Abstract
Objective:
Cognitive dysfunction is common in Parkinson disease (PD), even early in its clinical course. This disease manifestation has been associated with impaired verbal learning performance as well as abnormal expression of a specific PD-related cognitive spatial covariance pattern (PDCP). It is not known, however, how this metabolic network relates to the cognitive response to dopaminergic therapy on the individual patient level.
Methods:
We assessed treatment-mediated changes in verbal learning and PDCP expression in 17 patients with PD without dementia who underwent cognitive testing and metabolic imaging in the unmedicated and levodopa-treated conditions. We also determined whether analogous changes were present in 12 other patients with PD without dementia who were evaluated before and during the treatment of cognitive symptoms with placebo.
Results:
Levodopa-mediated changes in verbal learning correlated with concurrent changes in PDCP expression (r = −0.60, p < 0.01). The subset of patients with meaningful cognitive improvement on levodopa (n = 8) exhibited concurrent reductions in PDCP expression (p < 0.01) with treatment; network modulation was not evident in the remaining subjects. Notably, the levodopa cognitive response correlated with baseline PDCP levels (r = 0.70, p = 0.002). By contrast, placebo did not affect PDCP expression, even in the subjects (n = 7) with improved verbal learning during treatment.
Conclusions:
These findings suggest that cognitive dysfunction in PD may respond to treatment depending upon the degree of baseline PDCP expression. Quantification of treatment-mediated network changes can provide objective information concerning the efficacy of new agents directed at the cognitive manifestations of this disease.
Although Parkinson disease (PD) is defined clinically by its motor manifestations, substantial cognitive difficulties are also evident.1,2 Cognition can be further affected by dopaminergic therapy, improving or impairing different aspects of cognitive functioning.3,4 Indeed, the variability of these effects suggests that the cognitive response to dopaminergic treatment is determined by subject-specific factors such as baseline learning capacity.5,6
Resting state imaging studies of cerebral function in PD have proven valuable in delineating the effects of treatment at the network level.7 Spatial covariance analysis of resting metabolic imaging data has been used to characterize PD-related motor and cognitive patterns (PDRP and PDCP, respectively).8 Normalization of baseline abnormalities in PDRP expression has been found to be a consistent feature of therapeutic interventions directed at PD motor symptoms.7,9–11 However, it is not known whether treatment-mediated changes in cognitive functioning in patients with PD are associated with reductions in PDCP expression. While treatment-mediated PDCP modulation has not been demonstrated at the group mean level,8 baseline-dependent functional responses are still possible.6 For this reason, we explored the cognitive impact of levodopa in patients with PD at the individual subject level. In this study, we used neuropsychological testing in conjunction with metabolic brain imaging to determine 1) whether cognitive improvement with levodopa was associated with a significant degree of PDCP modulation, 2) whether the cognitive response to levodopa correlated with baseline levels of PDCP expression, and 3) whether analogous network changes occurred when the cognitive response was elicited through the administration of a placebo.
METHODS
Study 1: Network correlates of the cognitive response to levodopa.
Hypothesis.
In individual patients with PD, the cognitive response to levodopa is associated with specific treatment-mediated changes in PDCP expression. The magnitude of this response is determined by the degree of pattern expression present in the baseline unmedicated condition.
Subjects and procedures.
We studied 17 right-handed patients with PD without dementia (14 men and 3 women; mean ± SD age 58.4 ± 8.2 years) who underwent repeat psychometric testing and 18F-fluorodeoxyglucose (FDG) PET on and off levodopa treatment. These evaluations were conducted in 2 one-day sessions (“on” on 1 day; “off” on the other) as described elsewhere.9,11 In each session, the subjects underwent FDG PET imaging in 3-dimensional mode using the GE Advance tomograph (General Electric Medical Systems, Milwaukee, WI) at North Shore University Hospital. The details of the scanning procedure are provided elsewhere.9,12 The studies were performed in an awake resting state, with the subjects' eyes open in a dimly lit room and with minimal auditory stimulation.
