Abstract
Objective:
To determine whether functional connectivity is altered in subjects with mutations in the microtubule associated protein tau (MAPT) gene who were asymptomatic but were destined to develop dementia, and to compare these findings to those in subjects with behavioral variant frontotemporal dementia (bvFTD).
Methods:
In this case-control study, we identified 8 asymptomatic subjects with mutations in MAPT and 8 controls who screened negative for mutations in MAPT. Twenty-one subjects with a clinical diagnosis of bvFTD were also identified and matched to 21 controls. All subjects had resting-state fMRI. In-phase functional connectivity was assessed between a precuneus seed in the default mode network (DMN) and a fronto-insular cortex seed in the salience network, and the rest of the brain. Atlas-based parcellation was used to assess functional connectivity and gray matter volume across specific regions of interest.
Results:
The asymptomatic MAPT subjects and subjects with bvFTD showed altered functional connectivity in the DMN, with reduced in-phase connectivity in lateral temporal lobes and medial prefrontal cortex, compared to controls. Increased in-phase connectivity was also observed in both groups in the medial parietal lobe. Only the bvFTD group showed altered functional connectivity in the salience network, with reduced connectivity in the fronto-insular cortex and anterior cingulate. Gray matter loss was observed across temporal, frontal, and parietal regions in bvFTD, but not in the asymptomatic MAPT subjects.
Conclusions:
Functional connectivity in the DMN is altered in MAPT subjects before the occurrence of both atrophy and clinical symptoms, suggesting that changes in functional connectivity are early features of the disease.
Frontotemporal dementia (FTD) is a progressive neurodegenerative disorder characterized by behavioral and language deficits.1,2 A large proportion of subjects with FTD have an autosomal dominant pattern of inheritance,3,4 with many showing mutations in the microtubule associated protein tau (MAPT) gene.5 Subjects with mutations in MAPT typically present with behavioral variant FTD (bvFTD), with poor semantic abilities,6–8 and show predominant temporal atrophy, with less severe involvement of frontal and parietal lobes.9,10 Families characterized by the presence of mutations in MAPT provide the ideal construct to identify preclinical disease changes. Case studies assessing imaging in asymptomatic MAPT carriers show mixed results, with atrophy and hypometabolism observed in some asymptomatic subjects, but not others.11–15
Resting-state fMRI has recently emerged as a sensitive biomarker to detect changes in brain functional connectivity.16–18 Functional connectivity is altered in patients with bvFTD,19 with reduced connectivity observed in the salience network that plays a role in processing social-emotional and homeostatically relevant stimuli,20 and increased connectivity in the anti-correlated default mode network (DMN), involved in episodic memory function.18 It has been proposed that changes in functional connectivity may precede the occurrence of atrophy,21 and may therefore constitute one of the earliest features of the disease.
We aimed to determine whether functional connectivity is altered in asymptomatic MAPT subjects, and to compare changes to those observed in clinically diagnosed bvFTD.
METHODS
Subjects.
We identified all subjects seen at Mayo Clinic, Rochester, MN, between January 2009 and July 2010 who had screened positive for mutations in MAPT, were asymptomatic, and had a volumetric MRI and resting-state fMRI scan. Eight subjects, from 4 families, were identified, including 3 with the N279K (c.1842T>G; p.Asn279Lys) mutation from a pallido-ponto-nigral degeneration (PPND) family,22 3 with the V337M (c.2014G>A; Val337Met) mutation, 1 with the P301L mutation (c.1907C>T; p.Pro301Leu), and 1 with the R406W (c.2221C>T; p.Arg406Trp) mutation. All subjects had been enrolled and followed prospectively with annual clinical examinations. None of these subjects have yet developed any symptoms of bvFTD or any other neurodegenerative disease. Eight subjects who had screened negative for mutations in MAPT and were cognitively normal were identified and used as a control group (referred to as MAPT controls). These subjects were members of 3 families that had screened positive for mutations in MAPT.
We also identified 21 subjects seen at Mayo Clinic, Rochester, MN, who fulfilled clinical criteria1 for a diagnosis of bvFTD and had a volumetric MRI and a resting-state fMRI scan. Of these, 4 had mutations in MAPT (1 with the P301L mutation [c.1907C>T; p.Pro301Leu] and 3 with the V337M mutation [c.2014G>A; Val337Met]). These 21 subjects were age and gender-matched to 21 healthy control subjects (referred to as bvFTD controls). Subject demographics are shown in the table.
Table.
