Abstract
AIMS
To assess the safety, tolerability, pharmacokinetics (PK) and immunogenicity of sirukumab (CNTO 136) following intravenous (i.v.) infusion in healthy subjects.
METHODS
Forty-five healthy adult subjects (38 men and seven women) were randomly assigned to receive a single i.v. dose of placebo or sirukumab (0.3, 1, 3, 6 or 10 mg kg−1 in a dose-escalating manner). All treated subjects were observed for 96 h post infusion and underwent 20-week follow-up evaluations. Serum samples were collected to measure sirukumab concentrations, pharmacodynamic biomarkers and antibodies to sirukumab. Non-compartmental analysis and population PK modelling were conducted to characterize the PK of sirukumab.
RESULTS
Adverse events were generally brief in duration, mild or moderate in intensity and non-dose-dependent. No serious adverse events were observed in the sirukumab-treated subjects. Both Cmax and AUC(0,∞) increased in an approximately dose-proportional manner. Median terminal half-life ranged from 18.5 to 29.6 days. A two-compartment model adequately described the PK of sirukumab following i.v. administration. Population estimates for the clearance (CL), the central volume of distribution (V1), the inter-compartmental clearance (Q) and the peripheral volume of distribution (V2) were 0.364 l day−1, 3.28 l, 0.588 l day−1 and 4.97 l, respectively. Compared with placebo subjects, a sustained decrease from baseline in C-reactive protein was observed in all sirukumab-treated dose groups, although no clear dose–response relationship was observed. No subjects were positive for antibodies to sirukumab.
CONCLUSIONS
Sirukumab had a well-tolerated safety profile, desirable PK characteristics and a low incidence of immunogenicity following an i.v. infusion of 0.3 to 10 mg kg−1 in healthy subjects.
Keywords: first-in-human study, interleukin-6, monoclonal antibody, pharmacokinetics, sirukumab
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Interleukin (IL)-6 is a cytokine known for pleiotropic and pro-inflammatory functions. IL-6 is involved in various disease processes including lupus erythematosus, rheumatoid arthritis, insulin resistance and malignancy.
Anti-IL-6 receptor therapy has recently been demonstrated to be effective in the treatment of patients with rheumatoid arthritis.
WHAT THIS STUDY ADDS
Sirukumab, a human monoclonal antibody against soluble IL-6, has been found to bind to human IL-6 with high affinity and specificity and thus suppress the biological activity of IL-6. Preclinical studies have demonstrated the safety of sirukumab in cynomolgus monkeys, a toxicologically relevant animal species, following repeated intravenous and subcutaneous administrations.
This study shows that sirukumab has desirable pharmacokinetic characteristics (linear pharmacokinetics with long half-life), a low incidence of immunogenicity and a well-tolerated safety profile in healthy subjects, supporting further development of sirukumab as a potentially valuable therapeutic agent.
Introduction
Interleukin (IL)-6 is a cytokine known for pleiotropic and pro-inflammatory functions [1, 2]. The physiological and pathological role of IL-6 has been illustrated in many in vitro and in vivo studies collectively suggesting that IL-6 induces the differentiation of B cells into antibody-producing cells, promotes the development of cytotoxic T cells, affects macrophage differentiation [3], increases hepatic acute-phase reactants and promotes mesangial cell proliferation, keratinocyte growth, megakaryocytic differentiation and thrombosis [4]. IL-6 concentrations are increased in obesity and increased concentrations of IL-6 also correlate with insulin resistance [5]. Mice deficient in IL-6 have a normal phenotype, are viable and fertile, but have a slightly decreased number of T cells and a decreased acute-phase protein response to tissue injury [6]. In contrast, transgenic mice that overexpress IL-6 in the brain develop neurologic diseases such as neurodegeneration, astrocytosis and proliferative angiopathy [7]. Treatment with an anti-murine IL-6 monoclonal antibody has been shown to reduce the incidence and severity of arthritis in an animal model of collagen-induced arthritis [8]. In humans, IL-6 is a known component in the pathogenesis of a wide variety of disease processes including lupus erythematosus [9], rheumatoid arthritis [10], anaemia of chronic inflammation [11], insulin resistance [12] and cancer [13]. The development of therapies for these areas of unmet medical needs is highly desirable. Tocilizumab, a monoclonal antibody that targets the IL-6 receptor, has been approved for the treatment of rheumatoid arthritis [14].
