Abstract
Conformity refers to the act of changing one’s behaviour to match that of others. Recent studies in humans have shown that individual differences exist in conformity and that these differences are related to differences in neuronal activity. To understand the neuronal mechanisms in more detail, animal tests to assess conformity are needed. Here, we used a test of conformity in rats that has previously been evaluated in female, but not male, rats and assessed the nature of individual differences in conformity. Male Wistar rats were given the opportunity to learn that two diets differed in palatability. They were subsequently exposed to a demonstrator that had consumed the less palatable food. Thereafter, they were exposed to the same diets again. Just like female rats, male rats decreased their preference for the more palatable food after interaction with demonstrator rats that had eaten the less palatable food. Individual differences existed for this shift, which were only weakly related to an interaction between their own initial preference and the amount consumed by the demonstrator rat. The data show that this conformity test in rats is a promising tool to study the neurobiology of conformity.
Keywords: Conformity, Individual differences, Rats, Rattus norvegicus, Social learning
Introduction
Living in a social system has the advantage of obtaining information from other members in the social group in addition to acquiring information from one’s own experience (Day et al. 2001; Kendal et al. 2004). For example, animals may acquire information related to where, what and how to eat (Galef and Giraldeau 2001). In some cases, the motivation to copy the behaviour of others may be so strong that it overrides individual preferences. Thus, subjects change their own behaviour or preferences to match that of others, a prime example being the experiment by Asch in humans (1956), in which subjects chose an overtly false alternative while viewing the right alternative as a result of group normative behaviour (see e.g. Bond and Smith 1996 for a meta-analysis). This behaviour is labelled conformity (Cialdini and Goldstein 2004; Whiten and van Schaik 2007).
Recently, several studies have addressed the neurobiological basis of conformity. For instance, it has been shown that cingulate areas are involved in monitoring differences between private and public information (Burke et al. 2010; Klucharev et al. 2009), while the ventral striatum is involved in the tendency to adjust behaviour to (the amount of) public information (Burke et al. 2010; Campbell-Meiklejohn et al. 2010; Klucharev et al. 2009). The latter may be related to rewarding aspects of being aligned to the behaviour of other individuals (Burke et al. 2010; Campbell-Meiklejohn et al. 2010; Klucharev et al. 2009). Furthermore, some individuals are more prone to adjust their behaviour to social information conflicting with private information than others (Klucharev et al. 2009). Such individual differences are especially relevant to situations where conformity may lead to conform to criminal or addictive behaviour and thus warrants further study. As neurobiological studies are of a limited nature in humans, valid animal tests of conformity are necessary. Here, we study individual differences in a rat test of conformity.
A recent study suggested the existence of conformity in rats (Galef and Whiskin (2008) in line with data in primates (Dindo et al. 2009; Whiten et al. 2005). In particular, it was shown that rats ignored their personal experience in favour of the information of demonstrator rats: rats consumed less palatable or presumably toxic food items after interacting with demonstrator rats that had consumed these items, regardless of their earlier negative personal experience with these items. However, only female rats were studied (Galef and Whiskin 2008). It is suggested that in humans women show stronger tendencies to conform than men (Bond and Smith 1996; Hansson et al. 1980; Reysen and Reysen 2004). Therefore, the first goal was to study whether we could observe conformity in male rats. Second, it has not been examined whether individual differences exist in the levels of conformity in rats and how these differences relate to the initial preference of rats as well the behaviour of the demonstrator. Thus, the second goal was to assess whether we could observe individual differences in conformity behaviour of rats in this test and address their origin.
Materials and methods
Thirty male Wistar rats (10 weeks old upon arrival in the laboratory; Harlan, Horst, the Netherlands) served as experimental subjects. A further twenty (26–27 weeks old) male Wistar rats that had been used in earlier behavioural experiments served as demonstrators. Rats were housed in a temperature- and climate-controlled (T = 23 ± 2°C, 45–65% humidity) room with reversed day/night cycle (lights on from 19:00 to 07:00 h). A radio provided background noise 24 h a day, 7 days a week. All subjects were handled 2–3 times per week prior to testing. We assigned 10 of the subjects to an experimental condition and 10 to each of two control conditions (social control and individual-experience control). Animals were 17–18 weeks old at time of testing.
During habituation to the animal facilities, subjects were pair-housed under enriched conditions, i.e. a Macrolon type IV cage with a shelter and tissues. Food (Special Diets Services, Witham, Essex, England) and water were freely available. Two days prior to the start of the experiment and during the experiment, subjects were individually housed in enriched Macrolon type III cages with powdered diet and water freely available.