Following scan preprocessing, individual images were nonlinearly warped into Talairach space using a standard PET template, and smoothed with an isotropic Gaussian kernel (10 mm) in all directions to improve the signal-to-noise ratio. Single case computations were performed to quantify PDCP and PDRP expression in each subject/condition using an automated voxel-wise procedure (available at http://www.fil.ion.ucl.ac.uk/spm/ext/#SSM), as described elsewhere.12–14 Network values were z-scored with reference to healthy controls as described previously.8,12,14,15
Behavioral assessment.
In each session, the subjects were rated according to the motor portion of the Unified Parkinson's Disease Rating Scale (UPDRS).16 The cognitive response to treatment was assessed using the standard administration of the verbal learning paradigm.17 In PD, this neuropsychological test has been used to document cognitive dysfunction at early clinical stages of the disorder and to predict the subsequent development of dementia in these patients.2,18 Because this paradigm does not have a substantial motor component, changes in performance with antiparkinsonian interventions are considered to be specific for cognitive treatment effects.
Overall, verbal learning performance in each treatment condition was quantified as the sum across trials; this measure was z-scored with respect to age-matched normative data.17 In addition to providing a behavioral covariate for correlation with treatment-mediated changes in PDCP expression, this measure was used to divide the subjects into 2 cognitive response categories. This was accomplished by computing the Reliable Change Index (RCI)19,20 for the verbal learning performance measure as described in appendix e-1 on the Neurology® Web site at www.neurology.org. An observed verbal learning response above the RCI cannot be ascribed to measurement imprecision (test-retest variability) or to between-session practice effects.
Based on the computed RCI of +0.44 units for the verbal learning test, each of the 17 levodopa recipients was classified dichotomously as either having a positive verbal learning treatment response or not. In 8 of these participants, the observed treatment-mediated change in verbal learning was above RCI threshold (>0.44 units). These subjects were classified as responders (LDR). In the remaining 9 levodopa recipients, the verbal learning changes did not exceed criterion (≤0.44 units). These subjects were classified as nonresponders (LDNR). The LDR and LDNR subjects did not differ (p > 0.13) with respect to their baseline age, duration, levodopa equivalent daily dose,21–23 UPDRS motor score, verbal learning, and network activity (table 1A).
Table 1.
Baseline characteristics of the subjectsa
Abbreviations: PDCP=Parkinson disease–related cognitive pattern; PDRP=Parkinson disease–related motor pattern; UPDRS=Unified Parkinson’s Disease Rating Scale.
Values are mean (SD).
Data analysis.
Relationships between verbal learning and PDCP/PDRP expression were assessed by computing Pearson product-moment correlations between 1) baseline measures of verbal learning performance [LD(−)] with the corresponding network values; 2) treatment-mediated changes in verbal learning [LD(+) − LD(−)] with corresponding changes in pattern expression; and 3) treatment-mediated verbal learning changes [LD(+) − LD(−)] with baseline network values. The effects of both networks on the changes in verbal learning performance were assessed using multiple regression analysis. Correlations were considered significant for p ≤ 0.01, correcting for multiple independent observations.
Network modulation with levodopa was further evaluated by comparing treatment-mediated changes in PDCP/PDRP expression in verbal learning responders with the corresponding changes in nonresponders (i.e., LDR vs LDNR). This was done using repeated-measures analysis of variance (RMANOVA) followed by post hoc Bonferroni tests. The results were considered significant for p < 0.05 (2-tailed).
Study 2: Network changes in response to placebo.
Hypothesis.
PDCP modulation can also be seen during the treatment of PD cognitive symptoms with placebo.