Subject demographicsa
Abbreviation: bvFTD = behavioral variant frontotemporal dementia; MAPT = microtubule associated protein tau.
Data shown as median (range) unless otherwise stated.
Years before expected symptom onset (calculated using average symptom onset in each family).
Standard protocol approvals.
Informed consent was obtained from all subjects for participation in the studies, which were approved by the Mayo institutional review board.
Genetic analysis.
Analysis of MAPT exons 1, 7, and 9–13 was performed using primers and conditions that were previously described.5 PCR amplicons were purified using the Multiscreen system (Millipore, Billerica, MA) and then sequenced in both directions using Big Dye chemistry following manufacturer's protocol (Applied Biosystems, Foster City, CA). Sequence products were purified using the Montage system (Millipore) prior to being run on an ABI 3730 DNA Analyzer. Sequence data were analyzed using either SeqScape (Applied Biosystems) or Sequencher software (Gene Codes, Ann Arbor, MI).
Image acquisition.
A standardized protocol was performed on a 3T GE scanner. The resting-state fMRI signal time series was acquired using a gradient echoplanar sequence (repetition time [TR]/echo time [TE] = 3,000/30 msec, 90° flip angle, slice thickness 3.3, and 103 volumes). Subjects were instructed to keep their eyes open during scanning. A 3-dimensional magnetization-prepared rapid acquisition gradient echo (MPRAGE) was also acquired (TR/TE/inversion time, 2,300/3/900 msec; flip angle 8°, 26-cm field of view [FOV]; 256 × 256 in-plane matrix with a phase FOV of 0.94, slice thickness of 1.2 mm). All MPRAGE images underwent preprocessing correction for gradient nonlinearity and intensity nonuniformity.23
Processing of resting-state fMRI.
Preprocessing and data analysis were performed utilizing SPM5 and the resting-state fMRI data analysis toolkit (REST) (http://www.restfmri.net).24 Preprocessing steps included discarding the first 3 volumes to obtain steady state magnetization, realignment, slice time correction, normalization to template, smoothing with 4-mm full width at half maximum Gaussian kernel, linearly detrending to correct for signal drift, and 0.01–0.08 Hz bandpass filtering to reduce non-neuronal contributions to blood oxygenation level–dependent (BOLD) fluctuations. In addition, regression correction for spurious variables included rigid-body transformation motion effects, global mean signal, white matter, and CSF.17,25 These preprocessed images were first analyzed using group independent component analysis (ICA) and dual regression in order to identify the DMN and salience network. The ICA analysis was performed using the asymptomatic MAPT subjects and MAPT controls since this was the comparison of primary interest. The results of the ICA were then used to select seeds for a seed-based connectivity analysis of each network.
ICA analysis.
Independent components were identified using model-free ICA (MELODIC)26 with a low-dimensional estimation of 20 independent components. The DMN and salience network were identified by visual inspection. Individual ICNs were then derived for all subjects utilizing spatial and temporal dual regression, and individual components were entered into statistical analysis. One-sample t tests were used to display voxelwise connectivity maps in the MAPT controls for each network with results assessed at p < 0.05 corrected for multiple comparisons using familywise error (FWE) at the cluster level.
Seed-based analysis.
The results of the ICA one-sample t tests of the DMN and salience networks were used to select seed regions of interest (ROIs) for the voxelwise connectivity analysis. We selected the voxel with the highest T score in the precuneus to represent the DMN network (MNI coordinates 0, −60, 27). Since the fronto-insular cortex is a critical salience network hub,27 we selected the voxel with the highest T score in the fronto-insular cortex to represent the salience network (MNI coordinates 54, 9, 3). Although the ICA was based on the younger MAPT subjects and controls, the seed locations remained identical when the ICA was run with the bvFTD controls. A 6-mm seed was placed for each location. The average BOLD signal time course within each seed was correlated to every voxel in the brain for each subject in the study using Pearson correlation coefficient. The correlation coefficients were converted to z scores using the Fisher r-to-z transformation. Voxels that have positive z scores relative to the seed time courses indicate “in-phase” connections and the voxels with negative z scores indicate “out-of-phase” connections. These z score images were entered into the statistical analysis.
