: 5′-ATGTGCGACGARGAAGTTGC-3′ (forward) and 5′-TTAGAAGCACTTCCTGTGNAC-3′ (reverse). The full-length sequence (Accession no. AB283042) was determined using a SMART RACE cDNA amplification kit (Clontech).
RT-PCR analysis
Various tissues were dissected from day 3 sixth instar larvae and day 1 pupae of females reared in SD. Total RNAs were extracted using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and then subjected to Poly (A)+ RNA purification using Dynabeads Oligo (dT)25 (Dynal Biotech LLC.). Poly (A)+ RNA from the brain-SG complex was directly purified using Dynabeads Oligo (dT)25. First-strand DNA was synthesized using a SMART RACE amplification kit (Clontech). PCR amplification was carried out on mRNAs for DH-PBAN and actin genes. The DH-PBAN cDNA was amplified from nucleotides +1 to +759, and the actin cDNA from +238 to +722. The PCR products were subjected to electrophoresis, and then visualized with ethidium bromide staining.
In situ hybridization and Immunohistochemistry
In situ hybridization was performed as described by [51] with some modifications. Brain-SG and retrocerebral, corpus cardiacum (CC)-corpus allatum (CA) complexes were treated for 5 min or 3.5 min with 10 µg/ml proteinase K (Roche) at 37°C on larval or pupal tissues, respectively. The DIG-labeled RNA probes were prepared with a DIG RNA labeling kit (Roche, Mannheim, Germany) using DH-PBAN cDNA as a template. DH-PBAN cDNA encoding from nucleotides +1 to +759 was amplified by PCR and inserted into the pCR-XL-TOPO vector (Invitrogen) in an antisense direction from the T7 promoter. DIG-labeled RNA was detected with an alkaline phosphatase-conjugated anti-DIG antibody using a DIG nucleic acid detection kit (Roche), and by using the HNPP fluorescent detection set (Roche) as a substrate. The immunoreaction procedures were adapted from [29] by using an anti-FXPRLa polyclonal antibody [25], [29]. HNPP/FastRed TR fluorescence and anti-FXPRLamide immunofluorescent staining were detected using both a Radiance 2000 confocal microscope (Bio-Rad Lab., Hercules, CA, USA) and confocal microscope system A1 (Nikon, Tokyo, Japan). Images were adjusted and assembled in Adobe Photoshop CS3 (Adobe Systems, Inc., San Jose, CA, USA).
Injection of peptides and antibody
Purified synthetic peptides (DH, α-, β-, γ-SGNP, and PBAN) of >95% purity (HPLC area percentage) were provided from Operon Biotechnologies, KK. (Tokyo, Japan). Each peptide was dissolved in distilled water, and 10 µl solutions of each at various doses were injected into pupae on the day of pupation (day 0) of Orgyia, and 4 days after pupation in the N4 strain of Bombyx. Anti-FXPRLa antiserum was injected at 10 µl into Orgyia pupae on day 0 in accordance with [27].
Analyses of oogenesis
The ovaries were dissected out with phosphate-buffered saline (PBS) 1 day before eclosion when pupae integuments became white, and the wing color patterns of the adult forms were visualized. The paired ovaries were thoroughly separated into 8 ovarioles, then blotted dry, weighed quickly, and the number of follicles was counted. In each ovariole, 20 successively developing follicles from side of the lateral oviducts were collected to measure the maximum diameter, eggshell thickness, and glycogen content. The maximum diameter and eggshell thickness were measured by scanning on a SZX-12 microscope (Olympus, Tokyo, Japan) with a micrometer. Glycogen was extracted by digesting the homogenate with 30% (W/V) KOH in a boiling bath for 30 min, and was precipitated with ethanol at 4°C [52], and measured by the phenol-sulfuric acid method [53].