A separate group of 12 right-handed patients with PD without dementia (8 men and 4 women; age 62.3 ± 9.7 years) were also assessed with repeat cognitive testing and metabolic imaging conducted in 2 1-day sessions: at baseline [PL(−)] and again after 2 months of daily placebo treatment [PL(+)] as part of a small randomized clinical trial of an acetylcholinesterase inhibitor for PD cognitive symptoms.24 In this blinded study, the participants were told that the medication being investigated was designed to treat the cognitive and not the motor manifestations of the disorder and that there was an equal chance of receiving active drug or placebo. The demographic features of these subjects are presented in table 1B.
The same RCI criterion that was used in study 1 was applied to each subject. Seven of the subjects were classified as verbal learning responders (PLR); the remaining 5 subjects were classified as verbal learning nonresponders (PLNR). The PLR and PLNR subgroups (table 1B) did not differ with respect to the baseline measures listed above (p > 0.14). Network modulation with placebo was evaluated by comparing treatment-mediated changes in PDCP/PDRP expression in verbal learning responders (PLR) with those changes in nonresponders (PLNR) using RMANOVA as described above.
Standard protocol approvals, registrations, and patient consents.
Ethical permission was obtained from the Institutional Review Board of North Shore University Hospital. Written consent was obtained from each subject after detailed explanation of the procedures.
RESULTS
Network correlates of the response in verbal learning to levodopa.
Pairwise correlations between baseline measures of verbal learning and network activity, and between treatment-mediated changes in these variables, are presented in table 2. In the levodopa group (table 2A), a correlation (r = −0.70, p < 0.001) was present between baseline measures of verbal learning and PDCP expression (figure 1A, left). A correlation (r = −0.60, p < 0.01) was also noted between levodopa-mediated changes in verbal learning and those in PDCP expression, such that improvement in verbal learning with levodopa was associated with PDCP reductions. Changes in verbal learning with levodopa did not correlate with concurrent changes in UPDRS motor ratings (r = 0.32, p = 0.21). Multiple linear regression revealed that the levodopa-mediated changes in verbal learning correlated with changes in PDCP expression (p = 0.02) but not with concurrent PDRP changes (p = 0.09). Additionally, levodopa-mediated verbal learning responses were found to correlate (r = 0.70, p = 0.002) with baseline PDCP values (figure 1B), such that higher baseline network activity was associated with greater improvement in verbal learning during treatment. When baseline values for the 2 networks were entered together into a multiple regression model to predict levodopa-mediated changes in verbal learning, a correlation with cognitive outcome was present with PDCP (p = 0.02) but not with PDRP expression (p = 0.80).
Table 2.
Pairwise correlations between verbal learning performance and network activitya
Abbreviations: PDCP=Parkinson disease–related cognitive pattern; PDRP=Parkinson disease–related motor pattern.
Values are Pearson product-moment correlations.
Considered significant after correction for multiple comparisons.
p < 0.001.
p < 0.01.
p < 0.05.
Figure 1. Correlation between baseline measures of cognitive performance and Parkinson disease–related cognitive pattern (PDCP) activity.
(A) Relationship between baseline verbal learning performance and PDCP expression in the levodopa (left) and placebo (right) treatment groups. At baseline, higher PDCP expression was associated with more impaired verbal learning performance in both treatment groups. Squares and triangles refer respectively to cognitive responders (R) and nonresponders (NR) to treatment; see Methods. (B) Relationship between baseline PDCP expression and levodopa-mediated changes in verbal learning performance. Higher baseline PDCP scores correlated with greater improvement in cognitive functioning during levodopa treatment. The horizontal dashed line represents the cutoff (+0.44) for meaningful treatment-mediated change in verbal learning based on the reliable change index (see text). The vertical dashed line represents the baseline PDCP value (+1.01) that corresponded to this behavioral response cutoff. Baseline measures of network activity above this value were found to be associated with improved cognitive functioning during levodopa treatment.
Comparison of verbal learning responders and nonresponders to levodopa.
