Abstract
Objectives:
To identify structural connectivity change occurring during the first 6 months after traumatic brain injury and to evaluate the utility of diffusion tensor tractography for predicting long-term outcome.
Methods:
The participants were 28 patients with mild to severe traumatic axonal injury and 20 age- and sex-matched healthy control subjects. Neuroimaging was obtained 0–9 days postinjury for acute scans and 6–14 months postinjury for chronic scans. Long-term outcome was evaluated on the day of the chronic scan. Twenty-eight fiber regions of 9 major white matter structures were reconstructed, and reliable tractography measurements were determined and used.
Results:
Although most (23 of 28) patients had severe brain injury, their long-term outcome ranged from good recovery (16 patients) to moderately (5 patients) and severely disabled (7 patients). In concordance with the diverse outcome, the white matter change in patients was heterogeneous, ranging from improved structural connectivity, through no change, to deteriorated connectivity. At the group level, all 9 fiber tracts deteriorated significantly with 7 (corpus callosum, cingulum, angular bundle, cerebral peduncular fibers, uncinate fasciculus, and inferior longitudinal and fronto-occipital fasciculi) showing structural damage acutely and 2 (fornix body and left arcuate fasciculus) chronically. Importantly, the amount of change in tractography measurements correlated with patients' long-term outcome. Acute tractography measurements were able to predict patients' learning and memory performance; chronic measurements also determined performance on processing speed and executive function.
Conclusions:
Diffusion tensor tractography is a valuable tool for identifying structural connectivity changes occurring between the acute and chronic stages of traumatic brain injury and for predicting patients' long-term outcome.
Traumatic axonal injury (TAI) is strongly linked to high mortality and morbidity in traumatic brain injury (TBI).1 TAI is progressive with typical occurrence of cytoskeletal disruption within 4–6 hours postinjury and disconnection of axons between 1 and 7 days.2 Secondary axotomy, which damages brain structural connectivity and affects cognitive function, depending on the efficacy of structural connectivity, may persist for years.3 Diffusion tensor imaging (DTI)–associated tractography is an MRI technique for reconstructing white matter structures and assessing structural connectivity in vivo.4–6 It has shown promise in assessing TAI and predicting long-term outcome.7,8
Longitudinal changes in structural connectivity associated with TAI remain largely unknown. Only one study presented DTI tractography for reconstructing the corpus callosum (CC) in children with TBI and reported degenerative changes as well as changes consistent with continuous maturation/development from 3 to 18 months after TBI.9 Several studies applied DTI region-of-interest (ROI) or voxel-based analysis to examine structural integrity changes from the acute to chronic stage of injury.10–12 These studies found that many white matter areas were affected by TAI and showed discordant change patterns with either improved or deteriorated structural integrity. Longitudinal studies are needed to delineate the progression of TAI in major white matter fiber tracts. The aim of the present study was to provide a comprehensive assessment of structural connectivity change from the acute to chronic stages of TBI in 9 fiber tracts and evaluate the associations of tractography measurements with patients' long-term outcome. We hypothesized that DTI tractography would be able to detect TAI and predict patients' long-term outcome.
METHODS
Participants.
From 2005 to 2009, patients were recruited from Parkland Memorial Hospital, Dallas, TX. Inclusion criteria required that patients 1) had sustained closed-head injury during high-velocity motor vehicle collisions, with an injury mechanism consistent with TAI (absence of focal lesions with volume greater than 10 mL visible on cranial CT), 2) had stable medical conditions so that scans occurred within 14 days of injury, and 3) were at least 16 years old. Exclusion criteria comprised contraindications for MRI, preexisting neurologic impairment, a psychiatric condition, or a previous brain injury. Patients were contacted again 6 months later for the follow-up study. In addition, we enrolled approximate age- and sex-matched healthy participants without neurologic impairment or psychiatric disorders.
Standard protocol approvals, registrations, and patient consents.
Informed consent was obtained from all participants. Clinical research personnel not involved in patients' care obtained informed consent from patients' surrogates according to guidelines from the institutional review board and national standards.
Image acquisition.