During the experiment, diets were presented to subjects in stainless steel containers (length × width × height: 10 × 4 × 5 cm). Three diets were used (Galef and Whiskin, 2008): (1) ground food pellets (Special Diets Services, Witham, Essex, England) (‘powdered diet’); (2) 20 g of cocoa (Blooker Cacao, Amsterdam, the Netherlands) mixed with 980 g of powdered diet (‘diet coc’); (3) 10 g of ground cinnamon (Albert Heijn, Zaandam, The Netherlands) mixed with 100 g of ground sugar pellets (Bio-Serv, Frenchtown, NJ, USA) and 890 g of powdered diet (‘diet s-cin’).
The procedure was similar to that described by Galef and Whiskin (2008). In short, for 23 h, each of the experimental (n = 10) and social control subjects (n = 10; to control for interaction with demonstrators) received two containers, one containing diet coc and one containing diet s-cin, while individual-experience control subjects (n = 10; to control whether individual experience has an effect) were given powdered diet. The amount eaten of each diet was determined after 23 h (day-1). Subsequently, the experimental and individual-experience control subjects were allowed to interact for 30 min with a demonstrator, while the social control subjects remained alone in their cages. Thereafter, all subjects received two new containers for 24 h, one containing diet coc and the other diet s-cin. After 24 h, the amount eaten of each diet was determined (day-2). The demonstrators were placed on a feeding schedule, eating powdered diet for 1 h/day for 2 consecutive days. The day thereafter, demonstrators were given diet coc for 1 h. The amount eaten by each demonstrator was determined. Thereafter, they were placed with a rat from the experimental or individual-experience control group as indicated above. To assess whether the time spent in social interaction had an effect on the preference of experimental animals, social interaction time was determined in the first 10 min of interaction as in this period most interactions occurred.
All data were analysed using SPSS 17. Data on day-1 and day-2 are expressed as per cent diet s-cin eaten compared with the total amount eaten. A one-way ANOVA with planned contrasts was performed to compare the preferences of the different groups on day-2. Adjusted degrees of freedom were used whenever necessary. A paired t-test was used to assess whether preferences had changed between day-2 and day-1 in the experimental and social control group. To address how the preference on day-1, the amount eaten by the demonstrator or social interaction time affected the preference on day-2, Pearson correlations were run as well as a regression analysis. All values are means ± SEMs unless otherwise reported. Statistical tests are two-tailed with significance set at the 0.05 level.
Results
During the 23 h of testing on day-1, subjects assigned to both social control and experimental conditions showed a marked preference for diet s-cin (Fig. 1; Table 1). They consumed substantial amounts of diet s-cin (20.0 ± 0.9 g; range 8.3–25.0 g). During the hour preceding the interaction with subjects, demonstrators consumed, on average, 8.9 (±1.0) grams of diet coc (range: 1.1–17.6 g; Table 1).
Fig. 1.
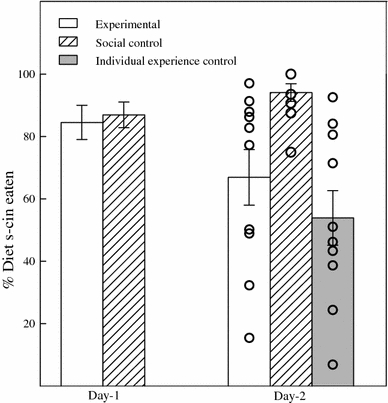
Mean ± SEM percentage of diet s-cin eaten by experimental and control subjects on day-1 and day-2. For day-2: circles indicate individual data points; due to overlapping values, the number of circles may not equal the number of rats in each group
Table 1.
