In the united states, nearly 60,000 patients per day receive general anesthesia for surgery.1 General anesthesia is a drug-induced, reversible condition that includes specific behavioral and physiological traits — unconsciousness, amnesia, analgesia, and akinesia — with concomitant stability of the autonomic, cardiovascular, respiratory, and thermoregulatory systems.2 General anesthesia produces distinct patterns on the electroencephalogram (EEG), the most common of which is a progressive increase in low-frequency, high-amplitude activity as the level of general anesthesia deepens3,4 (Fig. 1). How anesthetic drugs induce and maintain the behavioral states of general anesthesia is an important question in medicine and neuroscience.6 Substantial insights can be gained by considering the relationship of general anesthesia to sleep and to coma.
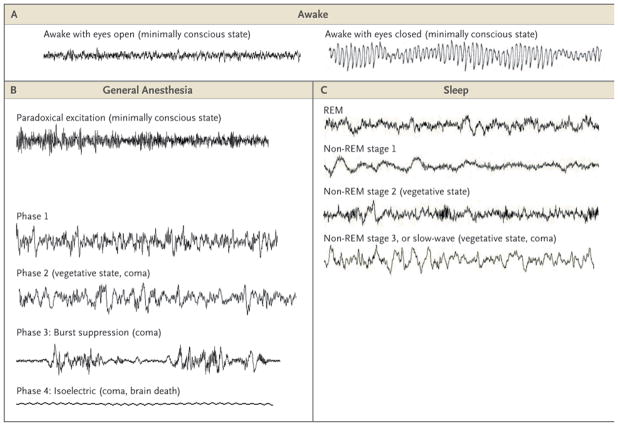
Figure 1. Electroencephalographic (EEG) Patterns during the Awake State, General Anesthesia, and Sleep.
Panel A shows the EEG patterns when the patient is awake, with eyes open (left) and the alpha rhythm (10 Hz) with eyes closed (right). Panel B shows the EEG patterns during the states of general anesthesia: paradoxical excitation, phases 1 and 2, burst suppression, and the isoelectric tracing. Panel C shows the EEG patterns during the stages of sleep: rapid-eye-movement (REM) sleep; stage 1 non-REM sleep; stage 2 non-REM sleep, and stage 3 non-REM (slow-wave) sleep. The EEG patterns during recovery from coma — coma, vegetative state, and minimally conscious state — resemble the patterns during general anesthesia, sleep, and the awake state. EEG tracings during sleep are from Watson et al.5
Humans spend approximately one third of their lives asleep. Sleep, a state of decreased arousal that is actively generated by nuclei in the hypothalamus, brain stem, and basal forebrain, is crucial for the maintenance of health.7,8 Normal human sleep cycles between two states — rapid-eye-movement (REM) sleep and non-REM sleep — at approximately 90-minute intervals. REM sleep is characterized by rapid eye movements, dreaming, irregularities of respiration and heart rate, penile and clitoral erection, and airway and skeletal-muscle hypotonia.7 In REM sleep, the EEG shows active high-frequency, low-amplitude rhythms (Fig. 1). Non-REM sleep has three distinct EEG stages, with higher-amplitude, lower-frequency rhythms accompanied by waxing and waning muscle tone, decreased body temperature, and decreased heart rate.
Coma is a state of profound unresponsiveness, usually the result of a severe brain injury.9 Comatose patients typically lie with eyes closed and cannot be roused to respond appropriately to vigorous stimulation. A comatose patient may grimace, move limbs, and have stereotypical withdrawal responses to painful stimuli yet make no localizing responses or discrete defensive movements. As the coma deepens, the patient’s responsiveness even to painful stimuli may diminish or disappear. Although the patterns of EEG activity observed in comatose patients depend on the extent of the brain injury, they frequently resemble the high–amplitude, low-frequency activity seen in patients under general anesthesia10 (Fig. 1). General anesthesia is, in fact, a reversible drug-induced coma. Nevertheless, anesthesiologists refer to it as “sleep” to avoid disquieting patients. Unfortunately, anesthesiologists also use the word “sleep” in technical descriptions to refer to unconsciousness induced by anesthetic drugs.11 (For a glossary of terms commonly used in the field of anesthesiology, see the Supplementary Appendix, available with the full text of this article at NEJM.org.)
This review discusses the clinical and neurophysiological features of general anesthesia and their relationships to sleep and coma, focusing on the neural mechanisms of unconsciousness induced by selected intravenous anesthetic drugs.
CLINICAL SIGNS AND EEG PAT TERNS OF UNCONSCIOUSNESS INDUCED BY GENER AL ANESTHESIA
The clinical signs and EEG patterns of general anesthesia–induced unconsciousness can be described in relation to the three periods in which they appear: induction, maintenance, and emergence.
INDUCTION PERIOD
Before induction, the patient has a normal, active EEG, with prominent alpha activity (10 Hz) when the eyes are closed (Fig. 1). Administration of a small dose of a hypnotic drug such as propofol, a barbiturate, or etomidate, all of which act on γ-aminobutyric acid type A (GABAA) receptors, induces a state of sedation in which the patient is calm and easily arousable, with the eyes generally closed.12 As the dose is slowly increased, the patient may enter a state of paradoxical excitation,13 characterized by purposeless or defensive movements, incoherent speech, euphoria or dysphoria, and an increase in beta activity on the EEG (13 to 25 Hz).3,4,13–16 This state is termed paradoxical because the drug that is intended to induce unconsciousness induces excitation instead.
As more of the hypnotic agent is administered — typically as a bolus over a period of 10 to 15 seconds — an increasingly irregular respiratory pattern develops that progresses to apnea, at which point bag-mask ventilation must be initiated to support breathing. There is a concomitant loss of response to oral commands and skeletal-muscle tone. Loss of consciousness can be easily assessed by having patients follow the movement of the anesthesiologist’s finger with their eyes. As unconsciousness ensues, eye tracking stops, nystagmus may appear, and blinking increases. The oculocephalic, eyelash, and corneal reflexes are lost,17 yet the pupillary light reflex remains.18 There can be either an increase or a decrease in blood pressure, whereas the heart rate typically increases. Administering an opioid or a benzodiazepine before or during induction may mitigate the increased heart-rate response, and vaso-pressors may be given to maintain blood pressure. Tracheal intubation is usually performed at the end of induction, after the administration of a muscle relaxant.
