Abstract
Objectives:
Experiments in animal models have identified specific subcortical anatomic circuits, which are critically involved in the pathogenesis and control of seizure activity. However, whether such anatomic substrates also exist in human epilepsy is not known.
Methods:
We studied 2 separate groups of patients with focal epilepsies arising from any cortical location using either simultaneous EEG-fMRI (n = 19 patients) or [11C]flumazenil PET (n = 18).
Results:
Time-locked with the interictal epileptiform discharges, we found significant hemodynamic increases common to all patients near the frontal piriform cortex ipsilateral to the presumed cortical focus. GABAA receptor binding in the same area was reduced in patients with more frequent seizures.
Conclusions:
Our findings of cerebral blood flow and GABAergic changes, irrespective of where interictal or ictal activity occurs in the cortex, suggest that this area of the human primary olfactory cortex may be an attractive new target for epilepsy therapy, including neurosurgery, electrical stimulation, and focal drug delivery.
Experimental evidence from animal models indicates that, independent of seizure induction, certain subcortical anatomic circuits act as critical modulators of seizure generation and propagation.1–5 Although epileptic seizures may result from a broad array of brain insults involving various brain areas, seizure activity does not spread diffusely throughout the brain but propagates along specific anatomic pathways.1–4 During focal cortical seizure activity, specific cortical-subcortical circuits contribute to sustaining and propagating the seizure discharge. Experiments in animal models have identified specific brain regions such as the substantia nigra and the deep anterior piriform cortex as important for controlling the initiation or propagation of both generalized and focal seizure activity.4,6–9 In rat and monkey, a discrete site within the deep piriform (primary olfactory) cortex, termed area tempestas or ventrostriatal anterior piriform cortex, is critical for modulating focal seizures.4,10 However, there is little experimental evidence to translate these observations to the human situation.11 Recent observations with deep brain stimulation in a variety of subcortical structures in patients with epilepsy12 suggest that cortical-subcortical circuits have the potential to be harnessed for therapeutic benefit.
We performed EEG combined with simultaneous fMRI in a group of patients with focal epilepsies arising from a wide variety of cortical locations to test whether specific interictal epileptiform discharge (IED)–correlated hemodynamic changes occur within the human equivalent of the area tempestas. Furthermore, in another group of patients with extratemporal epilepsy syndromes, we used 11C-labeled flumazenil (FMZ) PET to assess seizure-related metabolic γ-aminobutyric acid (GABA)–mediated changes within this region.
METHODS
Standard protocol approvals, registrations, and patient consents.
The study was approved by the joint ethics committee of the National Hospital for Neurology and Neurosurgery and University College London Institute of Neurology, London, UK. Subjects gave informed, written consent.
Patients.
Sixty-three patients with focal epilepsy underwent EEG-fMRI, after which IEDs were correlated with the fMRI data in an event-related fashion.11 Because IEDs occur spontaneously and unpredictably, the number of events captured varied widely across patients. To ensure the validity of the group analysis described below, i.e., to avoid any violation of homoscedasticity implicit in the loss of balance at the first level, it was mandatory only to include patients with a similar number of IEDs during fMRI data acquisition.12,13 Consequently, of the 63 patients with focal epilepsy, those with a spiking rate in the midrange level of activity in the group (between 1 and 20 IEDs/min) were selected, giving 19 patients (10 female; mean age 38 years, range 25–67 years) for the group analysis (for patient demographics, see table e-1a on the Neurology® Web site at www.neurology.org).
A different patient group was studied with [11C]FMZ PET: 18 patients (7 female; mean age 27 years, range 18–47 years) with MRI reported as normal by an experienced neuroradiologist were recruited (table e-1b). All of these subjects had focal or secondarily generalized seizures. Patients were excluded from the study if they were taking benzodiazepines. A group of 24 healthy subjects (3 female) of similar age (mean age 31 years, range 20–51 years), who had no evidence of a neurologic disorder and were taking no medication, were studied. Consumption of alcohol was not allowed during 48 hours preceding the scan. Written informed consent was obtained from all subjects, and approvals from local ethical committees and the UK Administration of Radioactive Substances Advisory Committee were obtained.