Individual data of the experimental rats (upper half, E) and individual-experience rats (lower half, IE)
| Rat number | Day-1% diet s-cin eaten | Demonstrator diet coc (gr) | Day-2% diet s-cin eaten | Difference day-2 and day-1 |
|---|---|---|---|---|
| E-40 | 100.0 | 12.2 | 97.0 | −3.0 |
| E-18 | 68.1 | 9.6 | 91.3 | 23.2 |
| E-39 | 92.5 | 3.8 | 87.9 | −4.7 |
| E-13 | 98.6 | 15.2 | 86.2 | −12.4 |
| E-15 | 97.9 | 9.4 | 82.8 | −15.1 |
| E-32 | 100.0 | 13.6 | 77.2 | −22.8 |
| E-20 | 85.9 | 13.4 | 50.2 | −35.7 |
| E-24 | 47.2 | 5.2 | 48.9 | 1.8 |
| E-26 | 79.8 | 6.5 | 32.4 | −47.4 |
| E-29 | 75.1 | 17.6 | 15.5 | −59.6 |
| IE-36 | 1.1 | 92.5 | ||
| IE-38 | 6.7 | 84.1 | ||
| IE-17 | 2.8 | 80.5 | ||
| IE-41 | 8.5 | 71.4 | ||
| IE-19 | 1.3 | 51.1 | ||
| IE-33 | 8.5 | 46.2 | ||
| IE-22 | 10.8 | 43.4 | ||
| IE-34 | 11.1 | 38.7 | ||
| IE-27 | 11.1 | 24.4 | ||
| IE-21 | 9 | 6.9 |
Data are organized in descending order for day-2. Only data are shown of the groups in which a demonstrator rat was used
On day-2, rats consumed substantial amounts of diet s-cin (17.4 ± 1.4 g; range 4.3–26.2 g). One animal in the social control group was discarded on day-2 because its preference was >2.5 SD below the group average. The three groups differed significantly for the per cent of diet s-cin eaten on day-2 (one-way ANOVA: F 2,14.1 = 12.03, P = 0.001, Fig. 1). Subjects in the social control group consumed a significantly higher percentage of diet s-cin compared with subjects in both the experimental (t 10.9 = 2.90, P = 0.02) and individual-experience control group (t 10.9 = 4.35, P = 0.001). Subjects in the experimental and individual-experience control group did not significantly differ in preference (t 18 = 1.04, P = 0.31). Whilst subjects in the experimental group showed a significantly lower preference on day-2 compared with day-1 (paired t-test: t 9 = 2.256, P = 0.05), subjects in the social control group showed a higher preference on day-2 compared with day-1 (paired t-test: t 8 = −2.429, P = 0.04).
In both the experimental and individual-experience control group, substantial differences were observed between subjects on day-2 (Table 1; Fig. 1). In the experimental group, 4 subjects with a low preference (range 15.5–50.2%) and 6 subjects with a high preference for diet s-cin (range 77.2–97%) appeared to exist. In the individual-experience control group, 6 subjects with a low preference for diet s-cin (range 6.9–51.1%) and 4 subjects with a high preference for diet s-cin (range 71.4–92.5%) appeared to exist. The preference for diet s-cin on day-2 of subjects of the individual-experience control group was significantly and negatively correlated (r = −0.643, N = 10, P = 0.05) with the amount of diet coc eaten by the demonstrators (Table 1). This was not the case for the experimental animals (r = −0.163, N = 10, P = 0.65). For the experimental animals, a non-significant positive correlation was observed for the preference for diet s-cin on day-2 versus day-1 (r =+0.496, N = 10, P = 0.15; Table 1). Social interaction time showed no correlation with diet s-cin preference on day-2 (r = −0.112, N = 10, P = 0.76). When all three factors were included in one model using regression analysis, a non-significant model effect was observed (F 3,6 = 2.813, P = 0.13; r-square = 0.584) with a significant positive contribution of the preference on day-1 (t = 2.668, P = 0.04), a weak negative contribution of the amount of diet coc eaten by the demonstrators (t = −2.153, P = 0.08) and no effect of social interaction (t = −1.753, P = 0.13). Thus, individual differences in the experimental group on day-2 were not simply the result of an interaction between these factors. Indeed, rat E-40 and rat E-32, which showed the same preference for diet s-cin on day-1 and were interacting with a demonstrator which consumed about the same amount of diet coc, showed a clear difference for their preference of diet s-cin on day-2 (change of −3 and −22.8% respectively), suggesting a difference in sensitivity to this conflicting information (Table 1).
Discussion
The main findings of this study are (1) that male rats decreased their preference for the more palatable food after interaction with demonstrator rats that had eaten the less palatable food and (2) that individual differences exist for this shift which were only weakly related to an interaction between their own initial preference and the amount consumed by the demonstrator rat. The data collectively show conformity in male rats and individual differences herein.
The group effects revealed on day-2 are in line with those observed by Galef and Whiskin (2008) in female rats: (1) the experimental group showed a lower preference for the more palatable food than the social control group, while no difference was observed with respect to the individual-experience control group and (2) the preference of the experimental group for the more palatable food on day-2 was lower than on day-1. Although both male and female rats show the same type of effect, the conformity effect in female rats (Galef and Whiskin 2008) seems to be stronger than in male rats (this study). This would be in line with human data (Bond and Smith 1996; Hansson et al. 1980; Reysen and Reysen 2004). Still, two crucial differences exist between the two studies which may preclude too strong a conclusion on gender differences in rats as (1) different strains were used (Long-Evans vs. Wistar rats) and (2) animals were tested at different ages (8–9 weeks vs. 17–18 weeks). Thus, more research is necessary to study whether these gender differences reflect true gender differences or are related to age and strains.