Based on the RCI criterion specified above, the levodopa recipients were subdivided into verbal learning responders (LDR: n = 8) and nonresponders (LDNR: n = 9). As expected, these 2 subgroups (figure 2A) differed in the effects of levodopa on verbal learning performance (F1,15 = 20.53, p < 0.0005; 2 × 2 RMANOVA, interaction effect). At baseline (table 1A), verbal learning scores were −1.27 ± 0.40 and −0.36 ± 0.40 (mean ± SE, p < 0.13) for the verbal learning responders and nonresponders, respectively. The change in performance with levodopa treatment (figure 2A) was 1.00 ± 0.18 for the verbal learning responders (p < 0.001; post hoc test) and −0.34 ± 0.23 for the verbal learning nonresponders (p = 0.11). Additionally, the LDR and LDNR subgroups differed in the degree of PDCP change (figure 2B) that occurred with treatment (F1,15 = 5.86, p = 0.03; 2 × 2 RMANOVA, interaction effect). At baseline, PDCP expression was 1.09 ± 0.32 and 0.36 ± 0.51 for the LDR and LDNR subjects, respectively. With levodopa treatment, the LDR exhibited a reduction in PDCP expression (−0.76 ± 0.22, p = 0.008; post hoc test), whereas there was no corresponding change (−0.07 ± 0.26, p = 0.78) in their nonresponder counterparts.
Figure 2. Cognition-related responses to levodopa treatment: Network effects.
(A) Changes in verbal learning performance with levodopa treatment differed (p < 0.001; 2 × 2 repeated-measures analysis of variance [RMANOVA], see Results) in cognitive responders (LDR; n = 8) and nonresponders (LDNR; n = 9). (B) Levodopa-mediated changes in Parkinson disease–related cognitive pattern (PDCP) expression differed for cognitive responders and nonresponders (p = 0.03; 2 × 2 RMANOVA, interaction effect), with treatment-mediated declines in the former (p = 0.008; post hoc Bonferroni test) but not the latter (p = 0.78). (C) Levodopa-mediated changes in Parkinson disease–related motor pattern (PDRP) expression also differed for the 2 subgroups (p = 0.001; 2 × 2 RMANOVA, interaction effect). For this motor-related metabolic network, levodopa-mediated reductions were present in both cognitive responders (p < 0.001; post hoc Bonferroni test) and nonresponders (p = 0.03). *p < 0.05, **p < 0.01, ***p < 0.001, Student t tests for comparisons of network activity in each subgroup with corresponding healthy control values; see Methods.
In contrast to the observed differences in the verbal learning response to levodopa, treatment-mediated motor responses were similar for the 2 subgroups (F1,15 = 0.16, p = 0.69; 2 × 2 RMANOVA, interaction effect). Levodopa resulted in improvement in UPDRS motor ratings in both subgroups (LDR −7.00 ± 1.45, p = 0.002; LDNR −8.06 ± 2.13, p < 0.001; post hoc tests). These changes were accompanied by concurrent reductions in PDRP expression (figure 2C) in both verbal learning response subgroups (R: p < 0.001, NR: p = 0.03; post hoc tests). Nonetheless, the degree of levodopa-mediated PDRP modulation was found to be greater in verbal learning responders relative to nonresponders (F1,15 = 16.85, p = 0.001; 2 × 2 RMANOVA, interaction effect). The results of a voxel-based comparison of the LD(+) and LD(−) scan data are provided in appendix e-2 (includes table e-1 and figure e-1).
Relationship between network activity and the response of verbal learning to placebo.
In the separate group of patients with PD who were examined before and after receiving placebo treatment for cognitive symptoms (table 2B), baseline verbal learning measures also correlated (r = −0.60, p = 0.04) with corresponding PDCP values (figure 1A, right). In contrast to levodopa treatment, there was no correlation between placebo-mediated changes in verbal learning and those in PDCP (r = 0.32, p = 0.32) or PDRP (r = 0.32, p = 0.31) expression. Similarly, the observed changes in verbal learning performance did not correlate with baseline measurements of network activity (PDCP: r = 0.17, p = 0.58; PDRP: r = 0.06, p = 0.86).