MAINTENANCE PERIOD
General anesthesia is maintained by a combination of hypnotic agents, inhalational agents, opioids, muscle relaxants, sedatives, and cardiovascular drugs, along with ventilatory and thermoregulatory support. During the maintenance period, changes in the heart rate and blood pressure are among the clinical signs used to monitor the level of general anesthesia (see the Supplementary Appendix). When the state of general anesthesia is inadequate for the level of nociceptive stimulation from surgery, the heart rate and blood pressure can increase dramatically, alerting the anesthesiologist to the possibility of increased nociception and arousal. Other indicators of inadequate general anesthesia are perspiration, tearing, changes in pupil size, the return of muscle tone, movement,19 and changes in EEG measures of brain activity.20 At levels appropriate for surgery, general anesthesia can functionally approximate brain-stem death,21 because patients are unconscious, have depressed brain-stem reflexes, do not respond to nociceptive stimuli, have no apneic drive, and require cardiorespiratory and thermoregulatory support.9
Four EEG patterns define the phases of the maintenance period (Fig. 1). Phase 1, a light state of general anesthesia, is characterized by a decrease in EEG beta activity (13 to 30 Hz) and an increase in EEG alpha activity (8 to 12 Hz) and delta activity (0 to 4 Hz).22 During phase 2, the intermediate state, beta activity decreases and alpha and delta activity increases, with so-called anteriorization — that is, an increase in alpha and delta activity in the anterior EEG leads relative to the posterior leads.22,23 The EEG in phase 2 resembles that seen in stage 3, non-REM (or slow-wave) sleep. Phase 3 is a deeper state, in which the EEG is characterized by flat periods interspersed with periods of alpha and beta activity — a pattern called burst suppression.15 As this state of general anesthesia deepens, the time between the periods of alpha activity lengthens, and the amplitudes of the alpha and beta activity decrease. Surgery is usually performed during phases 2 and 3. In phase 4, the most profound state of general anesthesia, the EEG is isoelectric (completely flat). An isoelectric EEG may be purposely induced by the administration of a barbiturate or propofol to protect the brain during neurosurgery24 or to stop generalized seizures.25,26
EMERGENCE PERIOD
Emergence from general anesthesia is a passive process that depends on the amounts of drugs administered; their sites of action, potency, and pharmacokinetics; the patient’s physiological characteristics; and the type and duration of the surgery. Recovery from general anesthesia is generally assessed by monitoring physiological and behavioral signs. The return of spontaneous respirations is typically one of the first clinical signs observed once peripheral neuromuscular blockade is decreased. This marks the patient’s return from a functional state that approximates brainstem death (Table 1). The heart rate and blood pressure typically increase, provided that these responses are not pharmacologically blocked. Salivation and tearing begin, followed by nonlocalizing responses to painful stimulation, suggesting that the patient’s state is most similar to a vegetative state with the notable exception that the eyes remain closed. As skeletal-muscle tone returns, the patient begins to grimace, swallow, gag, and cough and make defensive movements, such as reaching for the endotracheal or naso-gastric tube. At this point the anesthesiologist will perform extubation, provided that there is sufficient return of brain-stem reflexes to maintain spontaneous respirations and airway protection, even if there is no response to oral commands. The eyes may still not open spontaneously. As the patient emerges from general anesthesia, the EEG patterns proceed in approximately reverse order from phases 2 or 3 of the maintenance period to an active EEG that is consistent with a fully awake state (Fig. 1). Between extubation and discharge from the postanesthesia care unit, the patient passes through a minimally conscious state.27 Functional responses that are beyond a minimally conscious state must be evident before the patient is discharged from the postanesthesia care unit.27,28 The patient should be able to answer simple questions and to convey any discomfort, such as pain or nausea.
Table 1.
Emergence from General Anesthesia and Stages of Recovery from Coma.*
| Emergence from General Anesthesia | Recovery from Coma |
|---|---|
|
General anesthesia Stable administration of anesthetic drugs Arousal not possible, unresponsive; eyes closed, with reactive pupils Analgesia, akinesia Drug-controlled blood pressure and heart rate Mechanically controlled ventilation EEG patterns ranging from delta and alpha activity to burst suppression |
Brain-stem death No respiratory response to apneic oxygenation test Total loss of brain-stem reflexes Isoelectric EEG pattern |
|
Coma Structural brain damage to both cerebral hemispheres, with or without injuries to tegmental midbrain, rostral pons, or both Isolated bilateral injuries to midline tegmental midbrain, rostral pons, or both Arousal not possible, unresponsive Functionally intact brain stem, normal arterial blood gases EEG pattern of low-amplitude delta activity and intermittent bursts of theta and alpha activity or possibly burst suppression | |
|
Emergence, phase 1 Cessation of anesthetic drugs Reversal of peripheral-muscle relaxation (akinesis) Transition from apnea to irregular breathing to regular breathing Increased alpha and beta activity on EEG |
Vegetative state Spontaneous cycling of eye opening and closing Grimacing and nonpurposeful movements EEG pattern of high-amplitude delta and theta activity Absence of EEG features of sleep Usually able to ventilate without mechanical support |
|
Emergence, phase 2 Increased heart rate and blood pressure Return of autonomic responsiveness Responsiveness to painful stimulation Salivation (7th and 9th cranial nerve nuclei) Tearing (7th cranial nerve nuclei) Grimacing (5th and 7th cranial nerve nuclei) Swallowing, gagging, coughing (9th and 10th cranial nerve nuclei) Return of muscle tone (spinal cord, reticulospinal tract, basal ganglia, and primary motor tracts) Defensive posturing Further increase in alpha and beta activity on EEG Extubation possible |
|
|
Emergence, phase 3 Eye opening Responses to some oral commands Awake patterns on EEG Extubation possible |
Minimally conscious state Purposeful guarding movements, eye tracking Inconsistent communication, verbalizations Following oral commands Return of sleep–wake cycles Recovery of some EEG features of normal sleep–wake architecture |
EEG denotes electroencephalogram.
MECHANISMS OF UNCONSCIOUSNESS INDUCED BY GENER AL ANESTHESIA
CORTICAL CIRCUITS AND ALTERED AROUSAL
Observations from clinical practice and basic science indicate that anesthetic drugs induce unconsciousness by altering neurotransmission at multiple sites in the cerebral cortex,29–35 brain stem, and thalamus. If a procedure does not require full general anesthesia, it is standard clinical practice to use low doses of a hypnotic or sedative drug to achieve sedation, which is defined as diminished cognitive function (cortical activity)35 with intact respiratory and cardiovascular (brain-stem) function. Substantial decreases in neural activity in the cortex have been observed in a rodent model of general anesthesia.30 Similarly, positron-emission tomographic studies in humans under general anesthesia revealed appreciable decreases in cortical metabolic activity.31,32 Functional magnetic resonance imaging33 and local-field-potential recordings34 in humans have provided additional evidence of cortical mechanisms of unconsciousness induced by general anesthesia. In vivo and in vitro molecular pharmacologic studies have identified GABAA and N-methyl-D-aspartate (NMDA) receptors in the cortex, thalamus, brain stem, and striatum as two of the important targets of hypnotic drugs.36,37 Because small numbers of inhibitory interneurons control large numbers of excitatory pyramidal neurons, the enhanced GABAA inhibition induced by general anesthesia can efficiently inactivate large regions of the brain and contribute to unconsciousness (Fig. 2A).38,39
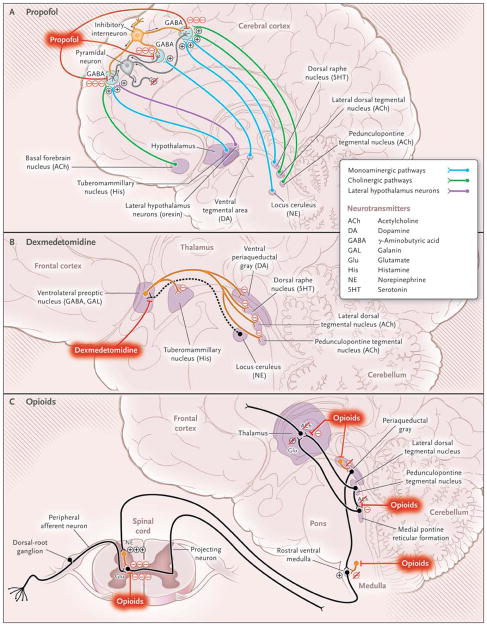
Figure 2. Possible Neural-Circuit Mechanisms of Altered Arousal Induced by Anesthetic Agents.