EEG and fMRI acquisition.
Methods and results pertaining to single-subject analyses have been reported elsewhere.13 In summary, using magnetic resonance–compatible equipment, 10 EEG channels were recorded using the International 10–20 System and bipolar EKGs. Over 35 minutes, 704 T2*-weighted single-shot gradient-echo echoplanar images (echo time = 40, repetition time = 3,000, 21 slices, voxel size 3.75 × 3.75 × 5 mm3) were acquired continuously on a 1.5-T Horizon EchoSpeed MRI scanner (General Electric, Milwaukee, WI). Patients were asked to rest with their eyes shut and to keep their head still. After removal of artifact on the in-scanner EEG, IEDs were marked by 2 trained observers. fMRI data were preprocessed and analyzed using statistical parametric mapping (SPM).14 After the first 4 image volumes were discarded, the echoplanar image time series was realigned and normalized (Montreal Neurological Institute [MNI] template brain), and images were spatially smoothed with a cubic Gaussian kernel of 8 mm full-width at half-maximum. The 3 datasets of patients in whom the presumed electroclinical location of the epileptic focus was right-sided were flipped along the x-axis before normalization.
Spike-correlated EEG-fMRI group analysis.
Onsets of IEDs were used to build a linear model of effects of interest by convolution with a canonical hemodynamic response function (event-related design) and its temporal derivative to account for variations in the blood oxygen level–dependent (BOLD) response delay. Motion realignment parameters were modeled as a confound.15 A single T-contrast image was generated per subject from the first (single-subject) level, and the images were used in a second-level analysis, to test for any common patterns across the group of patients. A random-effects model was used to identify any typical responses consistent across patients.16 We used this approach to test the hypothesis of activation in the region of the presumed area tempestas. Bilateral 0.7 × 1.4 × 1.4 cm search volumes (totaling 2,744 mm3) were each centered between the tip of the temporal pole and the orbitofrontal gyrus based on the aneurysm case report of Mizobuchi et al.,17 and, in these regions, fMRI signal changes were considered significant at p < 0.05 (family-wise error–corrected for multiple comparisons within the search volume). In addition, positive responses were explored across the whole brain at a significance threshold of p < 0.001 (uncorrected at the voxel level) to assess the presence of unspecific effects, e.g., subthreshold bilateral, or covering the entire region of interest or even beyond.
PET acquisition.
The method has been described in detail previously.18 In brief, scans were performed using an ECAT-953B PET scanner (CTI/Siemens, Knoxville, TN) in 3-dimensional mode, with the septa retracted to improve sensitivity. Scatter correction and attenuation correction were used in reconstruction to produce images with a resolution of 4.8 × 4.8 × 5.2 mm. Images containing 31 contiguous slices were produced with voxel dimensions of 2.09 × 2.09 × 3.43 mm. High specific activity [11C]FMZ tracer was injected IV. A dynamic image sequence of 20 frames was acquired over 90 minutes.
FMZ PET data analysis.