Sirukumab (formerly known as CNTO 136) is a human anti-IL-6 monoclonal antibody currently under development by Centocor Research & Development, Inc. It binds to IL-6 and inhibits IL-6-mediated signal transducers and activation of transcription-3 phosphorylation (STAT-3), a key component in the IL-6 signalling pathway [15]. Sirukumab has a high affinity and specificity for binding to IL-6 and, as a result, attenuates the biological activity of the cytokine. In addition, IL-6 has been identified as the primary inducer of C-reactive protein (CRP) synthesis by hepatocytes [16, 17]. Therefore, CRP suppression may serve as a surrogate pharmacodynamic (PD) biomarker for the inhibition of serum IL-6 bioactivity [18].
The objectives of this first-in-human study were to evaluate the safety, tolerability, pharmacokinetics (PK) and immunogenicity of a single, dose-ascending intravenous (i.v.) infusion of sirukumab in healthy subjects.
The proposed starting dose of sirukumab was 0.3 mg kg−1 and the proposed highest dose was 10 mg kg−1 for this study. Based on a 3-month toxicology study, no adverse effects in clinical signs, food consumption, bodyweight, physical examinations, vital signs, electrocardiograms and laboratory tests were observed in cynomolgus monkeys following weekly i.v. administration of sirukumab at doses of 10 mg kg−1 and 50 mg kg−1 (unpublished data). As a result, the no observed adverse effect level in monkeys was considered to be greater than 50 mg kg−1. The starting dose in humans (0.3 mg kg−1) was expected to have minimal pharmacological activity and to result in a drug exposure value predicted to be approximately 60 times lower than the mean steady-state exposure seen in monkeys following the 50 mg kg−1 dose. The highest dose of 10 mg kg−1 was predicted to have exposures of approximately 54% of the mean steady-state exposure observed with the 50 mg kg−1 i.v. dose in the 3-month toxicology study.
Methods
Study subjects
Healthy men, 18 to 45 years of age, and healthy women, 18 to 55 years of age, were considered eligible if they had no clinically relevant abnormalities as determined by medical history, physical examination, vital signs, serum chemistry, haematology, coagulation tests, urine dipstick and 12-lead electrocardiogram. Subjects were prohibited from the use of medication for concomitant illness within 2 weeks prior to randomization and from the use of over-the-counter [except paracetamol (acetaminophen) or pre-existing multivitamin use], herbal or ‘natural’ medications from 14 days prior to randomization until the completion of the study. Female subjects had to be of non-childbearing potential and had a negative pregnancy test on entry into the study.
The Ravenscourt Ethics Committee (Ravenscourt Park, London, UK) approved the protocol and written informed consent documents. The study was conducted in accordance with the Declaration of Helsinki and the regulations established in the USA for the Protection of Human Subjects (United States Code of Federal Regulations Title 21, Part 56) by Richmond Pharmacology Ltd at Mayday Hospital (Croydon, UK). All subjects provided informed consent before participating in any study-related procedures.
Study design and treatment
This study was conducted as a double-blind, placebo-controlled, ascending single-dose study in healthy subjects. Forty-five healthy adult subjects (38 men and seven women) were enrolled in six dose cohorts (cohort 1: 0.3 mg kg−1; cohort 2: 1 mg kg−1; cohort 3: 3 mg kg−1; cohort 4: 6 mg kg−1; cohort 5: 6 mg kg−1 in women only; cohort 6: 10 mg kg−1), and dose escalation occurred in a staggered parallel fashion. Cohort 5 consisted of only women in order to ensure the evaluation of safety, tolerability and PK of sirukumab in women, who comprise the majority of patients with lupus erythematosus [19] or rheumatoid arthritis [20], two potential target disease populations for sirukumab. In cohorts 1 to 4, a total of eight subjects each were randomized in a 6:2 ratio to receive a single i.v. infusion of either sirukumab or placebo. Cohort 5 comprised seven female subjects (six assigned to sirukumab, one to placebo). Cohort 6 comprised six subjects (four assigned to sirukumab, two to placebo). The study agent was administered in a 10-min i.v. infusion to cohorts 1 to 5, and in a 15-min i.v. infusion to cohort 6 as this cohort received the highest dose (10 mg kg−1) of sirukumab. The decision to proceed to each higher-dose cohort was made based on review of acute safety data collected for 4 days after study agent administration from the previous dose cohort.
Safety assessments
Subjects were monitored for 20 weeks after administration of the study agent. All treated subjects were observed in a hospital setting for 96 h post infusion and underwent follow-up evaluations at 1, 2, 3, 4, 6, 8, 10, 12, 14, 16 and 20 weeks after administration of study agent. Safety assessments included monitoring for all adverse events (AEs) and examination of vital signs, electrocardiograms and laboratory parameters. All untoward events, including serious AEs (SAEs), occurring between the time of obtaining informed consent and the 20-week study follow-up, were recorded regardless of whether they were considered study-related. The severity of an AE and its relationship to the study agent were determined by the investigators, who were blinded to the treatment assignments. A safety-evaluable subject was defined as any subject who received a dose of sirukumab or placebo.