Brain images were acquired using a GE Signa Excite 3-T MRI scanner with 8-channel phased array head coil (GE Healthcare, Milwaukee, WI). DTI sequences were obtained using a single-shot spin-echo, echoplanar imaging sequence in 45 axial slices of 3.0-mm thickness (no gap) with 240-mm field of view, b value of 1,000 s/mm2, 19 directions, 128 × 128 acquisition matrix, 256 × 256 image matrix, repetition time of 12,000 msec, echo time of 75.5 msec, 90° flip angle, and number of excitations of 2. Three additional images with minimum diffusion weighting were also acquired. T1-weighted structural images were acquired using a fast spoiled gradient recalled acquisition in steady-state sequence in 130 sagittal slices of 1.3-mm thickness (no gap) with 240-mm field of view, 256 × 256 matrix, repetition time of 8.1 msec, excitation time of 2.4 msec, 25° flip angle, and number of excitations of 2. We maximized patient safety by continuous monitoring of vital signs and used MRI-compatible respiration equipment as needed.
Image processing.
DTI image preprocessing consisted of skull stripping13 and eddy current and motion corrections using FSL (www.fmrib.ox.ac.uk/fsl/, University of Oxford, Oxford, UK). Tensor calculation and diffusion map generation were performed using DTI Studio (www.mristudio.org, Johns Hopkins Medical Institute, Baltimore, MD). To correct for individual differences in head size, fiber count and fiber volume were normalized by intracranial volume calculated from T1-weighted images using FSL.14
Over the 4-year period, 2 scanner upgrades caused mean diffusivity (MD) values to increase substantially. To adjust MD to its original level, 5 healthy subjects who had been scanned before the upgrades were scanned again to determine optimal b values for achieving no systematic change in MD. The optimal b values were determined to be 1,300 and 1,150 s/mm2 for images acquired after the first and second upgrades, respectively.
Tract reconstruction and quantification.
DTI tractography was performed in DTI Studio using a multiple ROI approach.6,15–17 The fractional anisotropy (FA) threshold was set to 0.25, and the angle threshold was set to 60°. We selected fiber tracts that could be reconstructed consistently across a majority of participants. The 9 fiber tracts included the CC, cingulum bundle (CB), fornix, angular bundle (AB), cerebral peduncular fibers (CPF), uncinate fasciculus (UF), inferior longitudinal fasciculus (ILF), inferior fronto-occipital fasciculus (IFO), and arcuate fasciculus (AF). A total of 28 fiber regions were reconstructed. The left and right sides were reconstructed separately. Fiber tracts that connect multiple brain regions (CC, CPF, and CB) were parceled into subregions. CC was first reconstructed as a whole and then parceled into 4 regions with equal length at the midsagittal slice corresponding to the genu (CCg), anterior and posterior body (CCab and CCpb), and splenium (CCs). The CPF were tracked separately as fibers to the cerebellum and to the anterior frontal, superior frontal, parietal (CPpa), and occipital lobes. The fornix body (FB) and left and right fornix crus (FC) were tracked separately because of the termination of fiber propagation at the superior FC caused by the large DTI spatial resolution relative to the size of fornix crus.18 Regions of the cingulum were reconstructed separately as left, right, anterior left, anterior right, posterior left, and posterior right. The right AF was excluded because it could not be reconstructed from some of the healthy participants. Fiber tracking was performed without knowledge of patients' injury severity or long-term outcome.
The 28 fiber regions were quantified using FA (representing diffusion directionality), MD (for packing density), fiber count, mean length, fiber volume (number of voxels occupied), and fiber density (fiber count/voxel). Two independent raters were used to establish interrater reliabilities for each fiber tract. The intraclass correlation coefficients were ≥0.90 for all fiber tracts, except for the fiber volume of the left FC, which was 0.87.
Test-retest reliability of tractography measurements is crucial in longitudinal studies. Thus, to identify reliable tractography measurements, we scanned 5 control subjects twice 3–8 months apart to find measurements with either high test-retest consistency or acceptable variance without systematic bias (i.e., one set of repeated measures is not consistently higher or lower than the other set). Table e-1 on the Neurology® Web site at www.neurology.org shows the 116 of 168 tractography measurements that were identified as reliable. Subsequent statistical analyses were based on these reliable measurements. The results were compared with those from the complete dataset.
Outcome assessment.
A project coordinator and a neuropsychologist assessed long-term cognitive outcomes of the patients with TBI on the day of the follow-up study. Cognitive domains evaluated comprised information processing speed, attention, verbal fluency, executive function, and learning and memory using the Processing Speed Index subtests from the Wechsler Adult Intelligence Scale–third edition,19 Trail Making Test,20 Dodrill Stroop Test,21 Controlled Oral Word Association Test,22 and California Verbal Learning Test–II (CVLT-II).23 Raw scores for each of these tasks were converted into appropriate standardized scores (i.e., age- and education-corrected where applicable).