The derivation of an arterial plasma input function was performed as described previously.19 Voxel-by-voxel parametric images of FMZ volume of distribution (FMZ-VT) were produced using spectral analysis.20 For group analysis, 8 datasets were flipped about the anteroposterior axis to ensure that the epileptogenic focus was on the same (left) side in all patients. SPM was used for spatial transformations and statistical analysis. First, all images were transformed into a standard space. An in-house created FMZ-VT template that occupies the standard stereotaxic space defined by the MNI/International Consortium for Brain Mapping (ICBM) 152 templates as supplied with SPM was right-left reversed (flipped), rigid-body coregistered onto itself, and averaged using a soft mean, thus creating a symmetric template approximating MNI/ICBM 152 space. Second, the images were smoothed using a (10 × 10 × 6 mm full-width at half-maximum) Gaussian kernel to reduce high spatial frequency noise. Third, effects were estimated according to the general linear model at every voxel. Global activity was included as a confounding covariate. Patients and normal subjects were compared using a voxel-wise t test. To test hypotheses about regionally specific effects, the estimates were compared using linear contrasts. The resulting set of voxel values for each contrast constituted a statistical parametric map of the t statistic (SPM{t}). For the comparison of the patient and normal groups, the SPM{t} was transformed to the unit normal distribution (SPM{Z}), and, because we had no a priori hypotheses with regard to the regions to be examined, an uncorrected threshold of p < 0.01 was subjected to a correction for multiple nonindependent comparisons in terms of peak height (μ), taking into account the shape of the thresholded volume (spatial extent [κ] at p < 0.05), to allow the entire brain volume to be interrogated.14 For the analysis of correlation between FMZ-VT and seizure frequency, the total number of seizures that occurred during the month before the PET scan (as determined from patients' prospectively compiled diaries) was included in the model in a voxel-wise linear regression. Effects were significant at p < 0.05 corrected for multiple comparisons using both μ and κ across the whole brain.14
RESULTS
EEG-fMRI.
We identified 19 patients who had well-defined focal epilepsy syndromes (table e-1a). We found a p < 0.05 (corrected for multiple comparisons) correlation between IED occurrence and BOLD increase common to all 19 patients (i.e., typical for the group studied with 1–20 IED/min) in an area near the frontal piriform cortex (X, Y, Z = −30, 6, −2, coordinates in Talairach space), on the same side as the presumed cortical epileptic focus (figure 1, table e-2).
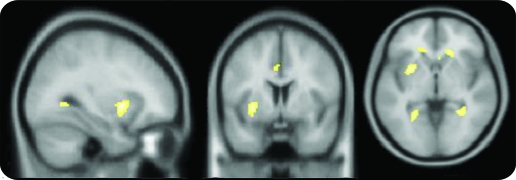
Figure 1. EEG-fMRI group analysis.
Results of a second-level random-effects group analysis of 19 patients with focal epilepsy syndromes. For visualization, consistent common activations (p < 0.001) are overlaid on axial slices of a mean T1-weighted template brain (X, Y, Z = −30, 6, −2, coordinates in Montreal Neurological Institute space). The activation within the region of interest near the presumed area tempestas was significant at p < 0.05 (family-wise error), when correcting for multiple comparisons across the search region (2,744 mm3).
[11C]FMZ PET.
The 18 patients had significant increases in FMZ-VT compared with that of the 24 control subjects in the ipsilateral putamen (z = 5.21) and the contralateral putamen (z = 4.4) (figure 2). These increases were apparent on a single-subject level in 13 of 18 patients. No regions of decreased FMZ-VT were found. For comparison with the fMRI data, we analyzed the data to look for regions in which FMZ-VT correlated significantly with seizure frequency, confining our attention only to those regions identified in the first analysis. The lower the FMZ-VT in the same area near the frontal piriform cortex, the higher was the seizure frequency over the preceding month (z = 3.97) (figure 3). This correlation remained significant, even when the subject with very frequent seizures (>70/month) was removed. There were no significant correlations between increasing FMZ-VT and seizure frequency.
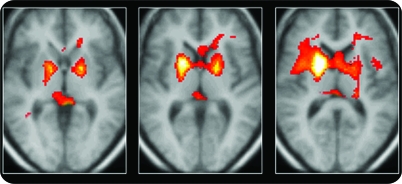
Figure 2. Flumazenil PET group comparison.
Regions of significantly increased flumazenil volume of distribution in 18 patients with focal epilepsy syndromes compared with those of 24 normal control subjects. The hot metal color scale displays all voxels falling below p < 0.01 for display; increasing intensity corresponds to increased significance.