Two-sample 2-sided t tests were performed to compare voxelwise connectivity between 1) asymptomatic MAPT subjects and MAPT controls, 2) subjects with bvFTD and bvFTD controls, and 3) asymptomatic MAPT subjects and subjects with bvFTD. These analyses were performed for both the DMN and salience networks. In order to assess only in-phase connections the group comparisons were masked by the out-of-phase connectivity map identified in a one-sample t test of the matched control group. Results were assessed at p < 0.05 corrected for multiple comparisons using FWE at the cluster level, and age and gender were included in the models as covariates. In addition, functional connectivity was assessed across specific ROIs defined using the AAL atlas.28 ROIs were placed to sample areas that showed altered functional connectivity in the disease groups (see Results). Therefore, DMN ROIs were placed in lateral temporal lobe, medial prefrontal cortex, and medial parietal lobes (precuneus + posterior cingulate), and a salience ROI was placed in anterior cingulate.
Processing of structural MRI.
Voxel-based morphometry using SPM5 was used to assess patterns of gray matter loss in the asymptomatic MAPT subjects compared to MAPT controls and in the subjects with bvFTD compared to bvFTD controls. Image processing was performed as previously described,29 and analyses were assessed corrected for multiple comparisons using the false discovery rate (p < 0.0005) with age and gender included as covariates. Atlas-based parcellation using the AAL atlas was used to generate gray matter volumes for each of the regions assessed in the fMRI analysis, as previously described.29 Total intracranial volume (TIV) was also calculated and used to correct regional gray matter volumes for differences in head size.
Statistics.
Subject demographics were compared between groups using χ2 tests and Wilcoxon rank sum test. For group comparisons of ROI-level data, we used analysis of covariance models, in which the ROI measurement was the response, age at imaging was an adjustment covariate, and group was a 2-level predictor. Analyses were performed using SAS version 9.2 (SAS Institute, Cary, NC) and R version 2.9.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Default mode network (precuneus seed).
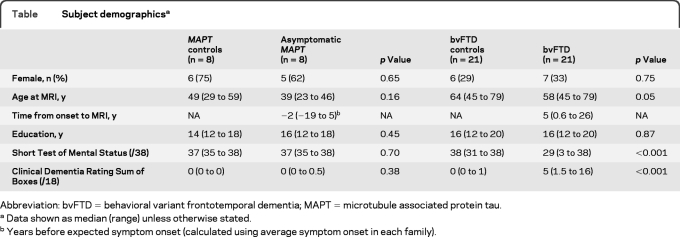
The precuneus seed showed in-phase connectivity with medial and lateral parietal lobe, medial prefrontal cortex, and lateral temporal lobe in controls (figure 1). The asymptomatic MAPT and bvFTD groups showed reduced in-phase connectivity in lateral temporal lobes and medial prefrontal cortex compared to controls (figures 1 and 2 ). The bvFTD group also showed reduced connectivity in dorsolateral frontal lobe. Increased in-phase connectivity was observed in both groups in medial parietal lobe. The asymptomatic MAPT group showed greater reduction in connectivity in lateral temporal lobe and basal ganglia than subjects with bvFTD (figure e-1 on the Neurology® Web site at www.neurology.org). The subjects with bvFTD did not show any regions of reduced connectivity compared to asymptomatic MAPT subjects.
Figure 1. Resting-state fMRI results from the default mode network seed-based analysis of the precuneus.
The top row shows the results for the asymptomatic MAPT subjects and the MAPT controls, while the bottom row shows the results for the subjects with behavioral variant frontotemporal dementia (bvFTD) and the bvFTD controls. Left: Patterns of in-phase voxelwise connectivity observed in control subjects. Middle: Patterns of in-phase voxelwise connectivity observed in the disease groups. Right: Patterns of reduced (shown in blue) and increased (shown in yellow) in-phase connectivity in the disease groups compared to the age-matched controls. Results are shown after cluster-level correction for multiple comparisons at p < 0.05.
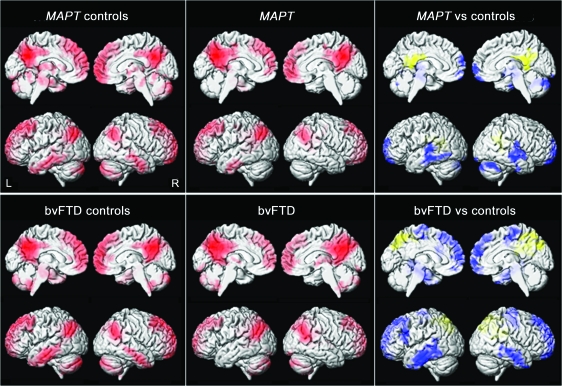
Figure 2. Box plots showing the distribution of mean functional connectivity (FC) and total intracranial volume–corrected gray matter (GM) volumes for the 4 regions of interest.