Statistical analyses.
Because a large number of tractography and neuropsychological measurements were collected, we selected statistical techniques tailored to handle multicollinearity. For group classification and TAI identification, discriminant correspondence analysis (DCA)24 was applied. DCA extends discriminant analysis to determine group membership of observations. It provides a set of discriminant factor scores (obtained as a linear combination of the variables) that best separate the groups. Like principal component analysis, these discriminant factor scores are used to plot observations as points on a map. The distance between an observation and the groups determines the group membership, and observations are assigned to their closest groups. DCA also provides statistics for identifying variables relevant for group discrimination.
Partial least-squares (PLS) regression25 was performed to evaluate the associations between acute and chronic tractography measurements with long-term outcome. PLS regression extends multiple regression, simultaneously decomposes the independent and dependent variables, and finds a set of latent variables that explains the maximum covariance between the independent and dependent variables. The quality of the prediction is evaluated with correlation coefficients between the original and predicted scores.
To assess the generalization of the findings, a jackknife procedure (leave one out)26 was performed on the analyses that were statistically significant. Because of the high variability in some of the fiber variables, tractography measurements were transformed to ranks before the analyses. In addition, because age and injury severity might affect patients' long-term outcome,27,28 PLS regression was repeated after controlling for these confounds. To account for multiple comparisons, α was set to 0.005.
RESULTS
From the 40 patients with TBI who met the inclusion criteria, 30 patients returned for the follow-up study (3 were deceased, 3 had incompatible MRI, and 4 were lost to contact). Two patients were excluded from the study because of poor image quality of their chronic scans. From the 28 pairs of scans examined in the study, 8 acute scans were analyzed in our previous DTI study,7 24 pairs of acute and chronic scans were used in the examination of the association between regional brain volumes and tractography measurements,29 and 28 chronic scans were used to investigate the sensitivity of 3 DTI methods for detecting TAI.30 Average age was 26.3 years (SD 10.6, range 16–58, 71% men). Time from injury to acute scan ranged from 0 to 9 days (mean 2.5, SD 2.6, median 1.0 days) and time to chronic scan ranged from 6 to 11 months (mean 7.7, SD 1.8, median 7.5 months).
Injury severity was classified according to the Glasgow Coma Scale (GCS) with scores of 3–8 as severe TBI, 9–12 as moderate TBI, and 13–15 as mild TBI. Most patients had severe TBI except for one with moderate TBI and 4 with mild TBI. Long-term functional outcome ranged from good recovery (16 patients) to moderately disabled (5 patients) and severely disabled (7 patients) (Glasgow Outcome Scale–Extended; range 3–8, mean 6.1, SD 2.0, median 7). Table e-2 provides additional acute characteristics of the patients. Twenty age- and sex-matched normal control subjects were also studied (age mean 29.9, SD 11.4, range 17–54, 70% men).
Most patients participated in all tests except for 5 native Spanish speakers who could not take the CVLT-II, and these patients were consequently excluded from analyses involving neuropsychological test scores. Thus, 23 patients participated in cognitive tests. As a group, the patients showed deficits in cognitive domains of information processing speed, attention, verbal fluency, and learning and memory (table e-3). Among the 5 cognitive domains, the most commonly impaired was the learning and memory function, with 25% of patients having scores 2 SD below the population mean.
DTI tractography.