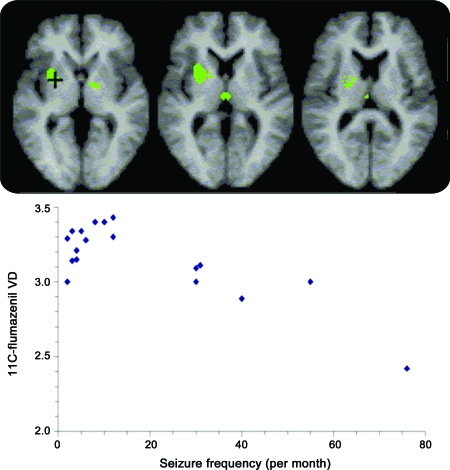
Figure 3. Flumazenil PET correlational analysis.
(Top row) Regions of increased flumazenil volume of distribution (VD) in 18 patients with focal epilepsy syndromes in a parametric analysis of patient data alone that showed reduced flumazenil binding with increased number of seizures per month (p < 0.05 corrected). (Bottom) Scattergraph of seizure frequency vs flumazenil volume of distribution at the voxel with a maximum z score (indicated by + in the left panel).
DISCUSSION
Our study is unique for the following 2 reasons. 1) By averaging the imaging data across a group of patients with different sites of seizure onset, we were able to eliminate signal changes associated with sites of seizure onset (which varied across the patients) and selectively detect signal changes common to all patients. 2) In 2 independent datasets using 2 different imaging modalities, we identified an area in the human piriform (primary olfactory) cortex that was active in association with interictal EEG spikes and where benzodiazepine-GABAA receptor complex expression was reduced as seizure frequency increased (figure 4). This region is located in close proximity to the physiologically defined deep piriform cortex (area tempestas) from which convulsants are known to initiate temporal lobe seizures,20,21 and blockade of glutamate4,20–22 or application of a GABA agonist in this area22 reduces limbic motor seizures in rodents and nonhuman primates.1
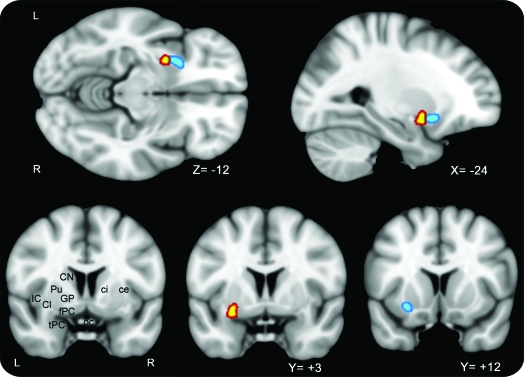
Figure 4. Combined EEG-fMRI/PET results.
Clusters around the peak voxels for EEG-fMRI group analysis (yellow) and correlation between flumazenil binding and seizure frequency (blue) are superimposed on a T1 template. ce = capsula externa; ci = capsula interna; Cl = claustrum; CN = caudate nucleus; fPC = frontal piriform cortex; GP = globus pallidus; IC = insular cortex; oc = optic chiasm; Pu = putamen; tPC = temporal piriform cortex.
The piriform/primary olfactory cortex, because of its unique intrinsic associative fiber system and its various connections to and from other limbic nuclei,23–25 might be part of an epileptic network that is pivotal in the genesis of focal seizures, facilitating and intensifying the spread of seizures from a focus in the hippocampus or other limbic sites to cortical and subcortical regions along pathways that are also used in normal movements.26–29 The deep piriform cortex is a site at which unilateral microinjection of a GABA receptor antagonist or glutamate receptor agonists triggered limbic motor seizures in rats and nonhuman primates, whereas enhancement of GABA-mediated mechanisms reduced seizure activity. Before our study, there was no direct evidence implicating the piriform cortex in the pathogenesis of human epilepsy.