Subjects receiving placebo were subdivided into verbal learning responders (PLR: n = 7) and nonresponders (PLNR: n = 5) according to the same RCI criterion used to classify the levodopa recipients (see above). As defined, the 2 groups (figure 3A) exhibited differing verbal learning responses to placebo (F1,10 = 15.54, p = 0.003). At baseline (table 1B), mean verbal learning was −1.66 ± 0.31 and −0.80 ± 0.47 (mean ± SE, p < 0.14) for the verbal learning responders and nonresponders, respectively. The mean change in verbal learning with placebo was 1.40 ± 0.29 (p < 0.001; post hoc test) for the PLR and −0.04 ± 0.14 (p = 0.89) for the PLNR. Of note, the treatment-mediated change in verbal learning performance observed in the PLR group did not differ from that seen in the verbal learning responders to levodopa (LDR = 1.00 ± 0.18, p = 0.24).
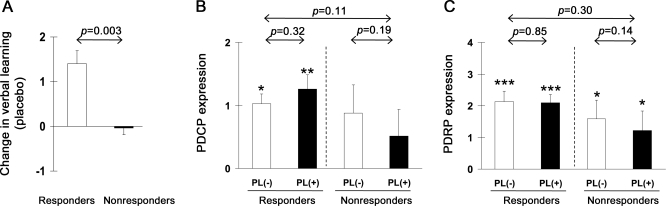
Figure 3. Cognition-related responses to placebo.
(A) Changes in verbal learning performance differed for cognitive responders (PLR; n = 7) and nonresponders (PLNR; n = 5) to placebo (p = 0.003; 2 × 2 repeated-measures analysis of variance [RMANOVA], interaction effect). (B) Treatment-mediated changes in Parkinson disease–related cognitive pattern (PDCP) expression did not differ for cognitive responders and nonresponders to placebo (p = 0.11; 2 × 2 RMANOVA, interaction effect). Network activity did not change with placebo in either response subgroup (p ≥ 0.19). (C) There was also no change in Parkinson disease–related motor pattern (PDRP) expression with placebo treatment (p ≥ 0.14) in either response subgroup. *p < 0.05, **p < 0.01, ***p < 0.001, Student t tests for comparisons of network expression in each subgroup to healthy control values.
Although significant changes in verbal learning performance were evident in the PLR group, there was no accompanying effect of placebo treatment on PDCP expression (PLR: p = 0.32, PLNR: p = 0.19; post hoc tests, figure 3B). Likewise, there was no placebo-mediated change in PDRP expression in either group (PLR: p = 0.85, PLNR: p = 0.14; figure 3C). The results of a voxel-based comparison of the PL(+) and PL(−) scan data are provided as appendix e-2.
DISCUSSION
In this study we show that dopaminergic treatment can improve verbal learning performance in patients with PD without dementia with baseline elevations in resting PDCP activity. That is, the cognitive response to levodopa was found to correlate with individual differences in baseline PDCP activity. In support of our first hypothesis, the cognitive changes associated with levodopa treatment paralleled the degree of PDCP modulation that was concurrently observed in the same subjects. Indeed, the LDR subjects, who had a meaningful improvement in verbal learning with levodopa, exhibited a significant reduction in this network abnormality during treatment. That said, levodopa-mediated PDCP modulation was not evident in the LDNR subjects, who did not exhibit cognitive improvement with treatment despite concurrent reductions in PDRP activity. Nonetheless, contrary to our second hypothesis, PDCP expression was not changed with placebo treatment, even in the PLR subjects, who exhibited treatment-mediated improvement in verbal learning that was not different from the cognitive responders to levodopa (p = 0.24). Indeed, the frequency of cognitive responders was similar for the 2 treatment groups (p = 0.55, χ2). In aggregate, the results suggest that the PDCP, a distinct metabolic cognition-related network associated with cognitive functioning in PD, can be modulated by dopaminergic therapy. By contrast, placebo treatment can give rise to cognitive benefit without concomitant PDCP modulation.