Comparison between the experimental and social control group shows that (1) repeated exposure to diets by itself does not decrease the preference of rats—if anything it actually increased preferences—and (2) interaction with the demonstrator is necessary for the change to occur. The data of the individual-experience control group also demonstrate the influence of the demonstrator as the rats do have ample time to assess the palatability of the two diets during the 24 h of diet exposure. The layout of the individual-experience control group has been used extensively in experiments on social transmission of food preferences in rats (see Galef 2009). The preference for the choice of the demonstrator’s diet in this group has been related to the olfactory cues of the demonstrator (Galef 2009) and explained by a reduction of food-related neophobia (e.g. Posadas-Andrews and Roper 1983). From the observation that the individual-experience control group and experimental group show the same preference on day-2, Galef and Whiskin (2008) concluded that prior experience with the food items was not a crucial factor for the preference on day-2. Food preference on day-2, and conformity in the experimental group, would then depend fully on information from the demonstrator. However, closer inspection using correlational analysis suggests otherwise.
In both the experimental and the individual-experience control group, individual differences were observed with about half of the number of subjects showing a weak and the other half a strong preference for the food consumed by the demonstrator (cf. Galef 1986, 1993). Preferences on day-2 in the individual-experience control group were correlated with the amount of diet coc consumed by the demonstrators, while in contrast this effect was not present in the experimental group. The amount of diet coc consumed by the demonstrators is likely to be related to the strength of the olfactory cues emanating from the demonstrators. Olfactory cues are of prime importance in affecting preferences (Galef 2009; Posadas-Andrews and Roper 1983). Accordingly, the data suggest that in the individual-experience control group, the preference of rats on day-2 is strongly dependent on the strength of the olfactory (social) information of the demonstrators, while this is not the case in the experimental group. Overall, therefore, the data of the two groups suggest that the preference on day-1 does have an effect in the experimental group. Indeed, in the experimental group, preferences on day-2 were related, albeit weakly, to preferences on day-1. In fact, preferences on day-2 were related to a weak interaction between preferences of day-1 and amount consumed by the demonstrator. However, as the variance on day-2 cannot be explained simply by initial preference and amount consumed by the demonstrator, the data also clearly indicate that individual differences emerge beyond this interaction. Thus, despite similar preferences and social information, some rats in the experimental group seemed more resistant to changing their preference in relation to private and social information than others, indicating a different sensitivity to conflicting information (cf. Klucharev et al. 2009). Whether these individual differences are related to, e.g., differences in activity in the cingulate areas and/or the ventral striatum (Burke et al. 2010; Campbell-Meiklejohn et al. 2010; Klucharev et al. 2009) remains then to be determined, i.e. whether some individuals are more prone than others to detect differences between their own behaviour and that of others (cingulate areas) and/or are more sensitive than other individuals to the rewarding properties of being aligned with the behaviour of other individuals (ventral striatum).
Three remarks need to be made finally. First, the possibility should be entertained that despite similarity in behavioural outcome between rats and humans to conform to the behaviour of others, underlying psychological and neural mechanisms may be different. For instance, humans may explicitly deliberate the difference between their own preference and that of others and subsequently conform to that behaviour or not, while rats obviously lack this capacity. This needs to be addressed in future studies. Secondly, a difference between human experiments and the present rat experiment is that in humans group norms are used, while in rats only one demonstrator rat is present. Although conformity to a group norm is thus not tested, the data revealed that a conformity effect can already be seen with an interaction with one demonstrator. In a version of the present task, which excluded the initial preference of rats, it was shown that the number of demonstrators (and uniformity of demonstrator behaviour) did have an effect on the preference of naive rats (reviewed in Galef 2009; conform humans: Bond and Smith 1996). In future experiments, more demonstrators may be used to study to what extent the number and/or uniformity of their behaviour affects the outcome. Thirdly, the sample size may have been too small to detect (strong) significant correlations in the experimental group. Nevertheless, we did detect a significant correlation in the individual-experience control group. Future studies are, however, needed to substantiate the conclusions.
In sum, we show that individual differences in sensitivity to conformity exist and that not only female rats (Galef and Whiskin 2008) but also male rats show conformity: changing one’s own behaviour to match that of others (Cialdini and Goldstein 2004; Whiten and van Schaik 2007). The test of conformity in rats offers a promising tool to study the neurobiology of conformity.