Our observed association of low FMZ-VT in the human frontal piriform (primary olfactory) cortex with increased seizure frequency is concordant with findings in animal models of focal epilepsies.30,31 FMZ-VT is directly correlated with central benzodiazepine receptor density (Bmax) and hence may act as an index of GABAA density. Postsynaptic increases in the number of GABAA receptors underlying the inhibitory potentiation in the kindling model have been described.32 Such an increase in available binding sites (Bmax) will lead to an increase in FMZ-VT. Likewise, a recent study using the pilocarpine model found presynaptic and postsynaptic changes of GABA transmission involving changes of GABAA receptor subunit composition.33 Thus, increased density or affinity of available receptors per neuron, either on abnormal nerve cells or as an adaptive response to the abnormal neuronal activity, may explain the observed increases of FMZ binding. If increased FMZ-receptor binding reflects increased GABAergic inhibition locally, the increased inhibition in this area would result in reduced cortical excitability in the lobe of seizure origin. Thus, we can speculate that the greater the increase in FMZ binding the fewer the seizures, as observed in this study. Likewise, greater reductions of FMZ binding were found as the interval since the last seizure got shorter.34 This potential plasticity of receptors after seizures is consistent with our observation of greater reductions of FMZ binding as the seizure frequency got higher. This observation holds true in particular for patients with frequent seizures (>10/month) (figure 4) but not necessarily for patients with very few seizures, in whom PET scans were performed at various intervals since the last seizure.
For group comparisons, the images of patients with clear right-sided focus were right-left reversed before normalization, making the focus appear on the same side in all patients. We have previously carefully investigated the influence of such right-left reversals before spatial normalization, and we did not find a difference in the statistical results.35 In both fMRI and PET groups, few patients had bilateral or no localizing features on MRI, EEG, or seizure semiology, but wrong lateralization would only reduce the likelihood of observing a unilateral (ipsilateral) effect.
Our findings from combined hemodynamic and neuroreceptor imaging studies support the concept of a network of cortical and subcortical structures modulating epileptiform activity. Our group analysis will be less sensitive to IED-correlated BOLD signal changes, reflecting potentially different irritative and seizure-onset zones, but will highlight common features (typical effects) in a group of patients. Despite exhibiting disparate sites of seizure foci, the patients in our study shared a common region of discharge-correlated activity. We restricted our analyses to EEG-fMRI studies with 1–20 IED/min. This enabled us to make valid inferences at the group level using a 2-stage procedure but limited the group size to 19 patients.36 Violations of homoscedasticity implicit in the loss of balance at the first level can make the second-level inference less efficient but would not bias or invalidate it.37
At the single-subject level, there may be other areas fulfilling such a role, which failed to reach significance as a result of group averaging. Interestingly, recent PET studies have suggested that increased FMZ binding in one of these areas, the retroventricular area (table e-2), is predictive of poor surgical outcome.38 Although there is likely to be considerable individual variability in potential epileptogenic networks, some areas are common to all networks and may be potential target areas for new therapeutic approaches. Our findings support an understanding of epilepsy moving on from the traditional zone concept to that of a network theory.39,40
Supplementary Material
GLOSSARY
- BOLD
blood oxygen level–dependent
- FMZ
flumazenil
- FMZ-VT
flumazenil volume of distribution
- GABA
γ-aminobutyric acid
- ICBM
International Consortium for Brain Mapping
- IED
interictal epileptiform discharge
- MNI
Montreal Neurological Institute
- SPM
statistical parametric mapping
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
H.L., M.R., L.L., J.D., and M.K. were involved in conception, analysis, and interpretation of the presented data as well as writing of the article. A.S.H. was involved in conception, acquisition, interpretation, and analysis of the single subject data. K.G., W.L., and C.V. were involved in interpretation of the presented data and preparation of figures and writing of the article. H.L. and M.R. performed statistical analysis, supported by Karl Friston, Wellcome Department for Cognitive Neuroscience.