Stratification of the levodopa recipients according to their verbal learning response highlights the complex relationship that exists between changes in dopaminergic neurotransmission, PDCP expression, and cognitive functioning in patients with PD without dementia. The effects of levodopa treatment on verbal learning and network activity proved to be individually variable in that not all subjects exhibited meaningful changes in these measures, which were not evident at the group level.8 The patient subgroup with improved cognitive functioning during levodopa treatment concurrently demonstrated a significant reduction in PDCP expression. Of note, these individuals displayed baseline abnormalities in task performance, with an average verbal learning score that was 1.3 SD below the normal mean, as well as a comparable 1.1 SD elevation in PDCP activity. In these cognitive responders, levodopa treatment corrected the functional abnormalities that were present at baseline. By contrast, patients who did not improve cognitively with levodopa administration (LDNR) exhibited baseline measures of verbal learning performance and associated network activity that were near normal. In these subjects, PDCP expression did not change significantly during levodopa treatment, despite a concurrent reduction in PDRP activity. Of note, 2 subjects exhibited substantial declines in verbal learning performance (>1 SD) with levodopa administration (figure 1B). Given that these individuals also exhibited the lowest (most normal) PDCP scores at baseline, it is possible that they experienced an “overdose” effect. While consistent with an inverted-U relationship between dopamine and cognition,25 more data will be needed to substantiate such a possibility.
Interestingly, although the levodopa recipients all improved motorically with treatment, those with the greatest cognitive response also had a greater degree of motor network (PDRP) modulation. The observation that patients with significant cognitive response (LDR) had more pronounced baseline verbal learning deficits, as well as relatively greater pretreatment PDCP and PDRP expression, suggests that the spatial extent of neurodegenerative change in the substantia nigra pars compacta is greater in these subjects. That is, in verbal learning responders, nigral cell loss is likely to extend beyond the “motoric” ventrolateral zone to involve the more “cognitive” dorsomedial aspect of this structure.26 This is expected to impact negatively on baseline cognitive functioning by reducing dopaminergic input to the caudate nucleus, with consequent changes in learning-related neural activation in the dorsolateral prefrontal cortex.27
It is well appreciated that substantial improvements in performance can occur in patients with PD treated with placebo.28,29 Thus, it is conceivable that the observed changes in verbal learning and PDCP activity are a manifestation of the placebo effect, rather than actual levodopa treatment. To examine this possibility, we evaluated a separate group of 12 patients who received placebo treatment as part of a blinded clinical trial of cholinesterase inhibition for cognitive dysfunction in PD. Of these subjects, 7 exhibited an improvement in verbal learning in response to placebo that was comparable in magnitude to that observed in their LDR counterparts. Nonetheless, despite achieving substantial improvement in verbal learning (mean change = 1.4 SD) with placebo, these individuals did not exhibit the significant PDCP changes seen with levodopa pharmacotherapy. This suggests that the network changes observed with levodopa treatment are unlikely to be a consequence of the placebo effect.
The current findings suggest that the PDCP metabolic network may be a viable biomarker for the assessment of treatment-mediated changes in cognitive functioning in patients with PD without dementia. In contrast to our prior group study,14 the current findings indicate that when assessed at the individual subject level, PDCP expression is sensitive to the change in cognition that occurred during levodopa treatment for the motor manifestations of the disease. Although levodopa is not viable as an effective treatment for PD cognitive dysfunction, the data illustrate how this network can serve as an objective biomarker of the cognitive response to treatment. The utility of PDCP quantification in clinical trials of new agents directed at the cognitive manifestations of PD is further highlighted by the absence of a discernible placebo effect on this measure.