Pharmacokinetic sampling and bioanalysis
For the measurement of sirukumab in the serum, blood samples were obtained prior to study agent infusion, at the completion of the infusion and at 1, 2, 4, 8, 24, 48, 72 and 96 h after infusion. Additional blood samples for the measurement of sirukumab were obtained at 1, 2, 3, 4, 6, 8, 10, 12, 14, 16 and 20 weeks after study agent administration.
Serum sirukumab concentrations were determined using a validated electrochemiluminescent immunoassay. Using this assay, the lowest quantifiable concentration in a sample after a twofold minimum dilution was 0.08 µg ml−1. The intra- and inter-assay precision values, expressed as per cent coefficient of variation (%CV), were 5.21% and 8.44%, respectively.
Non-compartmental pharmacokinetic analysis
Non-compartmental analysis (NCA) using WinNonlin® (Version 4.1, Pharsight Corporation, Mountain View, CA, USA) was used to determine the PK parameters of sirukumab following a single i.v. infusion. The maximum serum concentration (Cmax) was obtained from inspection of the individual serum concentration–time data. The terminal elimination rate constant (λz) was determined by least-squares regression analysis of the log-linear portion of the terminal phase. The area under the serum concentration–time curve from time zero to infinity [AUC(0,∞)] was determined as AUC(0,tz) +Cz/λz, where Cz is the last measurable serum concentration at time tz. The terminal half-life (t1/2) was calculated as 0.693/λz. The total systemic clearance (CL) was determined by dividing the dose by the AUC(0,∞). The volume of distribution based on the elimination phase (Vz) was determined as dose/[λz× AUC(0,∞)].
Population pharmacokinetic analysis
The phase 1 PK data were analysed using a population PK approach to develop a compartmental model for simulations of new dosing regimens and to quantify the inter-individual variability (IIV) for the PK parameters of sirukumab.
The PK data were analysed using non-linear mixed-effects modelling with the NONMEM software system (Version 7.1, ICON Development Solutions, Ellicott City, MD, USA). The first-order conditional estimation (FOCE) with interaction method was applied to all models tested. Models were accepted only if they yielded a successful covariance step with PK parameters consistent with prior knowledge. The selection of competing models was based on minimization of the objective function value (OFV), the Akaike information criterion (AIC), the precision and plausibility of parameter estimates and a variety of goodness-of-fit plots.
The final PK model was identified by comparing different structural models (e.g. one- vs. two-compartment models, two- vs. three-compartment models) with different elimination functions (e.g. first-order elimination, parallel first-order elimination, Michaelis-Menten elimination). Exponential models were used to fit IIV (η) in the PK parameters, as log-normal distribution is assumed for IIV in PK parameters. Correlation between two or more IIVs was also considered. The estimate of IIV was expressed as an approximate %CV. Several residual error models with different combinations of additive and/or proportional variance were examined during model development. The estimate of proportional error was expressed as an approximate %CV, and that of additive error was expressed as a standard deviation (SD).
Demographic characteristics (bodyweight, body surface area, age, gender and race) and baseline concentrations for several inflammatory biomarkers (CRP, IL-6, soluble (s) IL-6 receptor, CD40 ligand, vascular cell adhesion molecule-1, adiponectin, tumour necrosis factor (TNF) α and sTNFα receptor) were available in these healthy subjects. Due to the narrow distributions in demographic characteristics (e.g. small sample size, narrow ranges in age and weight, predominance of Caucasian race) and baseline biomarker concentrations for the healthy subjects enrolled in this study, a meaningful covariate analysis could not be performed. Nevertheless, body size has been identified as the most significant covariate on the PK (clearance and volume of distribution) of many monoclonal antibodies [21]. Therefore, the effects of bodyweight on the PK of sirukumab were still assessed using allometric equations, with the exponents for CL and Q fixed to 0.75, and the exponents for V1 and V2 fixed to 1.
Non-parametric bootstrap analysis [22, 23] and visual predictive checks [24] were performed on the final population PK model of sirukumab to test for model robustness, such as the precision of PK parameter estimates, model stability and predictability. The diagnostic plots and visual predictive checks were created using PSN 3.0.0 and Xpose 4.0.4 (Uppsala University, Uppsala, Sweden).
Pharmacodynamics
Serum samples were obtained for the high-sensitivity measurement of CRP at baseline (i.e. prior to study agent administration and at weeks 1, 2, 4, 8, 12, 16 and 20 after study agent administration). A highly sensitive immunoturbidimetric assay (Roche Diagnostics, Indianapolis, IN, USA) was used to detect CRP concentrations with a lower limit of quantification (LLOQ) of 0.1 mg l−1. The CRP assay was performed by The Doctors Laboratory (London, UK).