DISCLOSURE
Dr. Laufs has received funding for travel from Brain and the Institute of Neurology, UCL, UK; serves on the editorial board of Brain Topography; and receives research support from the Bundesministerium für Bildung und Forschung (BMBF) and the Deutsche Forschungsgemeinschaft (DFG). Prof. Richardson has served on scientific advisory boards for SCHWARZ PHARMA and UCB; has received funding for travel and/or speaker honoraria from UCB, Eisai Inc., and Janssen; and serves on the editorial board of the Journal of Neurology Neurosurgery and Psychiatry. Dr. Salek-Haddadi and Dr. Vollmar report no disclosures. Prof. Duncan serves on scientific advisory boards for GE Healthcare, Eisai Inc., and sanofi-aventis; has received funding for travel and/or speaker honoraria from Janssen, UCB, and Eisai Inc.; serves on the editorial boards of Seizure, Epilepsy Research, and Epilepsia; is co-inventor on a patent re: A miniaturized wearable apnea detector; receives publishing royalties for Eyelid Myoclonia and Typical Absences (Libbey, 1995); has served as a consultant for Cyberonics, UCB, GlaxoSmithKline, and Eisai Inc.; has an active practice in epilepsy surgery; and receives/has received research support from Medical Research Council UK and the Wellcome Trust. Prof. Gale has received research support from the NIH. Prof. Lemieux served on scientific advisory boards for Fonds de Recherche en Sante du Quebec (Canada), Agence Nationale de la Rechcherche (France), Canada Foundation for Innovation, Fundação para a Ciência e a Tecnologia–Portugal; has received funding for travel and speaker honoraria from BIAL; serves on the editorial boards of Neuroimage, Human Brain Mapping, Brain Topography, and Epilepsy Research and Treatment; receives publishing royalties for EEG-fMRI (Springer, 2009); and receives/has received research support from the Medical Research Council, Action Medical Research, the Brain Research Trust, and the Higher Education Funding Council. Prof. Löscher served on a scientific advisory board for UCB; has received funding for travel and/or speaker honoraria from GlaxoSmithKline and UCB; serves on the editorial boards of Naunyn-Schmiedeberg's Archives of Pharmacology and the European Journal of Pharmacology; serves as a consultant for Grünenthal GmbH; and receives research support from Grünenthal GmbH, the EU, and the Deutsche Forschungsgemeinschaft (DFG). Prof. Koepp served on a scientific advisory board for GE Healthcare; has received honoraria from UCB, Eisai Inc., and BIAL and funding for travel from UCB, Pfizer Inc, and Desitin Pharmaceuticals, GmbH; serves on the editorial board of Epilepsy Research; received research support from MRC, Wellcome Trust Foundation, and EU-Framework 7 programme; and holds shares in GlaxoSmithKline.