The observation that the degree of treatment-mediated cognitive change is correlated with baseline network activity suggests the potential utility of this measure at the individual subject level. In our sample, patients with PD were more likely to experience an improvement in verbal learning performance with levodopa if PDCP expression is elevated at baseline. Indeed, the majority of verbal learning responders to levodopa were found to have baseline PDCP scores greater than 1.01 (i.e., greater than 1 SD above the normal mean), which corresponds to the prespecified RCI threshold of 0.44 (figure 1B, dashed lines). By contrast, individuals with small, or even negative, PDCP values would be less likely to improve with treatment. Indeed, as can be seen in the lower left-hand quadrant of figure 1B, the 2 subjects with negative PDCP values exhibited substantial worsening (declines greater than 1 SD) in verbal learning performance with levodopa treatment. Whether the predictive value of baseline PDCP expression applies solely to dopaminergic treatment or has utility in the assessment of other interventions targeting cognitive symptoms of PD is a topic for future study.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Thomas Chaly and Claude Margouleff for technical support and Toni Fitzpatrick for assistance with copyediting.
GLOSSARY
- PD
Parkinson disease
- PDCP
Parkinson disease–related cognitive pattern
- PDRP
Parkinson disease–related motor pattern
- PLNR
verbal learning nonresponder
- PLR
verbal learning responder
- RCI
Reliable Change Index
- RMANOVA
repeated-measures analysis of variance
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
D.E. and P.M. designed the experiments. P.M. and V.D. performed the experiments. P.M., C.T., Y.M., and D.E. analyzed the data. P.M. and D.E. wrote the manuscript.
DISCLOSURE
Dr. Mattis has served as consultant for Neurologix, Inc. and receives research support from the NIH (NINDS), the Huntington Disease Society of America, and the Dana Foundation. Dr. Tang has received research support from Neurologix, Inc., and the NIH (NINDS). Dr. Ma has received research support from Neurologix, Inc., the NIH (NCRR, NINDS), and the High Q Foundation. Dr. Dhawan has received research support from Neurologix, Inc., the NIH (NIDCD, NIAID, NINDS), and the Dana Foundation. Dr. Eidelberg serves on scientific advisory boards for and has received honoraria from the Thomas Hartman Foundation for Parkinson's Research, Inc., the Michael J. Fox Foundation, and the Bachmann-Strauss Dystonia and Parkinson Foundation; has served as a consultant for Neurologix, Inc. and Merck & Co., Inc.; serves on the editorial board of Annals of Neurology and as Associate Editor for the Journal of Neuroscience; and is listed as coinventor of patents re: Markers for use in screening patients for nervous system dysfunction and a method and apparatus for using same; has received research support from the NIH (NINDS, NCRR, NIDCD, NIAID), High Q Foundation, the Dana Foundation, and the Bachmann-Strauss Dystonia and Parkinson Foundation; and has served as an expert in medico-legal cases.