Data are plotted separately for each of the 4 subject groups. Partial residuals from the analysis of covariance model are plotted. The value on the y-axis can be interpreted as the observed value minus what would be expected under the model given the subject's age. The boxes indicate the 25th, 50th (median), and 75th percentiles of the distributions while the horizontal lines extending from the boxes stop at the most extreme data points. The p values indicate the significance of differences between the asymptomatic MAPT subjects and their age-matched controls, and between the subjects with behavioral variant frontotemporal dementia (bvFTD) and their age-matched controls, using the Wilcoxon rank sum test. Four of the subjects with bvFTD had mutations in MAPT (shown in red).
Salience network (fronto-insular cortex seed).
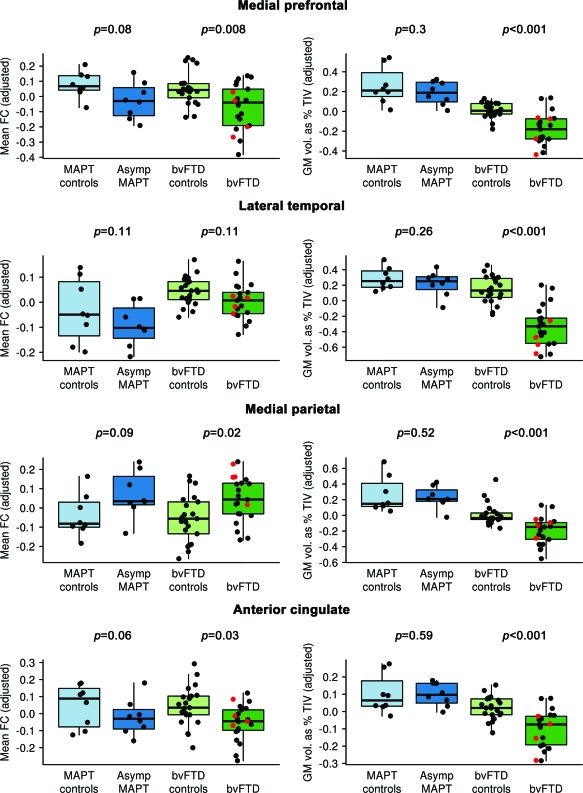
The fronto-insular cortex seed showed in-phase connectivity predominantly with bilateral fronto-insular cortex, anterior cingulate, and dorsolateral frontal lobes in controls (figure 3). In SPM, only the subjects with bvFTD showed alterations in this pattern, with reduced in-phase connectivity in bilateral fronto-insular cortex, anterior cingulate, dorsolateral frontal lobes, and striatum (figures 2 and 3). The asymptomatic MAPT subjects did, however, show a trend at the ROI level for reduced connectivity in anterior cingulate (figure 2). No regions showed increased connectivity in either group and no differences were observed between the asymptomatic MAPT subjects and subjects with bvFTD.
Figure 3. Resting-state fMRI results from the Salience network seed-based analysis of the fronto-insular cortex.
Results are shown only for the behavioral variant frontotemporal dementia (bvFTD) comparisons, since no significant changes in functional connectivity were observed in the asymptomatic MAPT subjects. Left: Patterns of in-phase voxelwise connectivity observed in the bvFTD control subjects. Middle: Patterns of in-phase voxelwise connectivity observed in the subjects with bvFTD. Right: Patterns of reduced (shown in blue) and increased (shown in yellow) in-phase connectivity in the subjects with bvFTD compared to the age-matched controls. Results are shown after cluster-level correction for multiple comparisons at p < 0.05.
Gray matter loss.
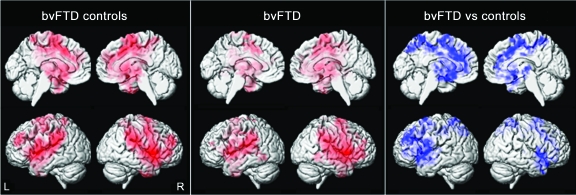
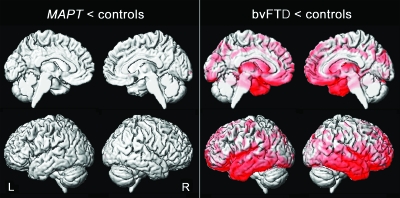
The subjects with bvFTD showed a widespread pattern of gray matter loss involving temporal, frontal, and parietal lobes compared to the bvFTD controls (figures 2 and 4). No gray matter loss was identified in the asymptomatic MAPT subjects compared to the MAPT controls (figure 2 and 4).