REFERENCES
- 1. Gale K, Zhong P, Miller LP, Murray TF. Amino acid neurotransmitter interactions in ‘area tempestas': an epileptogenic trigger zone in the deep prepiriform cortex. Epilepsy Res Suppl 1992;8:229–234 [DOI] [PubMed] [Google Scholar]
- 2. Löscher W, Ebert U. The role of the piriform cortex in kindling. Prog Neurobiol 1996;50:427–818 [DOI] [PubMed] [Google Scholar]
- 3. Gale K. Chemoconvulsant seizures: advantages of focally-evoked seizure models. Ital J Neurol Sci 1995;16:17–25 [DOI] [PubMed] [Google Scholar]
- 4. Piredda S, Gale K. A crucial epileptogenic site in the deep prepiriform cortex. Nature 1985;317:623–625 [DOI] [PubMed] [Google Scholar]
- 5. Steriade M. Sleep, epilepsy and thalamic reticular inhibitory neurons. Trends Neurosci 2005;28:317–324 [DOI] [PubMed] [Google Scholar]
- 6. Deransart C, Vercueil L, Marescaux C, Depaulis A. The role of basal ganglia in the control of generalized absence seizures. Epilepsy Res 1998;32:213–223 [DOI] [PubMed] [Google Scholar]
- 7. Depaulis A, Vergnes M, Marescaux C. Endogenous control of epilepsy: the nigral inhibitory system. Prog Neurobiol 1994;42:33–52 [DOI] [PubMed] [Google Scholar]
- 8. Biraben A, Semah F, Ribeiro MJ, Douaud G, Remy P, Depaulis A. PET evidence for a role of the basal ganglia in patients with ring chromosome 20 epilepsy. Neurology 2004;63:73–77 [DOI] [PubMed] [Google Scholar]
- 9. Bouilleret V, Semah F, Biraben A, et al. Involvement of the basal ganglia in refractory epilepsy: an 18F-fluoro-l-DOPA PET study using 2 methods of analysis. J Nucl Med 2005;46:540–547 [PubMed] [Google Scholar]
- 10. Ekstrand JJ, Domroese ME, Johnson DM, et al. A new subdivision of anterior piriform cortex and associated deep nucleus with novel features of interest for olfaction and epilepsy. J Comp Neurol 2001;434:289–307 [DOI] [PubMed] [Google Scholar]
- 11. Blumenfeld H, McNally KA, Anderhill SD, et al. Positive and negative network correlations in temporal lobe epilepsy. Cereb Cortex 2004;14:892–902 [DOI] [PubMed] [Google Scholar]
- 12. Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures? Curr Opin Neurol 2006;19:164–168 [DOI] [PubMed] [Google Scholar]
- 13. Salek-Haddadi A, Diehl B, Hamandi K, et al. Haemodynamic correlates of epileptiform discharges: a study of 63 patients with focal epilepsy. Brain Res 2006;9:148–166 [DOI] [PubMed] [Google Scholar]
- 14. Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp 1994;1:214–220 [DOI] [PubMed] [Google Scholar]
- 15. Friston KJ, Williams S, Howard R, Frackowiak RS, Turner R. Movement-related effects in fMRI time-series. Magn Reson Med 1996;35:346–355 [DOI] [PubMed] [Google Scholar]
- 16. Laufs H, Hamandi K, Salek-Haddadi A, Kleinschmidt AK, Duncan JS, Lemieux L. Temporal lobe interictal epileptic discharges affect cerebral activity in “default mode” brain regions. Hum Brain Mapp 2007;28:1023–1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mizobuchi M, Ito N, Tanaka C, Sako K, Sumi Y, Sasaki T. Unidirectional olfactory hallucination associated with ipsilateral unruptured intracranial aneurysm. Epilepsia 1999;40:516–519 [DOI] [PubMed] [Google Scholar]
- 18. Koepp MJ, Richardson MP, Brooks DJ, et al. Cerebral benzodiazepine receptors in hippocampal sclerosis: an objective in vivo analysis. Brain 1996;119:1677–1687 [DOI] [PubMed] [Google Scholar]
- 19. Cunningham VJ, Jones T. Spectral analysis of dynamic PET data. J Cereb Blood Flow Metab 1993;13:15–23 [DOI] [PubMed] [Google Scholar]
- 20. Piredda S, Gale K. Anticonvulsant action of 2-amino-7-phosphonoheptanoic acid and muscimol in the deep prepiriform cortex. Eur J Pharmacol 1986;12:115–119 [DOI] [PubMed] [Google Scholar]