REFERENCES
- 1. Green J, McDonald WM, Vitek JL, et al. Cognitive impairments in advanced PD without dementia. Neurology 2002;59:1320–1324 [DOI] [PubMed] [Google Scholar]
- 2. Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology 2009;72:1121–1126 [DOI] [PubMed] [Google Scholar]
- 3. Gotham AM, Brown RG, Marsden CD. ‘Frontal' cognitive function in patients with Parkinson's disease ‘on' and ‘off’ levodopa. Brain 1988;111:299–321 [DOI] [PubMed] [Google Scholar]
- 4. Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson's disease as a function of dopaminergic medication and task demands. Cereb Cortex 2001;11:1136–1143 [DOI] [PubMed] [Google Scholar]
- 5. Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson's disease. Neurosci Biobehav Rev 2006;30:1–23 [DOI] [PubMed] [Google Scholar]
- 6. Argyelan M, Carbon M, Ghilardi MF, et al. Dopaminergic suppression of brain deactivation responses during sequence learning. J Neurosci 2008;28:10687–10695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Poston KL, Eidelberg D. Network biomarkers for the diagnosis and treatment of movement disorders. Neurobiol Dis 2009;35:141–147 [DOI] [PubMed] [Google Scholar]
- 8. Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson's disease. Neuroimage 2007;34:714–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Asanuma K, Tang C, Ma Y, et al. Network modulation in the treatment of Parkinson's disease. Brain 2006;129:2667–2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Feigin A, Kaplitt MG, Tang C, et al. Modulation of metabolic brain networks after subthalamic gene therapy for Parkinson's disease. Proc Natl Acad Sci USA 2007;104:19559–19564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hirano S, Asanuma K, Ma Y, et al. Dissociation of metabolic and neurovascular responses to levodopa in the treatment of Parkinson's disease. J Neurosci 2008;28:4201–4209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma Y, Tang C, Spetsieris PG, Dhawan V, Eidelberg D. Abnormal metabolic network activity in Parkinson's disease: test-retest reproducibility. J Cereb Blood Flow Metab 2007;27:597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eidelberg D. Metabolic brain networks in neurodegenerative disorders: a functional imaging approach. Trends Neurosci 2009;32:548–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Spetsieris PG, Eidelberg D. Scaled subprofile modeling of resting state imaging data in Parkinson's disease: methodological issues. Neuroimage 2011;54:2899–2914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology 2008;70:1470–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fahn S, Elton RL. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Calne DB, eds. Recent Developments in Parkinson's Disease. Florham Park: MacMillan Health Care Information; 1987:153–163 [Google Scholar]
- 17. Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: adult version. San Antonio: The Psychological Corporation; 1987 [Google Scholar]
- 18. Woods SP, Troster AI. Prodromal frontal/executive dysfunction predicts incident dementia in Parkinson's disease. J Int Neuropsychol Soc 2003;9:17–24 [DOI] [PubMed] [Google Scholar]
- 19. Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991;59:12–19 [DOI] [PubMed] [Google Scholar]
- 20. Maassen GH. The standard error in the Jacobson and Truax Reliable Change Index: the classical approach to the assessment of reliable change. J Int Neuropsychol Soc 2004;10:888–893 [DOI] [PubMed] [Google Scholar]
- 21. Fine J, Duff J, Chen R, et al. Long-term follow-up of unilateral pallidotomy in advanced Parkinson's disease. N Engl J Med 2000;342:1708–1714 [DOI] [PubMed] [Google Scholar]
- 22. Rabinak CA, Nirenberg MJ. Dopamine agonist withdrawal syndrome in Parkinson disease. Arch Neurol 2010;67:58–63 [DOI] [PubMed] [Google Scholar]
- 23. Minguez-Castellanos A, Escamilla-Sevilla F, Katati MJ, et al. Different patterns of medication change after subtha-lamic or pallidal stimulation for Parkinson's disease: target related effect or selection bias? J Neurol Neurosurg Psychiatry 2005;76:34–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mentis MJ, Delalot D, Naqvi H, et al. Anticholinesterase effect on motor kinematic measures and brain activation in Parkinson's disease. Mov Disord 2006;21:549–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev 2000;31:295–301 [DOI] [PubMed] [Google Scholar]
- 26. Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain: II: patterns of loss of dopamine-containing neurons in Parkinson's disease. Brain 1999;122:1437–1448 [DOI] [PubMed] [Google Scholar]
- 27. Carbon M, Ma Y, Barnes A, et al. Caudate nucleus: influence of dopaminergic input on sequence learning and brain activation in parkinsonism. Neuroimage 2004;21:1497–1507 [DOI] [PubMed] [Google Scholar]
- 28. de la Fuente-Fernandez R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science 2001;293:1164–1166 [DOI] [PubMed] [Google Scholar]
- 29. Lidstone SC, Stoessl AJ. Understanding the placebo effect: contributions from neuroimaging. Mol Imaging Biol 2007;9:176–185 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.