Panel A shows a GABAergic inhibitory interneuron (orange) synapsing on a pyramidal neuron (gray) receiving excitatory inputs from ascending arousal pathways. The monoaminergic pathways arise from the locus ceruleus, which releases norepinephrine; the raphe, which releases serotonin; the tuberomammillary nucleus, which releases histamine; and the ventral teg-mental area, which releases dopamine. The cholinergic pathways, which release acetylcholine, arise from the basal forebrain, the lateral dorsal tegmental nuclei, and the pedunculopontine tegmental nuclei. Lateral hypothalamic neurons release orexin. Propofol binds post-synaptically and enhances GABAergic inhibition, counteracting arousal inputs to the pyramidal neuron, decreasing its excitatory activity, and contributing to unconsciousness. Dexmedetomidine binds to α2 receptors on neurons from the locus ceruleus, inhibiting norepinephrine release (dashed line) in the ventrolateral preoptic nucleus, as shown in Panel B. The disinhibited ventrolateral preoptic nucleus reduces arousal by means of GABAA-mediated and galanin-mediated inhibition of the midbrain, hypothalamic, and pontine arousal nuclei. As shown in Panel C, opioids reduce arousal by inhibiting the release of acetylcholine from neurons projecting from the lateral dorsal and pedunculopontine tegmental nuclei to the medial pontine reticular formation and to the thalamus, by binding to opioid receptors in the periaqueductal gray and rostral ventral medulla, and by binding presynaptically and postsynaptically to spinal cord opioid receptors at the synapses between peripheral afferent neurons in the dorsal-root ganglion and projecting neurons.
THE BRAIN STEM, SLEEP, AND ALTERED AROUSAL
A hypnotic drug administered as a bolus during induction of general anesthesia rapidly reaches brain-stem arousal centers, where it contributes to unconsciousness.40 The clinical signs cited above are consistent with actions in the brain stem. Losses of the oculocephalic and corneal reflexes are nonspecific indicators of impaired brain-stem function due to the actions of the hypnotic agent on the oculomotor, trochlear, abducens, trigeminal, and facial nuclei in the midbrain and pons.9
In a study in rodents, direct injection of a barbiturate into the mesopontine tegmental area led to unconsciousness.41 Such observations confirm brain-injury studies showing that brain-stem unconsciousness typically involves the lateral dorsal tegmental areas of the pons and the midbrain paramedian region.42 Apnea can be explained, in part, by the actions of the hypnotic agent on GABAA interneurons in the respiratory control network in the ventral medulla and pons.43
The rapid atonia that occurs after bolus administration of propofol is most likely due to the actions of this drug in the spinal cord44 and in the pontine and medullary reticular nuclei that control the antigravity muscles.45 Certain observations are consistent with this concept of propofol’s mechanism of action — for example, the atonia that follows the inadvertent injection of local anesthetics into the subarachnoid space or basilar artery during an interscalene block46,47 or the inadvertent injection of alcohol into the cervical spinal cord during a facet block.9 Furthermore, observations in patients who have pontine strokes and locked-in syndromes support this concept,9 as do observations with the muscle-relaxing and soporific effects of the GABA agonist baclofen.48 Such observations may explain why near the end of surgery, small doses of propofol can be used to provide rapid muscle relaxation of short duration and why, in this case, propofol is preferable to muscle relaxants that have a slower onset and longer duration of action. In contrast to the drug-induced atonia described above, rigidity and spasticity are typically seen in patients who are in a coma or a vegetative state,9 and muscle tone is preserved during slow-wave sleep.7
Signs of the loss of brain-stem function (apnea, atonia, and losses of the oculocephalic and corneal49 reflexes) and hence unconsciousness reliably indicate when to initiate bag-mask ventilation or to place a laryngeal mask airway during induction of general anesthesia.
Neural circuits involved in sleep provide additional insights into basal forebrain, brain-stem, and hypothalamic mechanisms of unconsciousness. During the awake state, the locus ceruleus provides norepinephrine-mediated inhibition of the ventrolateral preoptic nucleus in the hypothalamus.50,51 Therefore, GABAA-mediated and galanin-mediated inhibition of the ascending arousal circuits by the ventrolateral preoptic nucleus is inhibited and the awake state is promoted.51 Adenosine, one of the brain’s principal somnogens, accumulates from degradation of adenosine triphosphate during prolonged intervals of wakefulness.8 Binding of adenosine in the ventrolateral preoptic nucleus is associated with increased activity in this brain center.52 Adenosine binding and inhibition of the locus ceruleus lead to activation of the ventrolateral preoptic nucleus, which inhibits the ascending arousal circuits and promotes non-REM sleep. The sedative dexmedetomidine is an α2-adrenergic agonist that inhibits the release of norepinephrine from the locus ceruleus and thus allows the ventrolateral preoptic nucleus to reduce arousal by inhibiting the ascending arousal circuits (Fig. 2B).53,54 The EEG patterns of dexmedetomidine-induced sedation closely resemble those of non-REM sleep.55 Propofol also promotes unconsciousness, in part, by GABAA-mediated inhibition of release of the arousal-promoting neurotransmitter histamine in the cortex from the tuberomammillary nucleus in the hypothalamus.50
The active EEG patterns observed during REM sleep are due in part to strong cholinergic inputs from the lateral dorsal tegmental and pedunculopontine tegmental nuclei to the medial pontine reticular formation and thalamus and from the basal forebrain to the cortex.56 The synthetic opioid fentanyl decreases arousal by reducing acetylcholine in the medial pontine reticular formation, whereas morphine decreases arousal by inhibiting the neurons in the lateral dorsal teg-mental nucleus, medial pontine reticular formation,56 and basal forebrain57 (Fig. 2C). Opioids further contribute to unconsciousness by binding to opioid receptors in the periaqueductal gray,58 rostral ventral medulla,58,59 spinal cord,59 and possibly peripheral tissue60 to reduce nociceptive transmission in the central nervous system. That opioids act primarily in the nociceptive pathways rather than in the cortex to alter arousal and to partially alter cognition helps explain the high incidence of postoperative awareness in patients undergoing cardiac surgery, for which high-dose opioids have, until recently, been the primary anesthetic.61,62
Clinical studies have shown that unconsciousness induced by propofol can be reversed by the administration of the cholinomimetic agent physostigmine.63 A combination of imaging,31 molecular,37 and neurophysiological30 studies suggests that propofol acts, in part, by enhancing GABAA-mediated inhibition by interneurons of pyramidal neurons in the cortex and subcortical areas,50 whereas physostigmine counteracts this effect by enhancing cholinergic activity throughout the cortex (Fig. 2A). Physostigmine is a standard treatment for emergence delirium,64,65 a state of confusion seen on emergence from general anesthesia.