Because the high-sensitivity data on CRP revealed the best pharmacodynamic response among the aforementioned inflammatory biomarkers, only data on CRP suppression are described in this manuscript.
Immunogenicity assessment
The development of antibodies to sirukumab was determined from serum samples obtained prior to and following study agent administration at weeks 6 and 20. Antibodies to sirukumab were determined using a validated antigen bridging enzyme immunoassay. Briefly, the sera were screened and the optical densities (ODs) of the test samples were compared with an assay cut-off OD of 0.115 units. Potentially positive samples along with the subject's pre-treatment sample were then tested for titre. The specificity of the response to sirukumab was assessed by the addition of soluble sirukumab. Binding was considered to be specific for sirukumab if the test sample OD was reduced by at least 50% after pre-incubation with soluble sirukumab. Each assay performed also included positive and negative control samples. Subjects were classified as positive for antibodies to sirukumab if any post-treatment sample test result was above the assay cut-off OD. Subjects were classified as negative if antibodies to sirukumab were not detected in any post-treatment samples (i.e. all test results were below the assay cut-off OD).
Results
Study population
Forty-five healthy subjects were enrolled and comprised the safety-evaluable population. Subjects randomly assigned to cohorts 1 to 4 (0.3 mg kg−1, 1 mg kg−1, 3 mg kg−1, 6 mg kg−1) received the study agents per protocol, with each cohort consisting of six sirukumab-treated subjects and two placebo-treated subjects. Three subjects, one subject randomized to cohort 5 (6 mg kg−1, women only) and two subjects randomized to cohort 6 (10 mg kg−1) were not dosed due to technical reasons. Consequently, seven subjects were included in cohort 5, with six subjects receiving sirukumab and one subject receiving placebo, and cohort 6 (10 mg kg−1) included six subjects, with four sirukumab-treated subjects and two placebo-treated subjects. There were no safety findings that influenced the decision not to dose these individuals, and these subjects were not replaced with the enrolment of new subjects in the study population. Although per protocol cohorts 1 to 4 and cohort 6 (10 mg kg−1) allowed participation of both men and women, only men participated.
For the purpose of data analysis, placebo-treated subjects from each dose cohort were combined as the placebo treatment group (n = 11). The distribution of age, weight, race and gender for each treatment group is shown in Table 1. Overall, the study population included seven (16%) women and 38 (84%) men. The median age of the subjects was 30 years (range 18–54 years), and the median bodyweight was 71.3 kg (range 49–99 kg). The majority of subjects were Caucasian (n = 32, 71%). There were no noteworthy differences between cohorts in demographic characteristics with the exception of cohort 5, which comprised female subjects of non-childbearing potential with a median age of 49.5 years.
Table 1.
Baseline demographic characteristics of the study population
| Placebo*(n = 11) | Cohort 1 0.3 mg kg−1 (n = 6) | Cohort 2 1 mg kg−1 (n = 6) | Cohort 3 3 mg kg−1 (n = 6) | Cohort 4 6 mg kg−1 (n = 6) | Cohort 5†6 mg kg−1 (n = 6) | Cohort 6 10 mg kg−1 (n = 4) | |
|---|---|---|---|---|---|---|---|
| Age (years) | |||||||
| Mean ± SD | 28.3 ± 7.1 | 26.5 ± 7.0 | 23.8 ± 4.3 | 28.7 ± 5.1 | 23.0 ± 3.7 | 43.2 ± 13.1 | 28.0 ± 5.7 |
| Median | 29.0 | 27.0 | 24.5 | 29.5 | 22.5 | 49.5 | 26.0 |
| Range (min, max) | (18.0, 42.0) | (18.0, 36.0) | (19.0, 30.0) | (21.0, 35.0) | (20.0, 30.0) | (22.0, 54.0) | (24.0, 36.0) |
| Weight (kg) | |||||||
| Mean ± SD | 72.3 ± 10.8 | 78.7 ± 8.5 | 75.1 ± 12.8 | 69.3 ± 12.4 | 69.7 ± 8.5 | 65.8 ± 10.3 | 73.3 ± 10.6 |
| Median | 69.9 | 82.0 | 69.1 | 68.8 | 69.4 | 68.0 | 71.7 |
| Range (min, max) | (58.8, 96.5) | (66.8, 86.5) | (66.5, 98.9) | (55.2, 91.0) | (56.0, 79.3) | (48.7, 75.3) | (62.3, 87.3) |
| Gender, n (%) | |||||||
| Female | 1 (9.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 6 (100) | 0 (0.0) |
| Male | 10 (90.9) | 6 (100) | 6 (100) | 6 (100) | 6 (100) | 0 (0.0) | 4 (100) |
| Ethnicity, n (%) | |||||||
| Caucasian | 7 (63.6) | 5 (83.3) | 4 (66.7) | 4 (66.7) | 5 (83.3) | 4 (66.7) | 3 (75.0) |
| Black | 1 (9.1) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (16.7) | 1 (16.7) | 1 (25.0) |
| Asian | 1 (9.1) | 1 (16.7) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Other | 2 (18.2) | 2 (0.0) | 0 (0.0) | 2 (33.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Placebo subjects from each dose cohort were combined for analysis.