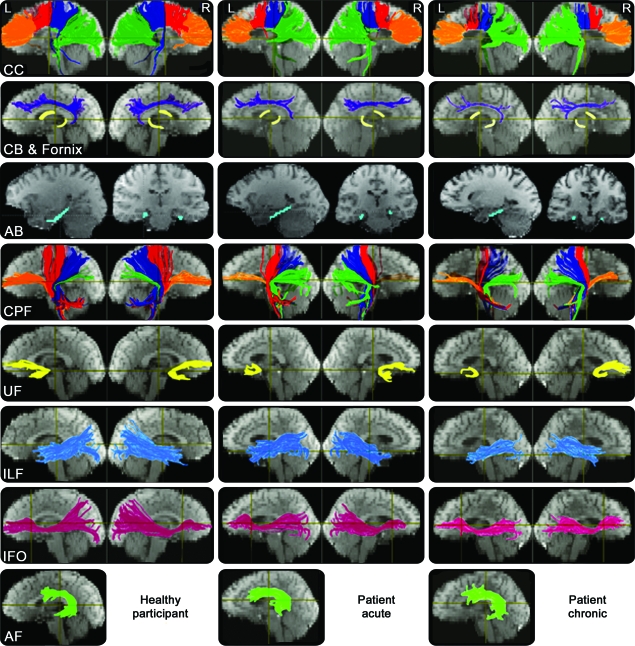
All 28 fiber regions were reconstructed successfully for all 20 healthy subjects. For the patients with TBI, however, one acute scan did not include the cerebellum. The left IFO from one acute scan, right UF from one follow-up scan, and right FC from another follow-up scan could not be reconstructed even after the FA threshold for fiber tracking was lowered to 0.15. Consequently, for the missing cerebellum, the average values of the acute patient group were used. For the remaining missing fiber tracts that could not be reconstructed mostly due to TBI, the MD was set to the patient group mean and the other 5 fiber variables were set to 0. Figure 1 shows the representative tractography results of a normal control subject and a patient at the acute and chronic stages of injury.
Figure 1. Representative fiber tracking results.
The reconstructed fiber tracts are overlaid on the images with minimum diffusion. The colors are assigned for the purpose of demarcating the fiber tracts and do not represent the principal diffusion direction as it is in the diffusion tensor imaging color-coded map. AB = angular bundle; AF = arcuate fasciculus; CB = cingulum bundle; CC = corpus callosum; CPF = cerebral peduncular fibers; IFO = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; UF = uncinate fasciculus.
Group classification and TAI identification.
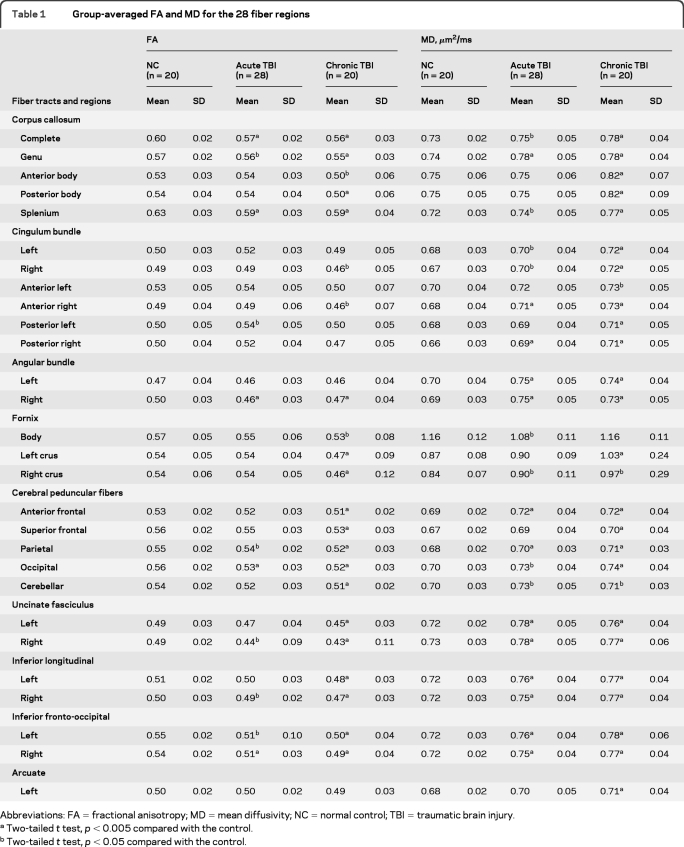
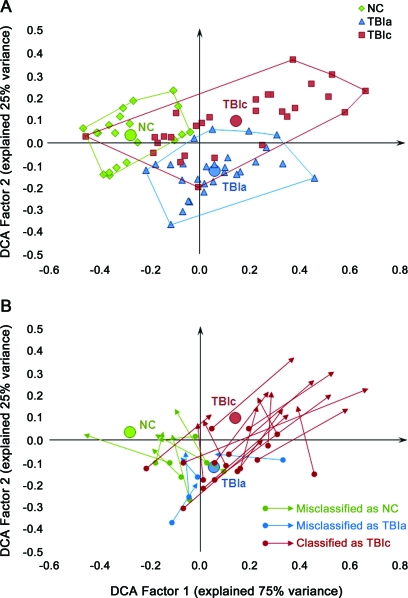
In general, patients exhibited reduced values in FA, mean length, fiber count, fiber volume, and fiber density, increased MD in most fiber tracts, and increased variability in the chronic scans (tables 1, e-4, and e-5). Figure 2A shows DCA group classification based on factor scores of individual observations. DCA misclassified 20 of 76 observations including 2 from the healthy participant group, 7 from the acute TBI group, and 11 from the chronic TBI group (56 of 76 correct classifications, p > 0.05). Figure 2B depicts how patients' DCA factor scores changed from the acute to chronic stages of TBI. To examine the relationship between patients' changes in tractography measurements from the acute to chronic stage of injury and long-term outcome, correlation analysis was conducted and revealed a strong negative relation between changes in DCA factor 1 score and average z scores of the 15 cognitive tests (Pearson r = −0.54, p = 0.004).