The EEG patterns and other features of general anesthesia generally differ from those of sleep (Fig. 1). The highly active state of the cortex during REM sleep is mediated by a cholinergic drive emanating from the basal forebrain and from the lateral dorsal and pedunculopontine tegmental nuclei to the cortex via the thalamus.56 As discussed below, paradoxical excitation under general anesthesia may represent GABA-mediated disinhibition in striatothalamic pathways. The skeletal-muscle hypotonia observed during REM sleep is partially due to cholinergic activation of pontomedullary networks, resulting in glycine-mediated inhibition of alpha motor neurons in the spinal cord,66 whereas during paradoxical excitation, motor tone is preserved.13
There is similarity between the EEG patterns seen in slow-wave sleep and those seen in phase 2 of the maintenance period of general anesthesia (Fig. 1). This period of general anesthesia is sufficiently deep to perform surgery. During sleep, the greatest decrease in pain perception occurs in the slow-wave stage. Arousal is possible during this stage, but it requires stronger stimulation than during other stages of sleep.67 Slow-wave sleep has been shown to represent the switch of the thalamus from its tonic to its bursting mode.68 The tonic mode favors transmission of somato-sensory information through the thalamus, whereas the bursting mode inhibits transmission of such information. Phase 2 of the maintenance period of general anesthesia and slow-wave sleep represent profound decreases in cortical activity achieved by pharmacologic means and by an endogenous circuit mechanism, respectively.
CENTRAL THALAMIC CIRCUITS AND CONTROL OF AROUSAL
The central thalamus plays an important role in normal arousal regulation.69 The convergence in this area of ascending pathways from the brain stem and basal forebrain and descending pathways from the frontal cortex helps regulate forebrain arousal and maintain organized behavior during wakefulness. Both direct injury of the central thalamus and marked deafferentation of its neurons due to diffuse brain insults are associated with the severe impairment of arousal and of forebrain functional integration observed in several brain disorders.70 Electrical stimulation of the central thalamus in a minimally conscious patient has been reported to improve cognitive function, mobility, and oral feeding.71 Similarly, unconsciousness induced by general anesthesia has been reversed experimentally by the direct injection of cholinergic agonists into the central thalamus.72
The central thalamus may mediate several of the phenomena seen in general anesthesia, as described above (Fig. 3). A possible explanation for the paradoxical excitation observed with a low dose of nearly every anesthetic drug3,4,13–16 is suggested by the circuit mechanism,69,73 which has been proposed to explain the paradoxical arousal of a patient from a minimally conscious state after administration of the GABAA1 agonist zolpidem.74 After administration of zolpidem, the marked downregulation of the anterior-forebrain network that accompanied this patient’s brain injury was reversed, with notable functional improvement. The globus pallidus interna normally provides tonic inhibitory inputs to the central thalamus that are opposed by striatal inhibition of the pallidum. Binding of zolpidem in the GABAA1-receptor–rich globus pallidus interna may suppress this tonic inhibitory input to the thalamus,75 fostering activation of thalamocortical and thalamostriatal circuits and, consequently, enhancing arousal (Fig. 3A). For anesthetic drugs that promote GABAA inhibition, paradoxical excitation may be explained by a similar action in these circuits. This hypothesis could also account for the paradoxical excitation observed during endoscopy and the delirium observed in intensive care units that is commonly associated with sedation induced with benzodiazepines, which are GABAA agonists.76,77 The fact that purpose-less movements are associated with paradoxical excitation is consistent with a possible basal ganglia mechanism.13
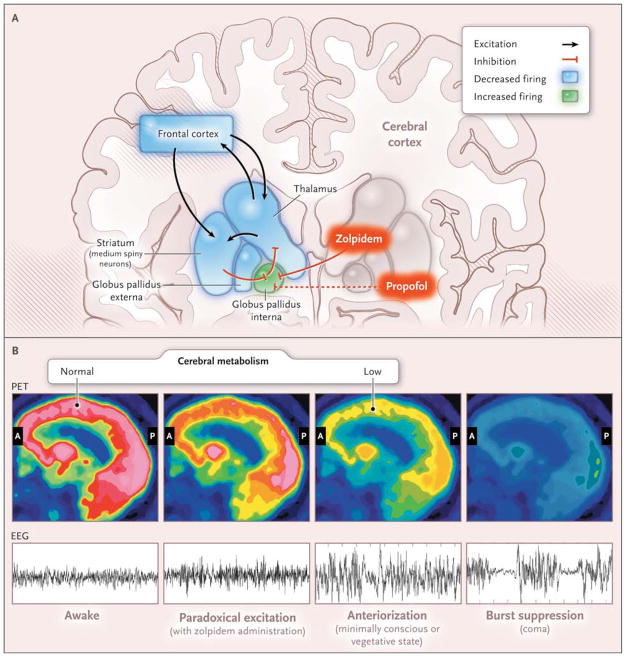
Figure 3. Paradoxical Excitation, Cerebral Metabolism, and Electroencephalographic (EEG) Activity in Stages of Coma Recovery.
Cortical damage causes loss of excitatory inputs from the frontal cortex to the median spiny neurons in the striatum, as shown in Panel A. Normal striatal inhibition of the globus pallidus interna is lost, and the globus pallidus interna tonically inhibits the thalamus. Zolpidem and propofol may bind to GABAA1 receptors in the globus pallidus interna, blocking its inhibitory inputs to the thalamus; as a result, excitatory cortical inputs from the thalamus are restored, causing paradoxical excitation.73 Panel B schematically depicts changes in cerebral metabolism as measured by positron emission tomographic (PET) scanning and on electroencephalography (EEG) at different stages of coma recovery. In the awake state, the EEG pattern is active and cerebral metabolism is globally active. Paradoxical excitation induced by the administration of zolpidem, which is associated with behavioral improvement in some minimally conscious patients, is reflected by an active EEG pattern with reduced prefrontal cortex metabolism. Patients in minimally conscious and vegetative states may show EEG anteriorization, with alpha, theta, and delta EEG patterns and decreased metabolism in the frontal cortex, striatum, and thalamus. Burst suppression in coma correlates with globally depressed metabolism. General anesthesia results in similar EEG patterns. A denotes anterior, and P posterior.
An imaging study in humans suggests a role for the corticobasal ganglia–thalamic circuit in propofol-induced unconsciousness.78 This circuit may also underlie anteriorization (Fig. 3B). The delta and alpha rhythms seen on the EEG during general anesthesia and sleep79 could arise from sustained hyperpolarization of pyramidal neurons in the lower output layers of the cortex owing to withdrawal (during sleep) or inhibition (during general anesthesia) of excitatory synaptic inputs.80 Initiation of these changes in the anterior forebrain could be a consequence of active inhibition of the central thalamus that occurs when cortical inputs to the striatum are insufficient (Fig. 3A). A theoretical model has shown that as propofol is increased to a dose beyond that which produces paradoxical excitation, its actions in the thalamocortical circuits lead to a coherent alpha oscillation between the thalamus and the anterior forebrain.81
Finally, burst suppression is believed to be a strong, synchronized outflow of thalamic discharges to a widely unresponsive cortex82 (Fig. 1). It is a deeper state of general anesthesia than is phase 2 of the maintenance period, which resembles the tonic bursting mode of the thalamus seen in slow-wave sleep. Bursts become more widely separated during burst suppression as the level of general anesthesia deepens. This suggests that a larger fraction of the cortex is inactive during burst suppression relative to phase 2 of general anesthesia or slow-wave sleep. Supporting this hypothesis is the observation that burst suppression is also seen in coma due to diffuse anoxic damage,83 induced hypothermia,84 and epilepsy due to the Ohtahara syndrome.85 The absence of burst suppression during sleep is an important electrophysiological distinction between sleep and general anesthesia.