All female cohort.
SD, standard deviation.
Safety and tolerability
Adverse events
A summary of AEs that occurred in two or more sirukumab-treated subjects is presented in Table 2.
Table 2.
Summary of all adverse events that occurred in two or more sirukumab-treated subjects by preferred term*
| Placebo†(n = 11) | Total sirukumab (n = 34) | Cohort 1 0.3 mg kg−1 (n = 6) | Cohort 2 1 mg kg−1 (n = 6) | Cohort 3 3 mg kg−1 (n = 6) | Cohort 4 6 mg kg−1 (n = 6) | Cohort 5‡6 mg kg−1 (n = 6) | Cohort 6 10 mg kg−1 (n = 4) | |
|---|---|---|---|---|---|---|---|---|
| Subjects with at least one AE, n (%) | 8 (72.7) | 19 (55.9) | 3 (50.0) | 2 (33.3) | 3 (50.0) | 4 (66.7) | 4 (66.7) | 3 (75.0) |
| Pharyngolaryngeal pain | 2 (18.2) | 5 (14.7) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 2 (33.3) | 2 (33.3) | 0 (0.0) |
| Headache | 2 (18.2) | 3 (8.8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 1 (25.0) |
| Nasopharyngitis | 3 (27.3) | 3 (8.8) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 2 (33.3) | 0 (0.0) | 0 (0.0) |
| Faeces discoloured | 0 (0.0) | 2 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (50.0) |
| Acne | 0 (0.0) | 2 (5.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 1 (16.7) | 0 (0.0) |
| Dyspepsia | 0 (0.0) | 2 (5.9) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) |
| Back pain | 1 (9.1) | 2 (5.9) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (25.0) |
Every subject was counted once within each preferred term.
Placebo subjects from each cohort were combined for analysis.
All female cohort.
Of the sirukumab-treated subjects, 19 (55.9%) of 34 had one or more AEs that appeared to be independent of the dose administered. Eight (72.7%) of 11 placebo-treated subjects had one or more AEs. Pharyngolaryngeal pain was the most common AE (seven subjects) and occurred in five sirukumab-treated subjects and two placebo-treated subjects. All AEs of pharyngolaryngeal pain were considered by the investigator to be of mild intensity and were resolved by each subject's completion of the study. One SAE occurred in a subject in the placebo group. Twenty-nine days after the administration of study agent, a 43-year-old Caucasian woman had a fall that resulted in a severe left calcaneum fracture for which she underwent surgery (subtalar fusion with bone graft). The SAE was not considered related to the study agent and resolved prior to the subject's completion of the study. No deaths occurred during the study period and no subjects withdrew from the study due to an AE.
Infections
Five (14.7%, 5/34) sirukumab-treated subjects and four (36.7%, 4/11) placebo subjects experienced infections or infestations. The types of infections or infestations reported by the sirukumab-treated subjects were nasopharyngitis (three subjects), acarodermatitis (one subject) and tooth abscess (one subject). None of the reported infections was opportunistic or serious.
Allergic and infusion reactions
Sirukumab administered as a 10- to 15-min i.v. infusion at doses ranging from 0.3 to 10 mg kg−1 was well tolerated. No anaphylaxis, severe allergic reactions, infusion reactions or delayed hypersensitivity reactions were observed. AEs such as pain, inflammation and infiltration were also not observed at the infusion sites.
Laboratory parameters, vital signs and electrocardiograms
There were no noteworthy differences in the haematology, clinical chemistry, vital signs, urinalysis or electrocardiogram measurements between sirukumab- and placebo-treated subjects or between baseline and post treatment with sirukumab for any dose group. There were no noteworthy differences between sirukumab and placebo or between sirukumab doses in post and baseline median values at any time point. Particularly, no trends were observed in the elevation of liver transaminases or lipid concentrations (total cholesterol, low-density lipoprotein cholesterol, very low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, high-density lipoprotein subclasses, low-density lipoprotein subclasses and triglycerides) in these healthy subjects. However, there was a dose-dependent decrease from baseline counts in neutrophils and platelets that reached a nadir at week 4, although median neutrophil and platelet counts remained within normal ranges for sirukumab-treated subjects (data not shown). Three sirukumab-treated subjects experienced markedly abnormal low absolute neutrophil counts (ANC, lower limit of the normal range: 2.0 × 109 l−1). One subject in the 1 mg kg−1 group had an abnormal ANC of 0.93 × 109 l−1 at week 4 through all follow-up visits, one subject in the 3 mg kg−1 group had an abnormal ANC of 0.90 × 109 l−1 at week 1 through all follow-up visits and one subject in the 6 mg kg−1 group had an abnormally low ANC of 0.81 × 109 l−1 at week 12, which returned to within normal range by week 16 (2.28 × 109 l−1). Of note, all three subjects had below normal ANC values at baseline, 1 day before dosing (1.63, 1.7 and 1.69 × 109 l−1, respectively). None of these low ANC values was considered AEs by the investigator, nor were they associated with infections.