Table 1.
Group-averaged FA and MD for the 28 fiber regions
Abbreviations: FA=fractional anisotropy; MD=mean diffusivity; NC=normal control; TBI=traumatic brain injury.
Two-tailed t test, p < 0.005 compared with the control.
Two-tailed t test, p < 0.05 compared with the control.
Figure 2. Observations and group boundaries in the discriminant correspondence analysis (DCA) space.
(A) Group boundaries. (B) Patients' progression on the DCA map from the acute to chronic stage of injury. The origins of arrows show the patients' positions at the acute stage of injury, and the terminations show their positions at the chronic stage. The colors of arrows indicate the group that the patients were assigned to in DCA at the chronic stage of traumatic brain injury (TBI). NC = normal control; TBIa = patients with acute TBI; TBIc = patients with chronic TBI.
DCA factor scores of the 116 tractography measurements identified 2 general categories of injury: 1) early damaged fiber tracts that exhibited signs of TAI acutely and 2) late damaged fiber tracts that were normal acutely but showed signs of injury chronically. Combining the change patterns in all measurements, the CCg, CCs, CB, AB, CPF, UF, ILF, and IFO were categorized as early damaged fiber tracts and the CCab, CCpb, FB, and AF were classified as late damaged tracts.
Outcome predictions.
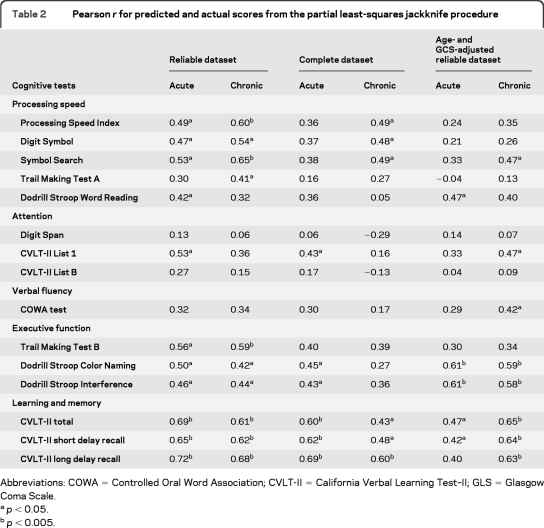
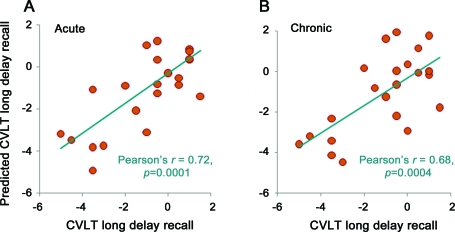
Using either the acute or chronic measurements, PLS regression was able to predict results for all 15 cognitive tests. In contrast, the jackknife procedure of PLS regression did not determine all scores (table 2). With use of the acute measurements, the jackknife procedure of PLS regression was able to predict all 3 scores of learning and memory. Substantial contributors for the prediction were measurements from the CC fiber regions except MD, FA of left CB, right AB, CPpa, right ILF, and right IFO and mean length of bilateral ILF. With use of the chronic measurements, the jackknife procedure was able to determine performance on processing speed, executive function, and learning and memory tasks, and measurements from all fiber regions made substantial contributions. Figure 3 plots the best correlation between PLS regression predicted against actual scores of CVLT-II Long Delay Recall. In comparison, with use of the acute or chronic measurements of the complete dataset, the jackknife procedure of PLS regression determined only learning and memory. After adjustment for age and GCS score, acute tractography measurements determined executive function, and the chronic tractography measurements determined executive function and learning and memory.