ACTIVE BR AIN STATES AND UNCONSCIOUSNESS
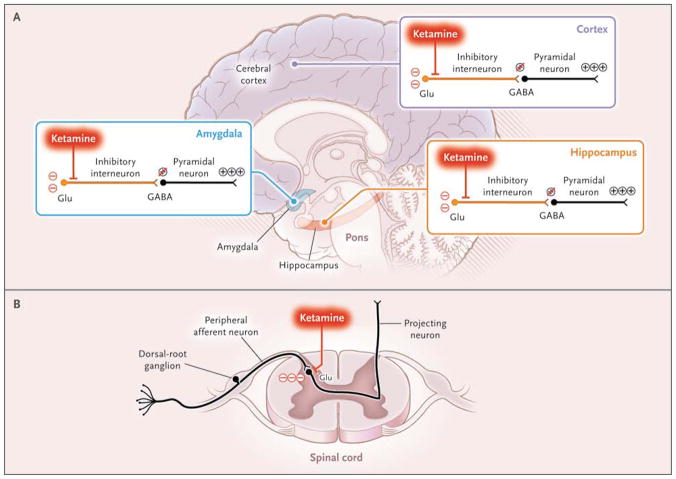
In contrast to unconsciousness induced by most hypnotic agents, which is predominantly associated with slow EEG patterns, unconsciousness induced by the NMDA antagonist ketamine is associated with active EEG patterns.86,87 Seizures are commonly associated with active, highly organized EEG patterns. Unconsciousness due to seizures most likely results from organized, aberrant brain activity that impedes the normal communications necessary to maintain arousal and cognition.88 Similarly, a highly active brain state most likely plays a role in unconsciousness induced by ketamine. Ketamine preferentially inhibits NMDA-mediated glutamatergic inputs to GABAergic interneurons, leading to aberrant excitatory activity in the cortex, hippocampus, and limbic system and ultimately unconsciousness (Fig. 4).89,90 Hallucinations may result because the aberrant activation allows the association of information in a manner that is inconsistent in time and space. The hallucinations can be mitigated by the concurrent administration of a benzodiazepine,91 which presumably acts to enhance GABAA-mediated activity of the interneurons and hence leads to sedation. The potent antinociceptive effects of ketamine on NMDA receptors in the spinal cord and its inhibition of acetylcholine release from the pons also contribute to unconsciousness (Fig. 4).92–94
Figure 4. Unconsciousness and Active Brain States.
Ketamine binds preferentially to N-methyl-D-aspartate (NMDA) receptors on inhibitory interneurons in the cortex, limbic system (amygdala), and hippocampus, promoting an uncoordinated increase in neural activity, an active electroencephalographic pattern, and unconsciousness, as shown in Panel A. In the spinal cord, ketamine decreases arousal by blocking NMDA glutamate (Glu)–mediated nociceptive signals from peripheral afferent neurons in the dorsal-root ganglion to projecting neurons, as shown in Panel B.
EMERGENCE FROM GENER AL ANESTHESIA AND RECOVERY FROM COMA
Recovery from an initial coma — if it occurs — may require hours to years. In contrast, emergence from general anesthesia typically requires minutes. Nevertheless, it is useful to compare emergence from general anesthesia and recovery from coma (Table 1). The early clinical signs of emergence from general anesthesia — return of regular breathing, salivation, tearing, swallowing, gagging, and grimacing — approximate the caudal–rostral progression in the brain stem of the return of sensory, motor, and autonomic function (Table 1). A later sign, such as response to oral commands, indicates the return of cortical function. The quantitative neurobehavioral metrics used to monitor recovery from coma could be used to track the emergence from general anesthesia from a functional state that can approximate brain-stem death to states similar to a vegetative state and, eventually, to a minimally conscious state.95 The fact that general anesthesia can be functionally equivalent to brain-stem death indicates how deeply general anesthesia can depress brain function and perhaps explains why some patients do not fully recover consciousness for several hours after general anesthesia and why postoperative cognitive dysfunction could persist in elderly patients for several months.96
In conclusion, a better understanding of sleep and coma may lead to new approaches to general anesthesia based on new ways to alter consciousness,29,97,98 provide analgesia,99,100 induce amnesia, and provide muscle relaxation.66
Supplementary Material
Acknowledgments
Supported by the Massachusetts General Hospital Department of Anesthesia, Critical Care, and Pain Medicine and by the National Institutes of Health (NIH) Director’s Pioneer Award (DP1OD003646, to Dr. Brown); by the University of Michigan, Department of Anesthesiology, and by NIH grants (HL40881 and HL65272, to Dr. Lydic); and by grants from the James S. McDonnell Foundation (to Dr. Schiff) and the NIH (HD51912, to Dr. Schiff).
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Sentinel event alert: preventing, and managing the impact of, anesthesia awareness. Oakbrook Terrace, IL: The Joint Commission; 2004. ( http://www.jointcommission.org/sentinel_event_alert_issue_32_preventing_and_managing_the_impact_of_anesthesia_awareness.) [PubMed] [Google Scholar]
- 2.Evers A, Crowder M. Cellular and molecular mechanisms of anesthesia. In: Barash PG, Cullen BF, Stoelting RK, Cahalan M, Stock MC, editors. Clinical anesthesia. 6. New York: Lippincott Williams & Wilkins; 2006. pp. 95–114. [Google Scholar]
- 3.Gibbs FA, Gibbs LE, Lennox WG. Effects on the electroencephalogram of certain drugs which influence nervous activity. Arch Intern Med. 1937;60:154–66. [Google Scholar]
- 4.Kiersey DK, Bickford RG, Faulconer A., Jr Electro-encephalographic patterns produced by thiopental sodium during surgical operations; description and classification. Br J Anaesth. 1951;23:141–52. doi: 10.1093/bja/23.3.141. [DOI] [PubMed] [Google Scholar]
- 5.Watson C, Bagdoyan H, Lydic R. A neurochemical perspective on states of consciousness. In: Hudetz AG, Pearce RA, editors. Suppressing the mind: anesthetic modulation of memory and consciousness. New York: Springer/Humana Press; 2010. pp. 33–80. [Google Scholar]
- 6.Kennedy D, Norman C. What don’t we know? Science. 2005;309:75. doi: 10.1126/science.309.5731.75. [DOI] [PubMed] [Google Scholar]
- 7.Kryger M, Roth T, Dement W. Principles and practice of sleep medicine. 5. New York: Elsevier Saunders; 2010. [Google Scholar]
- 8.McCarley RW. Neurobiology of REM and NREM sleep. Sleep Med. 2007;8:302–30. doi: 10.1016/j.sleep.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 9.Posner J, Saper C, Schiff N, Plum F. Plum and Posner’s diagnosis of stupor and coma. New York: Oxford University Press; 2007. [Google Scholar]
- 10.Young GB. The EEG in coma. J Clin Neurophysiol. 2000;17:473–85. doi: 10.1097/00004691-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Gawande A, Denno DW, Truog RD, Waisel DM. Physicians and execution — highlights from a discussion of lethal injection. N Engl J Med. 2008;358:448–51. doi: 10.1056/NEJMp0800378. [DOI] [PubMed] [Google Scholar]