Figure 4. Patterns of gray matter loss in the asymptomatic MAPT subjects and the subjects with behavioral variant frontotemporal dementia (bvFTD) when compared to their age-matched controls.
Results are shown on 3-dimensional renderings of the brain after correction for multiple comparisons using the false discovery rate at p < 0.0005.
DISCUSSION
We have demonstrated that functional connectivity in the brain is altered in subjects with mutations in MAPT even before the occurrence of gray matter atrophy and clinical symptoms. These findings increase our understanding of disease progression and suggest that changes in functional connectivity could be one of the earliest features of the disease.
The asymptomatic MAPT subjects showed reduced connectivity in the DMN predominantly between precuneus and lateral temporal lobe, but also medial prefrontal cortex, compared to age-matched controls. Gray matter loss has been observed in these regions in symptomatic MAPT subjects, with particularly severe involvement of the temporal lobes.9,10 However, no gray matter loss was observed in these regions in the asymptomatic subjects, suggesting, importantly, that changes in functional connectivity precede the occurrence of atrophy in these regions. Very similar patterns of reduced DMN functional connectivity were observed in bvFTD, involving both lateral temporal lobes and medial prefrontal cortex, again suggesting that these patterns are disease-related.
While the posterior DMN has been associated predominantly with episodic memory and visuospatial functions which are typically spared in bvFTD,1 components of the DMN, such as medial prefrontal cortex and lateral temporal lobes, have been shown to be associated with functions such as semantic memory30 and theory of mind,31 which show abnormalities both in subjects with bvFTD29,32 and MAPT subjects.6 It is therefore possible that deficits in the DMN could be responsible for some of the clinical features observed in bvFTD. The bvFTD group showed greater involvement of the frontal lobes, perhaps suggesting that disconnection of parietal and frontal lobes may relate directly to the development of behavioral symptoms. Our results contrast with a previous study that did not observe reduced DMN connectivity in bvFTD, and suggested that reduced DMN connectivity was a feature of Alzheimer disease (AD).19 However, given the similarity in the connectivity pattern between MAPT and bvFTD, and the fact that AD pathology is very unlikely in young individuals with MAPT mutations, we believe our findings are likely a feature of the primary tauopathy and not a manifestation of covert AD pathology. Discordance across the studies may be due to methodologic differences or inclusion of different anatomic subtypes of bvFTD.29
In contrast to the DMN findings, reduced functional connectivity in the salience network was a more striking feature of bvFTD rather than asymptomatic MAPT. Reduced connectivity was observed between the fronto-insular cortex seed and surrounding fronto-insular cortex and anterior cingulate in bvFTD. These reductions in the salience network are similar to those previously reported in bvFTD.19 The SPM analysis failed to identify any regions of reduced salience connectivity in the asymptomatic MAPT subjects, although there was a suggestion of reduced connectivity in anterior cingulate at the ROI level. The less striking findings in this group concur with the finding that MAPT mutations are associated with predominant temporal lobe involvement, with less involvement of frontal regions that are considered part of the salience network.9,10 Behavioral deficits that occur in symptomatic MAPT subjects could be due to deficits in the right temporal lobe.33
Increased functional connectivity was also observed in medial parietal regions of the DMN in both the asymptomatic MAPT subjects and subjects with bvFTD. This enhancement in posterior regions of the DMN has previously been observed in bvFTD and has been shown to be associated with reduced connectivity in the anti-correlated salience network.19 Impairment in the salience network is thought to alter the posterior DMN responses leading to enhanced parietal lobe functions.19 Our bvFTD findings concur with this hypothesis, although the asymptomatic MAPT subjects did not show strong reciprocal reductions in the salience network. This could suggest that the temporal sequence of events is reversed in MAPT subjects, with changes in the DMN occurring before the salience network. Alternatively, our sensitivity to detect network abnormalities could also be greater in the DMN.
Gray matter atrophy was observed in all regions that showed reduced functional network connectivity in bvFTD, suggesting an association between these 2 mechanisms. However, somewhat counterintuitively, we observed parietal lobe atrophy yet functional connectivity increases in bvFTD. Since parietal lobe is not commonly an early site of pathologic damage in bvFTD,34 atrophy is largely due to spreading of disease from the pathologically damaged frontal and temporal lobes.34 Increased functional connectivity in this region is likely due to a disconnection from pathologically affected salience regions. Therefore, atrophy and increased functional connectivity in this region may be resulting independently from pathologic changes occurring elsewhere in the brain.