Table 2.
Pearson r for predicted and actual scores from the partial least-squares jackknife procedure
Abbreviations: COWA = Controlled Oral Word Association; CVLT-II = California Verbal Learning Test–II; GLS = Glasgow Coma Scale.
p < 0.05.
p < 0.005.
Figure 3. Partial least-squares regression predicted and actual scores of California Verbal Learning Test (CVLT) long delay recall.
The jackknife procedure was used to make the predictions using (A) acute measurements and (B) chronic measurements.
DISCUSSION
The current study examined longitudinal changes in 9 major fiber tracts and demonstrated that tractography-based measurements can detect deterioration of structural connectivity associated with TAI and determine long-term outcome from both acute and chronic measurements. Tractography measurements were able to differentiate patients with acute TBI from healthy participants but could not distinguish patients at the chronic stage from the other 2 groups because of the diverse change patterns from the acute to chronic stages of TBI. Patients experienced improved, unchanged, or deteriorated tractography measurements. Importantly, the amount of change in tractography measurements was found to be related to patients' long-term outcome. Factors contributing to diverse outcomes of TBI need to be identified to develop effective treatment.
Despite the heterogeneous change pattern of tractography measurements in individual patients, tractography measurements showed systematic deterioration over the 6-month recovery period after TBI that were generally consistent with previous longitudinal studies.10,12 These previous studies examined the continuous changes in DTI measurements within the chronic stage of TBI and reported decreased FA and increased MD in many white matter areas. However, they also reported increased FA in the internal capsule and centrum semiovale and decreased MD in many white matter areas (e.g., internal capsule, ILF, and corona radiata). Methodologic differences in obtaining the DTI measurements may explain this divergent finding, because the present study used tractography-based as opposed to ROI- or voxel-based measurements. Quantitative tractography has the benefit of obtaining measurements pertaining to a particular tract even in areas (e.g., centrum semiovale and corona radiate) where more than one fiber tract is present. In addition, tractography measurements cover the whole extent of fiber tracts in contrast to small voxel clusters in white matter. Another contributing factor could be the diverse change patterns in individual patients. The percentage of patients who showed improved measurements in a study could affect the result of group analysis; however, the varied individual patterns of white matter change have not been reported in prior studies and need to be further investigated.
The current study examined the test-retest reliability and correlation with outcome of tractography measurements to determine whether DTI images acquired with 19 directions were sufficient for fiber tracking. We identified 116 of 168 reliable tractography measurements. Notably, these reliable measurements showed stronger association with long-term outcome of TBI compared with the complete dataset. Our results extended previous studies,8,9,31–33 which showed correlation between DTI measurements and long-term cognitive outcome. In the current cohort, the most commonly affected cognitive function was learning and memory. The jackknife procedure of PLS regression demonstrated that acute tractography measurements predicted performance in this domain, and chronic tractography measurements also determined performance on information processing speed and executive function tasks and indicated good generalization of the results.
An interesting finding is the change in the direction of correlation with outcome between the acute and chronic MD of the CC, CB, and AB. The correlation was positive acutely but became negative chronically. Figure e-1 illustrates such a correlation pair. Previous studies34,35 suggest that cytotoxic edema causes MD to decrease initially and then normalize or elevate because of either recovery or gliosis during the Wallerian degeneration process. In contrast, extracellular edema is accompanied by an increase in MD at the acute stage of injury and may signal a less severe injury compared with cytotoxic edema. Abnormally low MD at the acute stage of TBI might indicate cytotoxic edema, which would be expected to lead to poor prognosis. Studies are underway in our laboratory to test this hypothesis.
Although 11 fiber tracts were reconstructed, some fiber tracts important in TBI are still missing, including the anterior and posterior components of the superior longitudinal fasciculus36 and superior fronto-occipital fasciculus. The deterministic fiber-tracking algorithms we used could not resolve the crossing fiber issue and may erroneously indicate early termination of fiber propagation or absence of fibers. The timing of the acute scans could be another limitation. Most of the acute scans were acquired within 2 days of injury except for 8 patients who were scanned 2–10 days posttrauma. It is likely that secondary degeneration in white matter might have started during this time period and that the changes in tractography measurement only partially reflected the earliest cellular alternations associated with TAI.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the patients and volunteers who participated in this study, the nursing staff at Parkland Health and Hospital System, and the imaging staff, in particular Dr. Evelyn Babcock, at the Meadow's Imaging Center of Parkland Health and Hospital System.