Observation of embryogenesis and diapause
In both Orgyia and Bombyx, eclosed female moths were allowed to copulate with males overnight, and then to lay eggs overnight. The laid eggs were kept at 25°C, under which condition the non-diapause eggs hatched on day 7 to 8 after oviposition. Some eggs became light white or yellow in a few days and were recognized as non-fertilized eggs. Diapause eggs were identified as eggs that were colored dark brown on day 3 and remained unhatched at 2 weeks after oviposition, at which time all non-diapause eggs had hatched. Diapause egg inducing activity was estimated by counting the number of diapausing and non-diapausing eggs after the non-diapause eggs hatched, and the results were expressed as a percentage of diapause eggs [54]. The embryos were stained with carbolic thionin solution according to [55]. Eggs were mounted on a slide glass sealed with adhesive tape, and then eggs were sliced with a razor and observed under a microscope.
Acknowledgments
We thank Mrs. Eiji Yoshizawa and Shuichi Kato of Nagano Fruit Tree Experiment Station (Suzaka, Japan) for kindly providing the Orgyia thyellina larvae, and Mrs. Mika Jitsukawa for kindly providing the illustration in Fig. 1. We are also indebted to the Division of Gene Research, Research Center for Human and Environmental Sciences, Shinshu University, for providing facilities for these studies.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by a Grant-in-Aid for Scientific Research (20380033) from the Japan Society for the Promotion of Science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.West-Eberhard MJ. Developmental Plasticity and Evolution: Oxford Univ Pr (Sd) 2003.
- 2.Gilbert SF, Epel D. Gilbert SF, Epel D, editors. How agents in the environment effect molecular changes in development. Ecological Developmental Biology: Integrating Epigenetics, Medicine, and Evolution: Sinauer Associates Inc. 2009. pp. 38–78.
- 3.Kimura T, Masaki S. Brachypterism and seasonal adaptation in Orgyia thyellina Butler (Lepidoptera, Lymantriidae). Kontyu. 1977;45:97–106. [Google Scholar]
- 4.Sato T. Life history and diapause of the white-spotted tussock moth, Orgyia thyellina Butler (Lepidoptera: Lymantriidae). Odokon. 1977;21:6–14. [Google Scholar]
- 5.Hafez M, El-Said L. On the bionomics of Orgyia dubia judaea Stgr. (Lepidoptera: Lymantriidae). Bulletin de la Societe Entomologique d' Egypte. 1969;62:161–183. [Google Scholar]
- 6.Nardi JB, Godfrey GL, Bergstrom RA. Programmed cell death in the wing of Orgyia leucostigma (Lepidoptera: Lymantriidae). Journal of morphology. 1991;209:121–131. doi: 10.1002/jmor.1052090110. [DOI] [PubMed] [Google Scholar]
- 7.Gu S-H, Tsai R-S, Chow Y-S, Lin F-J. Sexual dimorphism in developmental rate and ecdysteroid titre in Orgyia postica. Journal of Insect Physiology. 1992;38:1043–1049. [Google Scholar]
- 8.Niitsu S. Wing Degeneration Due to Apoptosis in the Female of the Winter Moth Nyssiodes lefuarius (Lepidoptera, Geometridae). Entomological science. 2001;4:1–7. [Google Scholar]
- 9.Umeya Y. Embryonic hibernation and diapause in insects from the viewpoint of the hibernating-eggs of the silkworm. Bull Sericul Exp Sta. 1946;12:393–480. [Google Scholar]
- 10.Hodin J. Whiteman DW, Ananthakrishnan TN, editors. She shapes events as they come: Plasticity in female insect reproduction. Phenotypic plasticity of insects: Mechanisms and Consequences: Science Pub Inc. 2009. pp. 423–521.
- 11.Gatehouse AG, Zhang X-X. Drake VA, Gatehouse AG, editors. Migratory potential in insects: variation in an uncertain environment. Insect migration: tracking resources through space and time: Cambridge University Press. 1995. pp. 193–242.