Acknowledgments
We wish to thank Bennet Galef, Judith Homberg, Simon Reader and Louk Vanderschuren for helpful suggestions on the manuscript and experimental procedures. We also wish to thank three anonymous referees for comments that helped to improve the quality of the manuscript. All procedures in the present study conformed to the Dutch guidelines on animal care, with approval from the University of Utrecht Animal Ethics Committee.
Conflict of interest
The authors declare no conflict of interest.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Asch SE. Studies of independence and conformity: I a minority of one against a unanimous majority. Psychol Monogr. 1956;70:1–70. [Google Scholar]
- Bond R, Smith PB. Culture and conformity: a meta-analysis of studies using Asch’s (1952b, 1956) line judgment task. Psychol Bull. 1996;119:111–137. doi: 10.1037/0033-2909.119.1.111. [DOI] [Google Scholar]
- Burke CJ, Tobler PN, Schultz W, Baddeley M. Striatal BOLD response reflects the impact of herd information on financial decisions. Frontiers in Human Neuroscience. 2010;4:1–11. doi: 10.3389/fnhum.2010.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell-Meiklejohn DK, Bach DR, Roepstorff A, Dolan RJ, Frith CD. How the opinion of others affects our valuation of objects. Curr Biol. 2010;20:1165–1170. doi: 10.1016/j.cub.2010.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdini RB, Goldstein NJ. Social influence: compliance and conformity. Annual Reviews in Psychology. 2004;55:591–621. doi: 10.1146/annurev.psych.55.090902.142015. [DOI] [PubMed] [Google Scholar]
- Day RL, MacDonald T, Brown C, Laland KN, Reader SM. Interactions between shoal size and conformity in guppy social foraging. Anim Behav. 2001;62:917–925. doi: 10.1006/anbe.2001.1820. [DOI] [Google Scholar]
- Dindo M, Whiten A, de Waal FBM. In-group conformity sustains different foraging traditions in Capuchin Monkeys (Cebus apella) PLoS ONE. 2009;4(11):e7858. doi: 10.1371/journal.pone.0007858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef BG. Social interaction modifies learned aversions, sodium appetite, and both palatability and handling-time induced dietary preference in rats (R. norvegicus) J Comparative Psychol. 1986;100:432–439. doi: 10.1037/0735-7036.100.4.432. [DOI] [PubMed] [Google Scholar]
- Galef BG. Individual differences in responses of Norway rats to social induction of food preferences. Behav Process. 1993;30:309–316. doi: 10.1016/0376-6357(93)90143-F. [DOI] [PubMed] [Google Scholar]
- Galef BG. Strategies for social learning: testing predictions from formal theory. Adv Study Behav. 2009;39:117–151. doi: 10.1016/S0065-3454(09)39004-X. [DOI] [Google Scholar]
- Galef BG, Giraldeau L. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim Behav. 2001;61:3–15. doi: 10.1006/anbe.2000.1557. [DOI] [PubMed] [Google Scholar]
- Galef BG, Whiskin EE. ‘Conformity’ in Norway rats? Anim Behav. 2008;75:2035–2039. doi: 10.1016/j.anbehav.2007.11.012. [DOI] [Google Scholar]
- Hansson RO, Allen MM, Jones WH. Sex differences in conformity: instrumental or communal response? Sex Roles. 1980;6:207–212. doi: 10.1007/BF00287343. [DOI] [Google Scholar]
- Kendal RL, Coolen I, Laland KN. The role of conformity in foraging when personal and social information conflict. Behav Ecol. 2004;15(2):269–277. doi: 10.1093/beheco/arh008. [DOI] [Google Scholar]
- Klucharev V, Hytönen K, Rijpkema M, Smidts A, Fernández G. Reinforcement learning signal predicts social conformity. Neuron. 2009;61:140–151. doi: 10.1016/j.neuron.2008.11.027. [DOI] [PubMed] [Google Scholar]
- Posadas-Andrews A, Roper TJ. Social transmission of food-preferences in adult rats. Anim Behav. 1983;31:265–271. doi: 10.1016/S0003-3472(83)80196-1. [DOI] [Google Scholar]
- Reysen S, Reysen NB. Sex differences on a measure of conformity in automated teller machine Lines. Psychol Rep. 2004;95:443–446. doi: 10.2466/pr0.95.2.443-446. [DOI] [PubMed] [Google Scholar]
- Whiten A, van Schaik CP. The evolution of animal ‘cultures’ and social intelligence. Philos Transactions Royal Soc B. 2007;362:603–620. doi: 10.1098/rstb.2006.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten A, Horner V, de Waal FBM. Conformity to cultural norms of tool use in chimpanzees. Nature. 2005;437:737–740. doi: 10.1038/nature04047. [DOI] [PubMed] [Google Scholar]