- 21. Millan MH, Patel S, Mello LM, Meldrum BS. Focal injection of 2-amino-7-phosphonoheptanoic acid into prepiriform cortex protects against pilocarpine-induced limbic seizures in rats. Neurosci Lett 1986;70:69–74 [DOI] [PubMed] [Google Scholar]
- 22. Fornai F, Busceti CL, Kondratyev A, Gale K. AMPA receptor desensitization as a determinant of vulnerability to focally evoked status epilepticus. Eur J Neurosci 2005;21:455–463 [DOI] [PubMed] [Google Scholar]
- 23. Löscher W, Ebert U. Basic mechanisms of seizure propagation: targets for rational drug design and rational polypharmacy. Epilepsy Res Suppl 1996;11:17–43 [PubMed] [Google Scholar]
- 24. Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses 2001;26:551–556 [DOI] [PubMed] [Google Scholar]
- 25. Gottfried JA, Zald DH. On the scent of human olfactory orbitofrontal cortex: meta-analysis and comparison to non-human primates. Brain Res Rev 2005;50:287–304 [DOI] [PubMed] [Google Scholar]
- 26. Haberly LB, Bower JM. Olfactory cortex: model circuit for study of associative memory? Trends Neurosci 1989;12:258–264 [DOI] [PubMed] [Google Scholar]
- 27. McIntyre DC, Gilby KL. Parahippocampal networks, intractability, and the chronic epilepsy of kindling. Adv Neurol 2006;97:77–83 [PubMed] [Google Scholar]
- 28. Wilson DA, Stevenson RJ. Olfactory perceptual learning: the critical role of memory in odor discrimination. Neurosci Biobehav Rev 2003;27:307–328 [DOI] [PubMed] [Google Scholar]
- 29. Howard JD, Plailly J, Grueschow M, Haynes JD, Gottfried JA. Odor quality coding and categorization in human posterior piriform cortex. Nat Neurosci 2009;12:932–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Doherty J, Gale K, Eagles DA. Evoked epileptiform discharges in the rat anterior piriform cortex: generation and local propagation. Brain Res 2000;861:77–87 [DOI] [PubMed] [Google Scholar]
- 31. Gunderson VM, Dubach M, Szot P, et al. Development of a model of status epilepticus in pigtailed macaque infant monkeys. Dev Neurosci 1999;21:352–364 [DOI] [PubMed] [Google Scholar]
- 32. Nusser Z, Hajos N, Somogyi P, Mody I. Increased number of synaptic GABAA receptors underlies potentiation at hippocampal inhibitory synapses. Nature 1998;395:172–177 [DOI] [PubMed] [Google Scholar]
- 33. Brooks-Kayal AR, Shumate MD, Jin H, Rikhter TY, Coulter DA. Selective changes in single cell GABAA receptor subunit expression and function in temporal lobe epilepsy. Nat Med 1998;4:1166–1172 [DOI] [PubMed] [Google Scholar]
- 34. Bouvard S, Costes N, Bonnefoi F, et al. Seizure-related short-term plasticity of benzodiazepine receptors in partial epilepsy: a [11C]flumazenil-PET study. Brain 2005;128:1330–1343 [DOI] [PubMed] [Google Scholar]
- 35. Hammers A, Koepp MJ, Richardson MP, Hurlemann R, Brooks DJ, Duncan JS. Grey and white matter flumazenil binding in neocortical epilepsy with normal MRI: a PET study of 44 patients. Brain 2003;126:1300–1318 [DOI] [PubMed] [Google Scholar]
- 36. Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage 1999;10:385–396 [DOI] [PubMed] [Google Scholar]
- 37. Penny WD, Holmes AJ. Random effects analysis. In: Friston K, Kiebel S, Nichols T, Penny W. eds. Human Brain Function. San Diego, CA: Elsevier; 2006:156–165 [Google Scholar]
- 38. Hammers A, Koepp MJ, Brooks DJ, Duncan JS. Periventricular white matter flumazenil binding and postoperative outcome in hippocampal sclerosis. Epilepsia 2005;46:944–948 [DOI] [PubMed] [Google Scholar]
- 39. Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia 2002;43:219–227 [DOI] [PubMed] [Google Scholar]
- 40. Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010;51:676–685 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.