GLOSSARY
- AB
angular bundle
- AF
arcuate fasciculus
- CB
cingulum bundle
- CC
corpus callosum
- CCab
anterior body of the corpus callosum
- CCg
genu of the corpus callosum
- CCpb
posterior body of the corpus callosum
- CCs
splenium of the corpus callosum
- CPF
cerebral peduncular fibers
- CPpa
cerebral peduncular fibers to the parietal lobes
- CVLT-II
California Verbal Learning Test–II
- DCA
discriminant correspondence analysis
- DTI
diffusion tensor imaging
- FA
fractional anisotropy
- FB
fornix body
- FC
fornix crus
- GCS
Glasgow Coma Scale
- IFO
inferior fronto-occipital fasciculus
- ILF
inferior longitudinal fasciculus
- MD
mean diffusivity
- PLS
partial least-squares
- ROI
region of interest
- TAI
traumatic axonal injury
- TBI
traumatic brain injury
- UF
uncinate fasciculus
Footnotes
Editorial, page 810
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Wang designed the study, analyzed and interpreted the data, conducted statistical analysis, and drafted and revised the manuscript. Dr. Bakhadirov designed the study, analyzed the data, and revised the manuscript. Dr. Abdi designed the study, analyzed and interpreted the data, conducted statistical analysis, and revised the manuscript. Dr. Devous designed the study, interpreted the data, and revised the manuscript. Dr. Marquez de la Plata designed the study, interpreted the data, and revised the manuscript. C. Moore designed the study and revised the manuscript. Dr. Madden designed the study and revised the manuscript. Dr. Diaz-Arrastia designed the study, interpreted the data, and revised the manuscript.
DISCLOSURE
Dr. Wang, Dr. Bakhadirov, and Dr. Abdi report no disclosures. Dr. Devous serves on a scientific advisory board and as a consultant for Avid Radiopharmaceuticals, Inc.; served as an Associate Editor for the Journal of Experimental Biology; receives research support from Avid Radiopharmaceuticals, Inc., the NIH (NIA, NIDRR/US Dept. Ed.), and the Alzheimer's Association; and holds stock/stock options in Avid Radiopharmaceuticals, Inc. Dr. Marquez de la Plata, C. Moore, and Dr. Madden report no disclosures. Dr. Diaz-Arrastia serves on the speakers' bureau for and has received funding for travel and speaker honoraria from UCB; serves on the editorial board of the Journal of Neurotrauma; and receives research support from the NIH/NIDRR and the Alzheimer's Association.
REFERENCES
- 1. Graham DI, McIntosh TK, Maxwell WL, Nicoll JA. Recent advances in neurotrauma. J Neuropathol Exp Neurol 2000;59:641–651 [DOI] [PubMed] [Google Scholar]
- 2. Gaetz M. The neurophysiology of brain injury. Clin Neurophysiol 2004;115:4–18 [DOI] [PubMed] [Google Scholar]
- 3. Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. J Head Trauma Rehabil 2005;20:76–94 [DOI] [PubMed] [Google Scholar]
- 4. Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 1994;103:247–254 [DOI] [PubMed] [Google Scholar]
- 5. Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 1996;111:209–219 [DOI] [PubMed] [Google Scholar]
- 6. Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol 1999;45:265–269 [DOI] [PubMed] [Google Scholar]
- 7. Wang JY, Bakhadirov K, Devous MD, Sr, et al. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch Neurol 2008;65:6196–6126 [DOI] [PubMed] [Google Scholar]
- 8. Bigler ED, McCauley SR, Wu TC, et al. The temporal stem in traumatic brain injury: preliminary findings. Brain Imaging Behav 2010;4:270–282 [DOI] [PubMed] [Google Scholar]
- 9. Wu TC, Wilde EA, Bigler ED, et al. Longitudinal changes in the corpus callosum following pediatric traumatic brain injury. Dev Neurosci 2010;32:361–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bendlin BB, Ries ML, Lazar M, et al. Longitudinal changes in patients with traumatic brain injury assessed with diffusion-tensor and volumetric imaging. Neuroimage 2008;42:503–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar R, Husain M, Gupta RK, et al. Serial changes in the white matter diffusion tensor imaging metrics in moderate traumatic brain injury and correlation with neuro-cognitive function. J Neurotrauma 2009;26:481–495 [DOI] [PubMed] [Google Scholar]