Pharmacokinetic parameters from non-compartmental analysis
Mean sirukumab concentration vs. time curves for subjects in each dose group are presented in Figure 1. Following an i.v. infusion, sirukumab serum concentrations exhibited a typical biphasic PK profile with a relatively rapid distribution phase and a relatively slow elimination phase. Median t1/2 values ranged from 18.5 to 29.6 days and appeared to be independent of the dose administered.
Figure 1.
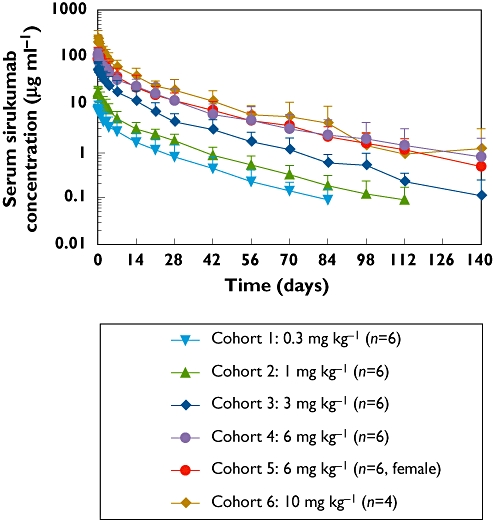
Mean (±SD) sirukumab serum concentration–time profiles following a single intravenous infusion of sirukumab in healthy subjects.
A summary of the pharmacokinetic parameters Cmax, AUC(0,∞), t1/2, CL and Vz values for each dose cohort is shown in Table 3. Mean Cmax concentrations of 7.9, 19.2, 60.1, 116.3:118.6 (male : female) and 248.8 µg ml−1 increased in an approximately proportional manner to doses of 0.3, 1, 3, 6 and 10 mg kg−1, respectively. Similarly, AUC(0,∞) values increased in an approximately dose-proportional fashion (Figure 2). Clearance appeared to be independent of dose, with mean values ranging from 3.8 to 6.1 ml day−1 kg−1 in all cohorts. The mean volume of distribution for sirukumab ranged from 121.3 to 247.6 ml kg−1.
Table 3.
Summary of pharmacokinetic parameters from non-compartmental analysis following a single intravenous dose of sirukumab in healthy subjects
| Cohort 1 0.3 mg kg−1 (n = 6) | Cohort 2 1 mg kg−1 (n = 6) | Cohort 3 3 mg kg−1 (n = 6) | Cohort 4 6 mg kg−1 (n = 6) | Cohort 5*6 mg kg−1(n = 6) | Cohort 6 10 mg kg−1 (n = 4) | |
|---|---|---|---|---|---|---|
| Cmax (µg ml−1) | ||||||
| Mean ± SD | 7.9 ± 1.4 | 19.2 ± 1.8 | 60.1 ± 14.0 | 116.3 ± 11.6 | 118.6 ± 19.2 | 248.8 ± 61.7 |
| Median (range) | 7.8 (5.8, 9.4) | 18.6 (17.4, 21.7) | 58.9 (42.4, 76.1) | 120.0 (98.9, 128.8) | 116.0 (99.5, 150.3) | 223.9 (206.9, 340.5) |
| AUC (µg ml−1 day) | ||||||
| Mean ± SD | 82.1 ± 20.4 | 167.3 ± 23.6 | 540.2 ± 175.2 | 1225 ± 378.2 | 1262.0 ± 307.0 | 2164.7 ± 658.5 |
| Median (range) | 79.1 (58.6, 116.1) | 168.8 (131.1, 193.6) | 559.7 (339.8, 831.0) | 1207.0 (771.0, 1891.9) | 1296.0 (755.5, 1695.4) | 2046.0 (1494.6, 3072.1) |
| t1/2 (days) | ||||||
| Mean ± SD | 23.4 ± 11.1 | 23.1 ± 9.8 | 18.6 ± 5.2 | 32.4 ± 12.1 | 24.5 ± 2.6 | 21.8 ± 5.6 |
| Median (range) | 20.9 (12.1, 42.9) | 19.9 (15.9, 42.2) | 18.5 (10.5, 26.6) | 29.6 (14.4, 48.0) | 25.0 (19.9, 28.0) | 21.0 (16.1, 29.2) |
| CL (ml kg−1 day−1) | ||||||
| Mean ± SD | 3.8 ± 0.9 | 6.1 ± 0.9 | 6.1 ± 2.0 | 5.3 ± 1.6 | 5.1 ± 1.5 | 4.9 ± 1.4 |
| Median (range) | 3.8 (2.6, 5.1) | 5.9 (5.2, 7.6) | 5.4 (3.6, 8.8) | 5.0 (3.2, 7.8) | 4.6 (3.5, 7.9) | 4.9 (3.3, 6.7) |
| Vz (ml kg−1) | ||||||
| Mean ± SD | 121.3 ± 42.7 | 196.6 ± 65.9 | 163.8 ± 87.8 | 247.6 ± 146.7 | 173.6 ± 29.2 | 149.0 ± 29.9 |
| Median (range) | 106.8 (89.3, 202.6) | 170.4 (150.2, 321.7) | 132.9 (101.1, 339.2) | 201.4 (126.4, 539.4) | 168.3 (142.8, 227.5) | 148.8 (114.5, 183.7) |
All female cohort.