The similarity of findings in the asymptomatic MAPT subjects to the bvFTD group provided an important validation of the results. A limitation, however, was that the bvFTD group did not consist solely of subjects with mutations in MAPT, and therefore cannot be strictly considered as being on the MAPT disease spectrum. Subjects with bvFTD have heterogeneous pathologic and genetic etiologies,2 which are typically associated with differing patterns of atrophy.10,35 Patterns of functional change in bvFTD may also differ according to pathology or genetics, although without pathologic confirmation we cannot draw firm conclusions concerning any such relationships. The 4 subjects with bvFTD with mutations in MAPT did however show very similar patterns of functional change to the rest of the bvFTD cohort.
The strengths of our study include the fact that the asymptomatic MAPT subjects and subjects with bvFTD were each matched to a control group that was as close as possible in age, and age was included in all analyses as a covariate to further reduce potential confounds. Functional connectivity alters during aging,36–38 and indeed we observed age-related differences in the lateral temporal lobe. The MAPT controls all screened negative for mutations in MAPT, but were from families that had screened positive, allowing us to inherently match for other genetic features. The number of subjects with MAPT mutations was however relatively small compared to the bvFTD group; this could have contributed to the negative findings and borderline ROI p values in this group. While resting state fMRI has an advantage over task-related fMRI in eliminating performance confounds, there are still some methodologic issues that need to be resolved.39 One key issue is the interpretability of out-of-phase correlations between regions introduced due to global signal removal.
The findings from this study have important implications for the study of FTD. They suggest that disruptions in functional connectivity may be a fundamental and critical early stage in the disease. Future studies, with larger numbers of subjects, test-retest assessments, and longitudinal imaging, will be needed to determine if functional connectivity fulfills criteria to be an early biomarker in FTD.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Guang Zeng for programming assistance and Audrey Strongosky for making travel arrangements for PPND family members.
GLOSSARY
- AD
Alzheimer disease
- BOLD
blood oxygenation level–dependent
- bvFTD
behavioral variant frontotemporal dementia
- DMN
default mode network
- FOV
field of view
- FTD
frontotemporal dementia
- FWE
familywise error
- ICA
independent component analysis
- MPRAGE
magnetization-prepared rapid acquisition gradient echo
- PPND
pallido-ponto-nigral degeneration
- ROI
region of interest
- TE
echo time
- TIV
total intracranial volume
- TR
repetition time
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Whitwell: drafting/revising the manuscript for content, study concept or design, analysis or interpretation of the data, acquisition of data, statistical analysis. Dr. Josephs: drafting/revising the manuscript for content, study concept or design, analysis or interpretation of the data, acquisition of data, study supervision. Dr. Avula: drafting/revising the manuscript for content, analysis or interpretation of the data. Nirubol Tosakulwong: drafting/revising the manuscript for content, statistical analysis. Stephen Weigand: drafting/revising the manuscript for content, statistical analysis. Matthew Senjem: drafting/revising the manuscript for content, analysis or interpretation of the data. Dr. Vemuri: drafting/revising the manuscript for content, analysis or interpretation of the data. Dr. Jones: drafting/revising the manuscript for content, analysis or interpretation of the data. Dr. Gunter: drafting/revising the manuscript for content, analysis or interpretation of the data. Matthew Baker: drafting/revising the manuscript for content, acquisition of data. Dr. Wszolek: drafting/revising the manuscript for content, acquisition of data, obtaining funding. Dr. Knopman: drafting/revising the manuscript for content, acquisition of data. Dr. Rademakers: drafting/revising the manuscript for content, acquisition of data, obtaining funding. Dr. Petersen: drafting/revising the manuscript for content, acquisition of data, obtaining funding. Dr. Boeve: drafting/revising the manuscript for content, acquisition of data. Dr. Jack: drafting/revising the manuscript for content, study concept or design, analysis or interpretation of the data, acquisition of data, study supervision, obtaining funding.