- 12.Roff DA. Theory and analysis: Chapman & Hall, Inc; 1992. The evolution of life histories. [Google Scholar]
- 13.Zera AJ, Harshman LG. The physiology of life history trade-offs in animals. Annu Rev Ecol Syst. 2001;32:95–126. [Google Scholar]
- 14.Flatt T, Tu MP, Tatar M. Hormonal pleiotropy and the juvenile hormone regulation of Drosophila development and life history. Bioessays. 2005;27:999–1010. doi: 10.1002/bies.20290. [DOI] [PubMed] [Google Scholar]
- 15.Yamashita O, Yaginuma T. Silkworm eggs at low temperatures: Implication for sericulture. In: Lee JRE, Denlinger DL, editors. Insects at Low Temperature. New York: Chapman and Hall; 1991. pp. 424–445. [Google Scholar]
- 16.Yamashita O. Diapause hormone of the silkworm, Bombyx mori: structure, gene expression and function. J Insect Physiol. 1996;42:669–679. [Google Scholar]
- 17.Homma T, Watanabe K, Tsurumaru S, Kataoka H, Imai K, et al. G protein-coupled receptor for diapause hormone, an inducer of Bombyx embryonic diapause. Biochem Biophys Res Commun. 2006;344:386–393. doi: 10.1016/j.bbrc.2006.03.085. [DOI] [PubMed] [Google Scholar]
- 18.Sato Y, Oguchi M, Menjo N, Imai K, Saito H, et al. Precursor polyprotein for multiple neuropeptides secreted from the suboesophageal ganglion of the silkworm Bombyx mori: characterization of the cDNA encoding the diapause hormone precursor and identification of additional peptides. Proc Natl Acad Sci U S A. 1993;90:3251–3255. doi: 10.1073/pnas.90.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi MY, Vander Meer RK. Identification of a new member of the PBAN family of neuropeptides from the fire ant, Solenopsis invicta. Insect Mol Biol. 2009;18:161–169. doi: 10.1111/j.1365-2583.2009.00867.x. [DOI] [PubMed] [Google Scholar]
- 20.Sato Y, Ikeda M, Yamashita O. Neurosecretory cells expressing the gene for common precursor for diapause hormone and pheromone biosynthesis-activating neuropeptide in the suboesophageal ganglion of the silkworm, Bombyx mori. Gen Comp Endocrinol. 1994;96:27–36. doi: 10.1006/gcen.1994.1156. [DOI] [PubMed] [Google Scholar]
- 21.Shiomi K, Fujiwara Y, Yasukochi Y, Kajiura Z, Nakagaki M, et al. The Pitx homeobox gene in Bombyx mori: regulation of DH-PBAN neuropeptide hormone gene expression. Mol Cell Neurosci. 2007;34:209–218. doi: 10.1016/j.mcn.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 22.Jurenka R, Nusawardani T. The pyrokinin/pheromone biosynthesis-activating neuropeptide (PBAN) family of peptides and their receptors in Insecta: evolutionary trace indicates potential receptor ligand-binding domains. Insect Mol Biol. 2010. [DOI] [PubMed]
- 23.Ichikawa T, Hasegawa K, Shimizu I, Katsuno K, Kataoka H, et al. Structure of neurosecretory cells with immunoreactive diapause hormone and pheromone biosynthesis activating neuropeptide in the silkworm, Bombyx mori. Zoolog Sci. 1995;12:703–712. [Google Scholar]
- 24.Davis NT, Homberg U, Teal PE, Altstein M, Agricola HJ, et al. Neuroanatomy and immunocytochemistry of the median neuroendocrine cells of the subesophageal ganglion of the tobacco hawkmoth, Manduca sexta: immunoreactivities to PBAN and other neuropeptides. Microsc Res Tech. 1996;35:201–229. doi: 10.1002/(SICI)1097-0029(19961015)35:3<201::AID-JEMT3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 25.Sato Y, Shiomi K, Saito H, Imai K, Yamashita O. Phe-X-Pro-Arg-Leu-NH(2) peptide producing cells in the central nervous system of the silkworm, Bombyx mori. J Insect Physiol. 1998;44:333–342. doi: 10.1016/s0022-1910(97)00140-6. [DOI] [PubMed] [Google Scholar]
- 26.Hahn DA, Denlinger DL. Energetics of insect diapause. Annu Rev Entomol. 2011;56:103–121. doi: 10.1146/annurev-ento-112408-085436. [DOI] [PubMed] [Google Scholar]