SD, standard deviation.
Figure 2.

Dose-proportionality assessment of sirukumab pharmacokinetics in healthy subjects. Left panel: Mean ± SD values for Cmaxvs. dose for subjects in each cohort with the linear regression line defined by f(x) = 22.5x, r2 = 0.973. Right panel: Mean ± SD values for AUC(0,∞) vs. dose for subjects in each cohort with the linear regression line defined by f(x) = 211x, r2 = 0.996
Values for the exposure parameters Cmax and AUC(0,∞) in the all-women 6 mg kg−1 dose cohort (cohort 5) were consistent with those from the all-men 6 mg kg−1 dose cohort (cohort 4). Other PK parameters (e.g. t1/2, CL, Vz) in the women-only cohort were also consistent with those from the other male cohorts (cohorts 1 to 4 and cohort 6). These results suggest that there were no apparent gender differences observed in the PK parameters in this study.
Population pharmacokinetic model
A total of 616 measurable serum concentration values of sirukumab from 34 healthy subjects were analysed using NONMEM. Using the FOCE with interaction method, a two-compartment model with first-order elimination and zero-order input (i.v. infusion) was found to be superior to other models (e.g. one-compartment model, three-compartment model and PK models with a non-linear Michaelis-Menten elimination pathway) for modelling sirukumab PK data in regard to the goodness of fit, model stability and plausibility of PK parameter estimates. In addition, the dose-proportionality of Cmax and AUC(0,∞) with increasing sirukumab dose also supported the use of a linear PK model. A residual error model including additive and proportional terms best fitted the observed data. As expected, incorporation of bodyweight as a covariate to the PK parameters resulted in a modest decrease (six points) in objective function value even though the number of parameters for the PK model was not increased, with the exponents for the allometric functions being fixed to 0.75 for CL and Q and to 1 for V1 and V2. The diagnostic plots, as shown in Figure 3, suggest that the population PK model adequately described the observed concentration–time data of sirukumab following i.v. administration.
Figure 3.