- 12.Davis MH, Coleman MR, Absalom AR, et al. Dissociating speech perception and comprehension at reduced levels of awareness. Proc Natl Acad Sci U S A. 2007;104:16032–7. doi: 10.1073/pnas.0701309104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bevan JC, Veall GR, Macnab AJ, Ries CR, Marsland C. Midazolam premedication delays recovery after propofol without modifying involuntary movements. Anesth Analg. 1997;85:50–4. doi: 10.1097/00000539-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy MM, Brown EN, Kopell N. Potential network mechanisms mediating electroencephalographic beta rhythm changes during propofol-induced paradoxical excitation. J Neurosci. 2008;28:13488–504. doi: 10.1523/JNEUROSCI.3536-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark DL, Rosner BS. Neurophysiologic effects of general anesthetics. I. The electroencephalogram and sensory evoked responses in man. Anesthesiology. 1973;38:564–82. [PubMed] [Google Scholar]
- 16.Rampil IJ. A primer for EEG signal processing in anesthesia. Anesthesiology. 1998;89:980–1002. doi: 10.1097/00000542-199810000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Coté CJ, Goudsouzian NG, Liu LM, Dedrick DF, Rosow CE. The dose response of intravenous thiopental for the induction of general anesthesia in unpremedicated children. Anesthesiology. 1981;55:703–5. doi: 10.1097/00000542-198155060-00023. [DOI] [PubMed] [Google Scholar]
- 18.Gray AT, Krejci ST, Larson MD. Neuromuscular blocking drugs do not alter the pupillary light reflex of anesthetized humans. Arch Neurol. 1997;54:579–84. doi: 10.1001/archneur.1997.00550170055014. [DOI] [PubMed] [Google Scholar]
- 19.Prys-Roberts C. Anaesthesia: a practical or impractical construct? Br J Anaesth. 1987;59:1341–5. doi: 10.1093/bja/59.11.1341. [DOI] [PubMed] [Google Scholar]
- 20.Palanca BJ, Mashour GA, Avidan MS. Processed electroencephalogram in depth of anesthesia monitoring. Curr Opin Anaesthesiol. 2009;22:553–9. doi: 10.1097/ACO.0b013e3283304032. [DOI] [PubMed] [Google Scholar]
- 21.Quality Standards Subcommittee of the American Academy of Neurology. Practice parameters: determining brain death in adults. Neurology. 1995;45:1012–4. doi: 10.1212/wnl.45.5.1012. [DOI] [PubMed] [Google Scholar]
- 22.Feshchenko VA, Veselis RA, Reinsel RA. Propofol-induced alpha rhythm. Neuropsychobiology. 2004;50:257–66. doi: 10.1159/000079981. [DOI] [PubMed] [Google Scholar]
- 23.Tinker JH, Sharbrough FW, Michenfelder JD. Anterior shift of the dominant EEG rhythm during anesthesia in the Java monkey: correlation with anesthetic potency. Anesthesiology. 1977;46:252–9. doi: 10.1097/00000542-197704000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Doyle PW, Matta BF. Burst suppression or isoelectric encephalogram for cerebral protection: evidence from metabolic suppression studies. Br J Anaesth. 1999;83:580–4. doi: 10.1093/bja/83.4.580. [DOI] [PubMed] [Google Scholar]
- 25.Bergey GK. Refractory status epilepticus: is EEG burst suppression an appropriate treatment target during drug- induced coma? What is the Holy Grail? Epilepsy Curr. 2006;6:119–20. doi: 10.1111/j.1535-7511.2006.00117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Claassen J, Hirsch LJ, Emerson RG, Mayer SA. Treatment of refractory status epilepticus with pentobarbital, propofol, or midazolam: a systematic review. Epilepsia. 2002;43:146–53. doi: 10.1046/j.1528-1157.2002.28501.x. [DOI] [PubMed] [Google Scholar]
- 27.Giacino JT, Ashwal S, Childs N, et al. The minimally conscious state: definition and diagnostic criteria. Neurology. 2002;58:349–53. doi: 10.1212/wnl.58.3.349. [DOI] [PubMed] [Google Scholar]
- 28.Aldrete JA. The post-anesthesia recovery score revisited. J Clin Anesth. 1995;7:89–91. doi: 10.1016/0952-8180(94)00001-k. [DOI] [PubMed] [Google Scholar]
- 29.Alkire MT, Hudetz AG, Tononi G. Consciousness and anesthesia. Science. 2008;322:876–80. doi: 10.1126/science.1149213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angel A. The G.L. Brown lecture. Adventures in anaesthesia. Exp Physiol. 1991;76:1–38. doi: 10.1113/expphysiol.1991.sp003471. [DOI] [PubMed] [Google Scholar]
- 31.Alkire MT, Haier RJ, Barker SJ, Shah NK, Wu JC, Kao YJ. Cerebral metabolism during propofol anesthesia in humans studied with positron emission tomography. Anesthesiology. 1995;82:393–403. doi: 10.1097/00000542-199502000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Fiset P, Paus T, Daloze T, et al. Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study. J Neurosci. 1999;19:5506–13. doi: 10.1523/JNEUROSCI.19-13-05506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purdon PL, Pierce ET, Bonmassar G, et al. Simultaneous electroencephalography and functional magnetic resonance imaging of general anesthesia. Ann N Y Acad Sci. 2009;1157:61–70. doi: 10.1111/j.1749-6632.2008.04119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velly LJ, Rey MF, Bruder NJ, et al. Differential dynamic of action on cortical and subcortical structures of anesthetic agents during induction of anesthesia. Anesthesiology. 2007;107:202–12. doi: 10.1097/01.anes.0000270734.99298.b4. [Erratum, Anesthesiology 2008;108:175.] [DOI] [PubMed] [Google Scholar]
- 35.Ferrarelli F, Massimini M, Sarasso S, et al. Breakdown in cortical effective connectivity during midazolam-induced loss of consciousness. Proc Natl Acad Sci U S A. 2010;107:2681–6. doi: 10.1073/pnas.0913008107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudolph U, Antkowiak B. Molecular and neuronal substrates for general anaesthetics. Nat Rev Neurosci. 2004;5:709–20. doi: 10.1038/nrn1496. [DOI] [PubMed] [Google Scholar]
- 37.Hemmings HC, Jr, Akabas MH, Gold-stein PA, Trudell JR, Orser BA, Harrison NL. Emerging molecular mechanisms of general anesthetic action. Trends Pharmacol Sci. 2005;26:503–10. doi: 10.1016/j.tips.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Bai D, Pennefather PS, MacDonald JF, Orser BA. The general anesthetic propofol slows deactivation and desensitization of GABA(A) receptors. J Neurosci. 1999;19:10635–46. doi: 10.1523/JNEUROSCI.19-24-10635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shepherd G. The synaptic organization of the brain. 4. New York: Oxford University Press; 1998. [Google Scholar]
- 40.Propofol. Bedford, OH: Bedford Laboratories; 2008. (package insert). ( http://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?id=6337) [Google Scholar]
- 41.Devor M, Zalkind V. Reversible analgesia, atonia, and loss of consciousness on bilateral intracerebral microinjection of pentobarbital. Pain. 2001;94:101–12. doi: 10.1016/S0304-3959(01)00345-1. [DOI] [PubMed] [Google Scholar]