DISCLOSURE
Dr. Whitwell receives research support from the NIH (NIDCD, NIA) and the Dana Foundation. Dr. Josephs receives research support from the NIH (NIDCD, NIA) and the Dana Foundation. Dr. Avula serves as a consultant to Medical Imaging Solutions. N. Tosakulwong, S.D. Weigand, and M.L. Senjem report no disclosures. Dr. Vemuri receives support from the Robert H. Smith Family Foundation Research Fellowship and the NIH. Dr. Jones and Dr. Gunter report no disclosures. M. Baker holds patents re: Methods and materials for detecting and treating dementia. Dr. Wszolek serves as Co-Editor-in-Chief of Parkinsonism and Related Disorders, Regional Editor of the European Journal of Neurology, and on the editorial boards of Neurologia i Neurochirurgia Polska, Advances in Rehabilitation, the Medical Journal of the Rzeszow University, and Clinical and Experimental Medical Letters; holds and has contractual rights for receipt of future royalty payments from patents re: A novel polynucleotide involved in heritable Parkinson's disease; receives royalties from publishing Parkinsonism and Related Disorders (Elsevier, 2007, 2008, 2009) and the European Journal of Neurology (Wiley-Blackwell, 2007, 2008, 2009); and receives research support from Allergan, Inc., the NIH, the Pacific Alzheimer Research Foundation (Canada), the CIHR, the Mayo Clinic Florida Research Committee CR program, and a gift from Carl Edward Bolch, Jr., and Susan Bass Bolch. Dr. Knopman serves as Deputy Editor for Neurology®; has served on a data safety monitoring board for Eli Lilly and Company; has served as a consultant for Elan/Janssen AI; is an investigator in clinical trials sponsored by Elan/Janssen AI, Baxter International Inc., and Forest Laboratories, Inc.; and receives research support from the NIH. Dr. Rademakers holds patents re: Methods and materials for detecting and treating dementia and receives research support from the NIH, the Pacific Alzheimer Research Foundation (Canada), the Association for Frontotemporal Dementia, the Amyotrophic Lateral Sclerosis Association, CurePSP, and the Consortium for Frontotemporal Dementia. Dr. Petersen serves on scientific advisory boards for the Alzheimer's Association, the National Advisory Council on Aging (NIA), Elan/Janssen AI, Pfizer Inc (Wyeth), and GE Healthcare; receives royalties from publishing Mild Cognitive Impairment (Oxford University Press, 2003); serves as a consultant for Elan/Janssen AI and GE Healthcare; and receives research support from the NIH/NIA. Dr. Boeve has served as a consultant to GE Healthcare; receives publishing royalties for The Behavioral Neurology of Dementia (Cambridge University Press, 2009); and receives research support from Cephalon, Inc., Allon Therapeutics, Inc., the NIH/NIA, the Alzheimer's Association, and the Mangurian Foundation. Dr. Jack serves on scientific advisory boards for Elan/Janssen AI, Eli Lilly & Company, GE Healthcare, and Eisai Inc.; receives research support from Baxter International Inc., Allon Therapeutics, Inc., Pfizer Inc., the NIH/NIA, and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation; and holds stock/stock options in Johnson & Johnson.
REFERENCES
- 1. Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 1998;51:1546–1554 [DOI] [PubMed] [Google Scholar]
- 2. Josephs KA. Frontotemporal dementia and related disorders: deciphering the enigma. Ann Neurol 2008;64:4–14 [DOI] [PubMed] [Google Scholar]
- 3. Chow TW, Miller BL, Hayashi VN, Geschwind DH. Inheritance of frontotemporal dementia. Arch Neurol 1999;56:817–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rizzu P, Van Swieten JC, Joosse M, et al. High prevalence of mutations in the microtubule-associated protein tau in a population study of frontotemporal dementia in the Netherlands. Am J Hum Genet 1999;64:414–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hutton M, Lendon CL, Rizzu P, et al. Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 1998;393:702–705 [DOI] [PubMed] [Google Scholar]
- 6. Pickering-Brown SM, Rollinson S, Du Plessis D, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain 2008;131:721–731 [DOI] [PubMed] [Google Scholar]
- 7. Beck J, Rohrer JD, Campbell T, et al. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain 2008;131:706–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boeve BF, Hutton M. Refining frontotemporal dementia with parkinsonism linked to chromosome 17: introducing FTDP-17 (MAPT) and FTDP-17 (PGRN). Arch Neurol 2008;65:460–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whitwell JL, Jack CR, Jr, Boeve BF, et al. Atrophy patterns in IVS10+16, IVS10+3, N279K, S305N, P301L, and V337M MAPT mutations. Neurology 2009;73:1058–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Whitwell JL, Jack CR, Jr, Boeve BF, et al. Voxel-based morphometry patterns of atrophy in FTLD with mutations in MAPT or PGRN. Neurology 2009;72:813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Miyoshi M, Shinotoh H, Wszolek ZK, et al. In vivo detection of neuropathologic changes in presymptomatic MAPT mutation carriers: a PET and MRI study. Parkinsonism Relat Disord 2010;16:404–408 [DOI] [PubMed] [Google Scholar]