- 27.Shiomi K, Ishida Y, Ikeda M, Sato Y, Saito H, et al. Induction of non-diapause eggs by injection of anti-diapause hormone rabbit serum into the diapause type of the silkworm, Bombyx mori. J Insect Physiol. 1994;40:693–699. [Google Scholar]
- 28.Su ZH, Ikeda M, Sato Y, Saito H, Imai K, et al. Molecular characterization of ovary trehalase of the silkworm, Bombyx mori and its transcriptional activation by diapause hormone. Biochimica et biophysica acta. 1994;1218:366–374. doi: 10.1016/0167-4781(94)90190-2. [DOI] [PubMed] [Google Scholar]
- 29.Hagino A, Kitagawa N, Imai K, Yamashita O, Shiomi K. Immunoreactive intensity of FXPRL amide neuropeptides in response to environmental conditions in the silkworm, Bombyx mori. Cell Tissue Res. 2010;342:459–469. doi: 10.1007/s00441-010-1083-4. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe K, Hull JJ, Niimi T, Imai K, Matsumoto S, et al. FXPRL-amide peptides induce ecdysteroidogenesis through a G-protein coupled receptor expressed in the prothoracic gland of Bombyx mori. Mol Cell Endocrinol. 2007;273:51–58. doi: 10.1016/j.mce.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Holman GM, Cook BJ, Nachman RJ. Primary structure and synthesis of a blocked myotropic neuropeptide isolated from the cockroach, Leucophaea maderae. Comp Biochem Physiol C. 1986;85:219–224. doi: 10.1016/0742-8413(86)90077-0. [DOI] [PubMed] [Google Scholar]
- 32.Raina AK, Jaffe H, Kempe TG, Keim P, Blacher RW, et al. Identification of a Neuropeptide Hormone That Regulates Sex Pheromone Production in Female Moths. Science. 1989;244:796–798. doi: 10.1126/science.244.4906.796. [DOI] [PubMed] [Google Scholar]
- 33.Matsumoto S, Kitamura A, Nagasawa H, Kataoka H, Orikasa C, et al. Functional diversity of a neurohormone produced by the suboesophageal ganglion: Molecular identity of melanization and reddish colouration hormone and pheromone biosynthesis activating neuropeptide. J Insect Physiol. 1990;36:427–432. [Google Scholar]
- 34.Zdarek J, Nachman RJ, Hayes TK. Insect neuropeptides of the pyrokinin/PBAN family accelerate pupariation in the fleshfly (Sarcophaga bullata) larvae. Ann N Y Acad Sci. 1997;814:67–72. doi: 10.1111/j.1749-6632.1997.tb46145.x. [DOI] [PubMed] [Google Scholar]
- 35.Xu WH, Denlinger DL. Molecular characterization of prothoracicotropic hormone and diapause hormone in Heliothis virescens during diapause, and a new role for diapause hormone. Insect molecular biology. 2003;12:509–516. doi: 10.1046/j.1365-2583.2003.00437.x. [DOI] [PubMed] [Google Scholar]
- 36.Swevers L, Raikhel AS, Sappington TW, Shirk P, Iatrou K. Gilbert LI, Iatrou K, Gill SS, editors. Vitellogenesis and post-vitellogenic maturation of the insect ovarian follicle. Comprehensive Molecular Insect Science: Elsevier Pergamon. 2005. pp. 87–155.
- 37.Chatani F, Ohnishi E. Effect of ecdysone on the ovarian development of Bombyx silkworm. Develop Growth and Differ. 1976;18:481–484. doi: 10.1111/j.1440-169X.1976.00481.x. [DOI] [PubMed] [Google Scholar]
- 38.Swevers L, Iatrou K. The ecdysone regulatory cascade and ovarian development in lepidopteran insects: insights from the silkmoth paradigm. Insect Biochem Mol Biol. 2003;33:1285–1297. doi: 10.1016/j.ibmb.2003.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Kawaguchi Y, Banno Y, Doira H, Fujii H. Manifestation of characteristics in the “Giant Egg” mutant of Bombyx mori (Lepidoptera: Bombycidae) 2. Artificial induction of large eggs by application of large amounts of 20-hydroxyecdysone. Odokon. 1989;33:63–68. [Google Scholar]
- 40.Heyland A, Hodin J, Reitzel AM. Hormone signaling in evolution and development: a non-model system approach. BioEssays: news and reviews in molecular, cellular and developmental biology. 2005;27:64–75. doi: 10.1002/bies.20136. [DOI] [PubMed] [Google Scholar]
- 41.Nijhout HF. Nijhout HF, editor. Polyphenisms. Insect hormones: Princeton Univ Pr. 1994. pp. 176–196.