- 42.Parvizi J, Damasio AR. Neuroanatomical correlates of brainstem coma. Brain. 2003;126:1524–36. doi: 10.1093/brain/awg166. [DOI] [PubMed] [Google Scholar]
- 43.Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232–42. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kungys G, Kim J, Jinks SL, Atherley RJ, Antognini JF. Propofol produces immobility via action in the ventral horn of the spinal cord by a GABAergic mechanism. Anesth Analg. 2009;108:1531–7. doi: 10.1213/ane.0b013e31819d9308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pontine (medial) and medullary (lateral) reticulospinal tracts. Laboratory 12: tract systems I. University of Pennsylvania School of Veterinary Medicine; 2006. ( http://cal.vet.upenn.edu/projects/neurology/lab12/lab12_rspt.htm.) [Google Scholar]
- 46.Dutton RP, Eckhardt WF, III, Sunder N. Total spinal anesthesia after interscalene blockade of the brachial plexus. Anesthesiology. 1994;80:939–41. doi: 10.1097/00000542-199404000-00028. [DOI] [PubMed] [Google Scholar]
- 47.Durrani Z, Winnie AP. Brainstem toxicity with reversible locked-in syndrome after intrascalene brachial plexus block. Anesth Analg. 1991;72:249–52. doi: 10.1213/00000539-199102000-00020. [DOI] [PubMed] [Google Scholar]
- 48.Penn RD, Savoy SM, Corcos D, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med. 1989;320:1517–21. doi: 10.1056/NEJM198906083202303. [DOI] [PubMed] [Google Scholar]
- 49.Mourisse J, Gerrits W, Lerou J, van Egmond J, Zwarts MJ, Booij L. Electromyo-graphic assessment of blink and corneal reflexes during midazolam administration: useful methods for assessing depth of anesthesia? Acta Anaesthesiol Scand. 2003;47:593–600. doi: 10.1034/j.1399-6576.2003.00100.x. [DOI] [PubMed] [Google Scholar]
- 50.Nelson LE, Guo TZ, Lu J, Saper CB, Franks NP, Maze M. The sedative component of anesthesia is mediated by GABA(A) receptors in an endogenous sleep pathway. Nat Neurosci. 2002;5:979–84. doi: 10.1038/nn913. [DOI] [PubMed] [Google Scholar]
- 51.Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–63. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- 52.Morairty S, Rainnie D, McCarley R, Greene R. Disinhibition of ventrolateral preoptic area sleep-active neurons by adenosine: a new mechanism for sleep promotion. Neuroscience. 2004;123:451–7. doi: 10.1016/j.neuroscience.2003.08.066. [DOI] [PubMed] [Google Scholar]
- 53.Correa-Sales C, Rabin BC, Maze M. A hypnotic response to dexmedetomidine, an alpha 2 agonist, is mediated in the locus coeruleus in rats. Anesthesiology. 1992;76:948–52. doi: 10.1097/00000542-199206000-00013. [DOI] [PubMed] [Google Scholar]
- 54.Jorm CM, Stamford JA. Actions of the hypnotic anaesthetic, dexmedetomidine, on noradrenaline release and cell firing in rat locus coeruleus slices. Br J Anaesth. 1993;71:447–9. doi: 10.1093/bja/71.3.447. [DOI] [PubMed] [Google Scholar]
- 55.Huupponen E, Maksimow A, Lapinlampi P, et al. Electroencephalogram spindle activity during dexmedetomidine sedation and physiological sleep. Acta Anaesthesiol Scand. 2008;52:289–94. doi: 10.1111/j.1399-6576.2007.01537.x. [DOI] [PubMed] [Google Scholar]
- 56.Lydic R, Baghdoyan HA. Sleep, anesthesiology, and the neurobiology of arousal state control. Anesthesiology. 2005;103:1268–95. doi: 10.1097/00000542-200512000-00024. [DOI] [PubMed] [Google Scholar]
- 57.Osman NI, Baghdoyan HA, Lydic R. Morphine inhibits acetylcholine release in rat prefrontal cortex when delivered systemically or by microdialysis to basal forebrain. Anesthesiology. 2005;103:779–87. doi: 10.1097/00000542-200510000-00016. [DOI] [PubMed] [Google Scholar]
- 58.Heinricher MM, Morgan MM. Supra-spinal mechanisms of opioid analgesia. In: Stein C, editor. Opioids in pain control: basic and clinical aspects. Cambridge, United Kingdom: Cambridge University Press; 1999. pp. 46–69. [Google Scholar]
- 59.Cesselin FBJ, Bourgoin S, et al. Spinal mechanisms of analgesia. In: Stein C, editor. Opioids in pain control: basic and clinical aspects. Cambridge, United Kingdom: Cambridge University Press; 1999. pp. 70–95. [Google Scholar]
- 60.Stein C, Machelska H, Binder W, Schafer M. Peripheral opioid analgesia. Curr Opin Pharmacol. 2001;1:62–5. doi: 10.1016/s1471-4892(01)00005-4. [DOI] [PubMed] [Google Scholar]
- 61.Hug CC., Jr Does opioid “anesthesia” exist? Anesthesiology. 1990;73:1–4. doi: 10.1097/00000542-199007000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Hilgenberg JC. Intraoperative awareness during high-dose fentanyl–oxygen anesthesia. Anesthesiology. 1981;54:341–3. doi: 10.1097/00000542-198104000-00018. [DOI] [PubMed] [Google Scholar]
- 63.Meuret P, Backman SB, Bonhomme V, Plourde G, Fiset P. Physostigmine reverses propofol-induced unconsciousness and attenuation of the auditory steady state response and bispectral index in human volunteers. Anesthesiology. 2000;93:708–17. doi: 10.1097/00000542-200009000-00020. [DOI] [PubMed] [Google Scholar]
- 64.Lepousé C, Lautner CA, Liu L, Gomis P, Leon A. Emergence delirium in adults in the post-anaesthesia care unit. Br J Anaesth. 2006;96:747–53. doi: 10.1093/bja/ael094. [DOI] [PubMed] [Google Scholar]
- 65.Funk W, Hollnberger H, Geroldinger J. Physostigmine and anaesthesia emergence delirium in preschool children: a randomized blinded trial. Eur J Anaesthesiol. 2008;25:37–42. doi: 10.1017/S0265021507001159. [DOI] [PubMed] [Google Scholar]
- 66.Chase MH. Confirmation of the consensus that glycinergic postsynaptic inhibition is responsible for the atonia of REM sleep. Sleep. 2008;31:1487–91. doi: 10.1093/sleep/31.11.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lavigne GJ, Sessle BJ, Choiniere M, Soja PJ, editors. Sleep and pain. Seattle: IASP Press; 2007. [Google Scholar]
- 68.Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37:563–76. doi: 10.1016/s0896-6273(03)00065-5. [DOI] [PubMed] [Google Scholar]
- 69.Schiff ND. Central thalamic contributions to arousal regulation and neurological disorders of consciousness. Ann N Y Acad Sci. 2008;1129:105–18. doi: 10.1196/annals.1417.029. [DOI] [PubMed] [Google Scholar]
- 70.Schiff ND, Plum F. The role of arousal and “gating” systems in the neurology of impaired consciousness. J Clin Neurophysiol. 2000;17:438–52. doi: 10.1097/00004691-200009000-00002. [DOI] [PubMed] [Google Scholar]
- 71.Schiff ND, Giacino JT, Kalmar K, et al. Behavioural improvements with thalamic stimulation after severe traumatic brain injury. Nature. 2007;448:600–3. doi: 10.1038/nature06041. [DOI] [PubMed] [Google Scholar]