- 12. Arvanitakis Z, Witte RJ, Dickson DW, et al. Clinical-pathologic study of biomarkers in FTDP-17 (PPND family with N279K tau mutation). Parkinsonism Relat Disord 2007;13:230–239 [DOI] [PubMed] [Google Scholar]
- 13. Kishore A, Wszolek ZK, Snow BJ, et al. Presynaptic nigrostriatal function in genetically tested asymptomatic relatives from the pallido-ponto-nigral degeneration family. Neurology 1996;47:1588–1590 [DOI] [PubMed] [Google Scholar]
- 14. Alberici A, Gobbo C, Panzacchi A, et al. Frontotemporal dementia: impact of P301L tau mutation on a healthy carrier. J Neurol Neurosurg Psychiatry 2004;75:1607–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kantarci K, Boeve BF, Wszolek ZK, et al. MRS in presymptomatic MAPT mutation carriers: a potential biomarker for tau-mediated pathology. Neurology 2010;75:771–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Rev 2007;8:700–711 [DOI] [PubMed] [Google Scholar]
- 17. Fox MD, Snyder AZ, Vincent JL, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA 2005;102:9673–9678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 2003;100:253–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhou J, Greicius MD, Gennatas ED, et al. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer's disease. Brain 2010;133:1352–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007;27:2349–2356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seeley WW, Crawford RK, Zhou J, et al. Neurodegenerative diseases target large-scale human brain networks. Neuron 2009;62:42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wszolek ZK, Pfeiffer RF, Bhatt MH, et al. Rapidly progressive autosomal dominant parkinsonism and dementia with pallido-ponto-nigral degeneration. Ann Neurol 1992;32:312–320 [DOI] [PubMed] [Google Scholar]
- 23. Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chao-Gan Y, Yu-Feng Z. DPARSF: a MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Frontiers Syst Neurosci 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weissenbacher A, Kasess C, Gerstl F, et al. Correlations and anticorrelations in resting-state functional connectivity MRI: a quantitative comparison of preprocessing strategies. Neuroimage 2009;47:1408–1416 [DOI] [PubMed] [Google Scholar]
- 26. Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging 2004;23:137–152 [DOI] [PubMed] [Google Scholar]
- 27. Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA 2008;105:12569–12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 2002;15:273–289 [DOI] [PubMed] [Google Scholar]
- 29. Whitwell JL, Przybelski SA, Weigand SD, et al. Distinct anatomical subtypes of the behavioural variant of frontotemporal dementia: a cluster analysis study. Brain 2009;132:2932–2946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wirth M, Jann K, Dierks T, et al. Semantic memory involvement in the default mode network: a functional neuroimaging study using independent component analysis. NeuroImage 2011;54:3057–3066 [DOI] [PubMed] [Google Scholar]
- 31. Spreng RN, Grady CL. Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J Cogn Neurosci 2010;22:1112–1123 [DOI] [PubMed] [Google Scholar]
- 32. Kipps CM, Hodges JR. Theory of mind in frontotemporal dementia. Soc Neurosci 2006;1:235–244 [DOI] [PubMed] [Google Scholar]
- 33. Josephs KA, Whitwell JL, Knopman DS, et al. Two distinct subtypes of right temporal variant frontotemporal dementia. Neurology 2009;73:1443–1450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kril JJ, Macdonald V, Patel S, et al. Distribution of brain atrophy in behavioral variant frontotemporal dementia. J Neurol Sci 2005;232:83–90 [DOI] [PubMed] [Google Scholar]
- 35. Whitwell JL, Jack CR, Jr, Parisi JE, et al. Does TDP-43 type confer a distinct pattern of atrophy in frontotemporal lobar degeneration? Neurology 2010;75:2212–2220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Andrews-Hanna JR, Snyder AZ, Vincent JL, et al. Disruption of large-scale brain systems in advanced aging. Neuron 2007;56:924–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Biswal BB, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA 2010;107:4734–4739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Damoiseaux JS, Beckmann CF, Arigita EJ, et al. Reduced resting-state brain activity in the “default network” in normal aging. Cereb Cortex 2008;18:1856–1864 [DOI] [PubMed] [Google Scholar]
- 39. Van Dijk KR, Hedden T, Venkataraman A, et al. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 2010;103:297–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.