- 12. Sidaros A, Engberg AW, Sidaros K, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain 2008;131:559–572 [DOI] [PubMed] [Google Scholar]
- 13. Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002;17:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Smith SM, Zhang Y, Jenkinson M, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage 2002;17:479–489 [DOI] [PubMed] [Google Scholar]
- 15. Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage 2002;17:77–94 [DOI] [PubMed] [Google Scholar]
- 16. Huang H, Zhang J, van Zijl PC, Mori S. Analysis of noise effects on DTI-based tractography using the brute-force and multi-ROI approach. Magn Reson Med 2004;52:559–565 [DOI] [PubMed] [Google Scholar]
- 17. Wakana S, Caprihan A, Panzenboeck MM, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007;36:630–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Concha L, Gross DW, Beaulieu C. Diffusion tensor tractography of the limbic system. AJNR Am J Neuroradiol 2005;26:2267–2274 [PMC free article] [PubMed] [Google Scholar]
- 19. Wechsler D. WAIS-III Administration and Scoring Manual. San Antonio, TX: Psychological Corporation; 1997 [Google Scholar]
- 20. Reitan RM. The relation of the trail making test to organic brain damage. J Consult Psychol 1955;19:393–394 [DOI] [PubMed] [Google Scholar]
- 21. Dodrill CB. A neuropsychological battery for epilepsy. Epilepsia 1978;19:611–623 [DOI] [PubMed] [Google Scholar]
- 22. Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. New York: Oxford University Press; 1998 [Google Scholar]
- 23. Delis DC, Kramer JH, Kaplan E, Ober B. California Verbal Learning Test, 2nd edition. San Antonio, TX: Psychological Corporation; 2000 [Google Scholar]
- 24. Abdi H. Discriminant correspondence analysis. In: Salkind NJ, ed. Encyclopedia of Measurement and Statistics. Thousand Oaks, CA: Sage Publications; 2007:2702–2775 [Google Scholar]
- 25. Abdi H. Partial least squares regression and projection on latent structure regression (PLS-regression). Wiley Interdiscip Rev Comput Stat 2010;2:97–106 [Google Scholar]
- 26. Abdi H, Williams LJ. Jackknife. In: Salkind NJ, Dougherty DM, Frey B, eds. Encyclopedia of Research Designs. Thousand Oaks, CA: Sage Publications; 2010:6556–6560 [Google Scholar]
- 27. Mathias JL, Wheaton P. Changes in attention and information-processing speed following severe traumatic brain injury: a meta-analytic review. Neuropsychology 2007;21:212–223 [DOI] [PubMed] [Google Scholar]
- 28. Meythaler JM, Peduzzi JD, Eleftheriou E, Novack TA. Current concepts: diffuse axonal injury-associated traumatic brain injury. Arch Phys Med Rehabil 2001;82:1461–1471 [DOI] [PubMed] [Google Scholar]
- 29. Warner MA, Marquez de la Plata C, Spence J, et al. Assessing spatial relationships between axonal integrity, regional brain volumes, and neuropsychological outcomes after traumatic axonal injury. J Neurotrauma 2010;27:2121–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marquez de la Plata CD, Yang FG, Wang JY, et al. Diffusion tensor imaging biomarkers for traumatic axonal injury: analysis of three analytic methods. J Int Neuropsychol Soc 2011;17:24–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain 2007;130:2508–2519 [DOI] [PubMed] [Google Scholar]
- 32. Miles L, Grossman RI, Johnson G, Babb JS, Diller L, Inglese M. Short-term DTI predictors of cognitive dysfunction in mild traumatic brain injury. Brain Inj 2008;22:115–122 [DOI] [PubMed] [Google Scholar]
- 33. Salmond CH, Menon DK, Chatfield DA, et al. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage 2006;29:117–124 [DOI] [PubMed] [Google Scholar]
- 34. Huisman TA, Schwamm LH, Schaefer PW, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol 2004;25:370–376 [PMC free article] [PubMed] [Google Scholar]
- 35. Ito J, Marmarou A, Barzo P, Fatouros P, Corwin F. Characterization of edema by diffusion-weighted imaging in experimental traumatic brain injury. J Neurosurg 1996;84:97–103 [DOI] [PubMed] [Google Scholar]
- 36. Catani M, Jones DK, ffytche DH. Perisylvian language networks of the human brain. Ann Neurol 2005;57:81–86 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.