- 72.Alkire MT, McReynolds JR, Hahn EL, Trivedi AN. Thalamic microinjection of nicotine reverses sevoflurane-induced loss of righting reflex in the rat. Anesthesiology. 2007;107:264–72. doi: 10.1097/01.anes.0000270741.33766.24. [DOI] [PubMed] [Google Scholar]
- 73.Schiff ND, Posner JB. Another “Awakenings”. Ann Neurol. 2007;62:5–7. doi: 10.1002/ana.21158. [DOI] [PubMed] [Google Scholar]
- 74.Brefel-Courbon C, Payoux P, Ory F, et al. Clinical and imaging evidence of zolpidem effect in hypoxic encephalopathy. Ann Neurol. 2007;62:102–5. doi: 10.1002/ana.21110. [DOI] [PubMed] [Google Scholar]
- 75.Chen L, Savio Chan C, Yung WH. Electrophysiological and behavioral effects of zolpidem in rat globus pallidus. Exp Neurol. 2004;186:212–20. doi: 10.1016/j.expneurol.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 76.Fulton SA, Mullen KD. Completion of upper endoscopic procedures despite paradoxical reaction to midazolam: a role for flumazenil? Am J Gastroenterol. 2000;95:809–11. doi: 10.1111/j.1572-0241.2000.01866.x. [DOI] [PubMed] [Google Scholar]
- 77.Tung A, Tadimeti L, Caruana-Montaldo B, et al. The relationship of sedation to deliberate self-extubation. J Clin Anesth. 2001;13:24–9. doi: 10.1016/s0952-8180(00)00237-3. [DOI] [PubMed] [Google Scholar]
- 78.Mhuircheartaigh RN, Rosenorn-Lanng D, Wise R, Jbabdi S, Rogers R, Tracey I. Cortical and subcortical connectivity changes during decreasing levels of consciousness in humans: a functional magnetic resonance imaging study using propofol. J Neurosci. 2010;30:9095–102. doi: 10.1523/JNEUROSCI.5516-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Steriade M, Nuñez A, Amzica F. A novel slow (< 1 Hz) oscillation of neo-cortical neurons in vivo: depolarizing and hyperpolarizing components. J Neurosci. 1993;13:3252–65. doi: 10.1523/JNEUROSCI.13-08-03252.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Steriade M, Contreras D, Curro Dossi R, Nuñez A. The slow (< 1 Hz) oscillation in reticular thalamic and thalamocortical neurons: scenario of sleep rhythm generation in interacting thalamic and neocortical networks. J Neurosci. 1993;13:3284–99. doi: 10.1523/JNEUROSCI.13-08-03284.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ching S, Cimenser A, Purdon P, Brown EN, Kopell NK. Thalamocortical model for a propofol-induced alpha rhythm associated with loss of consciousness. Proc Natl Acad Sci U S A. 2010 doi: 10.1073/pnas.1017069108. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steriade M, Amzica F, Contreras D. Cortical and thalamic cellular correlates of electroencephalographic burst-suppression. Electroencephalogr Clin Neurophysiol. 1994;90:1–16. doi: 10.1016/0013-4694(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 83.Koenig MA, Kaplan PW, Thakor NV. Clinical neurophysiologic monitoring and brain injury from cardiac arrest. Neurol Clin. 2006;24:89–106. doi: 10.1016/j.ncl.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 84.Stecker MM, Cheung AT, Pochettino A, et al. Deep hypothermic circulatory arrest: I. Effects of cooling on electroencephalogram and evoked potentials. Ann Thorac Surg. 2001;71:14–21. doi: 10.1016/s0003-4975(00)01592-7. [DOI] [PubMed] [Google Scholar]
- 85.Yamatogi Y, Ohtahara S. Early-infantile epileptic encephalopathy with suppression-bursts, Ohtahara syndrome: its overview referring to our 16 cases. Brain Dev. 2002;24:13–23. doi: 10.1016/s0387-7604(01)00392-8. [DOI] [PubMed] [Google Scholar]
- 86.Maksimow A, Särkelä M, Långsjö JW, et al. Increase in high frequency EEG activity explains the poor performance of EEG spectral entropy monitor during S-ketamine anesthesia. Clin Neurophysiol. 2006;117:1660–8. doi: 10.1016/j.clinph.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 87.Tsuda N, Hayashi K, Hagihira S, Sawa T. Ketamine, an NMDA-antagonist, increases the oscillatory frequencies of alpha-peaks on the electroencephalographic power spectrum. Acta Anaesthesiol Scand. 2007;51:472–81. doi: 10.1111/j.1399-6576.2006.01246.x. [DOI] [PubMed] [Google Scholar]
- 88.Blumenfeld H, Taylor J. Why do seizures cause loss of consciousness? Neuroscientist. 2003;9:301–10. doi: 10.1177/1073858403255624. [DOI] [PubMed] [Google Scholar]
- 89.Seamans J. Losing inhibition with ketamine. Nat Chem Biol. 2008;4:91–3. doi: 10.1038/nchembio0208-91. [DOI] [PubMed] [Google Scholar]
- 90.Olney JW, Newcomer JW, Farber NB. NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–33. doi: 10.1016/s0022-3956(99)00029-1. [DOI] [PubMed] [Google Scholar]
- 91.Reboso Morales JA, Ketamine González Miranda F. Rev Esp Anestesiol Reanim. 1999;46:111–22. (In Spanish.) [PubMed] [Google Scholar]
- 92.Oye I, Paulsen O, Maurset A. Effects of ketamine on sensory perception: evidence for a role of N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 1992;260:1209–13. [PubMed] [Google Scholar]
- 93.Sinner B, Graf BM. Ketamine. Handb Exp Pharmacol. 2008;182:313–33. doi: 10.1007/978-3-540-74806-9_15. [DOI] [PubMed] [Google Scholar]
- 94.Lydic R, Baghdoyan HA. Ketamine and MK-801 decrease acetylcholine release in the pontine reticular formation, slow breathing, and disrupt sleep. Sleep. 2002;25:617–22. [PubMed] [Google Scholar]
- 95.Giacino JT, Kalmar K, Whyte J. The JFK Coma Recovery Scale–Revised: measurement characteristics and diagnostic utility. Arch Phys Med Rehabil. 2004;85:2020–9. doi: 10.1016/j.apmr.2004.02.033. [DOI] [PubMed] [Google Scholar]
- 96.Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. doi: 10.1097/01.anes.0000296071.19434.1e. [DOI] [PubMed] [Google Scholar]
- 97.Kelz MB, Sun Y, Chen J, et al. An essential role for orexins in emergence from general anesthesia. Proc Natl Acad Sci U S A. 2008;105:1309–14. doi: 10.1073/pnas.0707146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Franks NP. General anaesthesia: from molecular targets to neuronal pathways of sleep and arousal. Nat Rev Neurosci. 2008;9:370–86. doi: 10.1038/nrn2372. [DOI] [PubMed] [Google Scholar]
- 99.Ren J, Ding X, Funk GD, Greer JJ. Ampakine CX717 protects against fentanyl-induced respiratory depression and lethal apnea in rats. Anesthesiology. 2009;110:1364–70. doi: 10.1097/ALN.0b013e31819faa2a. [DOI] [PubMed] [Google Scholar]
- 100.Watson SL, Watson CJ, Baghdoyan HA, Lydic R. Thermal nociception is decreased by hypocretin-1 and an adenosine A1 receptor agonist microinjected into the pontine reticular formation of Sprague Dawley rat. J Pain. 2010;11:535–44. doi: 10.1016/j.jpain.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.