- 42.Zera AJ. Whiteman DW, Ananthakrishnan TN, editors. Wing polymorphism in Gryllus (Orthoptera: Gryllidae): Proximate endocrine, energetic and biochemical mechanisms underlying morph specialization for flight vs. reproduction. Phenotypic plasticity of insects: Mechanisms and Consequences: Science Pub Inc. 2009. pp. 609–653.
- 43.Nijhout HF, Emlen DJ. Competition among body parts in the development and evolution of insect morphology. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:3685–3689. doi: 10.1073/pnas.95.7.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao Z, Zera AJ. Differential lipid biosynthesis underlies a tradeoff between reproduction and flight capability in a wing-polymorphic cricket. Proc Natl Acad Sci U S A. 2002;99:16829–16834. doi: 10.1073/pnas.262533999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stern DL, Emlen DJ. The developmental basis for allometry in insects. Development. 1999;126:1091–1101. doi: 10.1242/dev.126.6.1091. [DOI] [PubMed] [Google Scholar]
- 46.Shingleton AW, Frankino WA, Flatt T, Nijhout HF, Emlen DJ. Size and shape: the developmental regulation of static allometry in insects. BioEssays: news and reviews in molecular, cellular and developmental biology. 2007;29:536–548. doi: 10.1002/bies.20584. [DOI] [PubMed] [Google Scholar]
- 47.Thomas RH. Ecology of body size in Drosophila buzzatii: untangling the effects of temperature and nutrition. Ecological Entomology. 1993;18:84–90. [Google Scholar]
- 48.Santos M, Fowler K, Partridge L. Gene-environment interaction for body size and larval density in Drosophila melanogaster: an investigation of effects on development time, thorax length and adult sex ratio. Heredity. 1994;72:515–521. doi: 10.1038/hdy.1994.69. [DOI] [PubMed] [Google Scholar]
- 49.Shingleton AW, Das J, Vinicius L, Stern DL. The temporal requirements for insulin signaling during development in Drosophila. PLoS biology. 2005;3:e289. doi: 10.1371/journal.pbio.0030289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mirth CK, Riddiford LM. Size assessment and growth control: how adult size is determined in insects. BioEssays: news and reviews in molecular, cellular and developmental biology. 2007;29:344–355. doi: 10.1002/bies.20552. [DOI] [PubMed] [Google Scholar]
- 51.Shiomi K, Fujiwara Y, Atsumi T, Kajiura Z, Nakagaki M, et al. Myocyte enhancer factor 2 (MEF2) is a key modulator of the expression of the prothoracicotropic hormone gene in the silkworm, Bombyx mori. Febs J. 2005;272:3853–3862. doi: 10.1111/j.1742-4658.2005.04799.x. [DOI] [PubMed] [Google Scholar]
- 52.Yamashita O, Irie K. Larval hatching from vitellogenin-deficient eggs developed in male hosts of the silkworm. Nature. 1980;283:385–386. [Google Scholar]
- 53.Dubois M, Gilles KA, Hamilton JK, Pebers PA, Smith F. Colorimetric method for determination of sugars and related substances. Analytical Chemistry. 1956;28:350–356. [Google Scholar]
- 54.Yamashita O, Hasegawa K. Embryonic diapause. In: Kerkut GA, Gilbert LI, editors. Comprehensive Insect Physiology, Biochemistry and Pharmacology. Oxford: Pergamon Press; 1985. pp. 407–434. [Google Scholar]
- 55.Yaginuma T, Kobayashi M, Yamashita O. Effects of low temperatures on NAD-sorbitol dehydrogenase activity and morphogenesis in non-diapause eggs of the silkworm, Bombyx mori. Comp Biochem Physiol. 1990;97B:495–506. [Google Scholar]