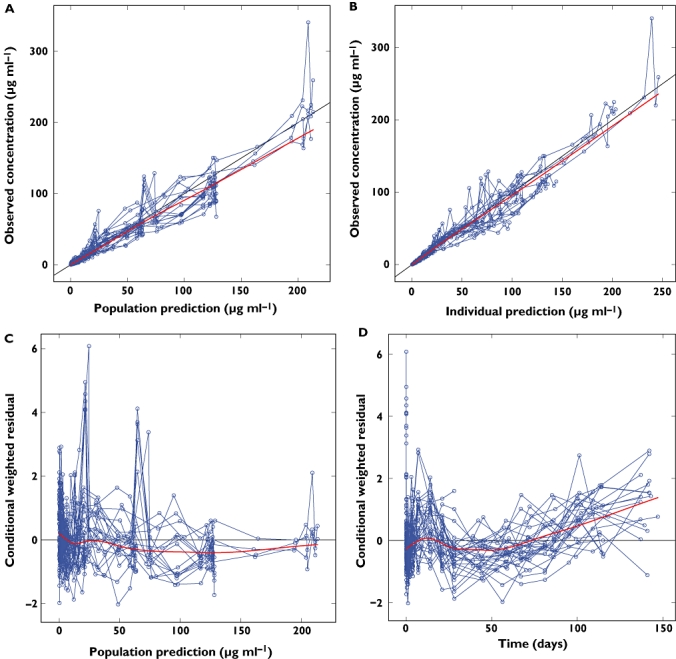
Goodness-of-fit plots for the final population pharmacokinetics model of sirukumab in healthy subjects. The thin solid line is the line of identity and the thick solid line is the trend line. A) Typical predicted vs. observed sirukumab serum concentrations, B) Bayesian-predicted vs. observed sirukumab serum concentration, C) conditional weighted residuals vs. predicted and D) conditional weighted residuals vs. time
The parameter estimates from the final population PK model are shown in Table 4. The population estimates (typical value ± SEM) of sirukumab PK parameters for a patient with a standard bodyweight of 70 kg were as follows: CL = 0.364 ± 0.017 l day−1 (i.e. 46.9 ± 1.5 ml day−1 kg−1), V1 = 3.28 ± 0.11 l (i.e. 5.2 ± 0.2 ml kg−1), Q = 0.59 ± 0.08 l day−1 (i.e. 8.4 ± 1.1 ml day−1 kg−1) and V2 = 4.97 ± 0.34 l (i.e. 71.4 ± 4.8 ml kg−1). The IIVs for CL, V1, Q and V2 were 24.3%, 19.3%, 53.4% and 28.3%, respectively. The volume of distribution at steady state (Vss=V1+V2) for a subject weighing 70 kg was 8.3 l. Based on these typical PK parameters, the typical half-life value was estimated to be 20.9 days.
Table 4.
Parameter estimates from population pharmacokinetic analysis for sirukumab following intravenous administration in healthy subjects
| Symbol | Estimate | RSE (%) | 2.5th percentile | 50th percentile | 97.5th percentile | |
|---|---|---|---|---|---|---|
| Pharmacokinetic parameters | ||||||
| CL (l day−1) | θ1 | 0.364 | 4.6 | 0.331 | 0.365 | 0.397 |
| V1 (l) | θ2 | 3.28 | 3.2 | 3.07 | 3.27 | 3.50 |
| Q (l day−1) | θ3 | 0.588 | 13.6 | 0.452 | 0.599 | 0.827 |
| V2 (l) | θ4 | 4.97 | 6.8 | 4.31 | 4.96 | 5.64 |
| Inter-individual variability (IIV) | ||||||
| IIV (%) on CL | ωCL | 24.3 | – | 17.3 | 23.9 | 29.9 |
| IIV (%) on V1 | ωVc | 19.3 | – | 13.1 | 18.9 | 24.6 |
| IIV (%) on Q | ωQ | 53.4 | – | 22.5 | 52.9 | 110 |
| IIV (%) on V2 | ωVp | 28.3 | – | 17.9 | 27.8 | 37.3 |
| Residual variability | ||||||
| Proportional error variability (%) | σProp | 21.7 | – | 17.6 | 21.2 | 24.6 |
| Additive error (μg l−1) | σAdd | 0.0228 | – | 0.00137 | 0.0272 | 0.0605 |
Parameter estimates: final parameter estimates for θ, ω, σ; RSE% = 100 × (standard error/estimate); IIV (%): inter-individual variability = 100 sqrt(estimate for ω2); ω2: random effect parameter that represents inter-individual variance; σ2: random effect parameter that represents residual variance; CL: clearance [CL =θ1× (weight/70)0.75]; V1: volume of distribution in the central compartment [V1=θ1× (weight/70)]; Q: inter-compartmental clearance [Q =θ3× (weight/70)0.75]; V2: volume of distribution in the peripheral compartment [V2=θ4× (weight/70)]; 2.5th, 50th, 97.5th percentiles: 2.5th, 50th, 97.5th percentiles from 1000 bootstrapping runs.
The median values for the PK parameters estimated from 1000 successfully converged bootstrapping runs were nearly identical to the typical parameter estimates from the final population PK model (Table 4). Additionally, the 95% confidence intervals (2.5th percentile, 97.5th percentile) of the parameter estimates were narrowly distributed. These results indicate that the final model had adequate stability and the population PK parameters were estimated with reasonable precision. In addition, visual predictive check using Monte Carlo simulations showed that the vast majority of the observed concentrations fell within the 5th to 95th percentiles of simulated values, with few exceptions (data not shown). Therefore, the final model adequately described the observed sirukumab PK data.
Pharmacodynamics
A substantial per cent decrease below baseline CRP concentrations was observed over time in all sirukumab cohorts compared with placebo-treated subjects (Figure 4). There was no clear dose-dependent CRP suppression by sirukumab, as the largest decrease in CRP was observed in the 3 mg kg−1 group. Nevertheless, the median CRP concentrations returned to baseline concentrations for the lower-dose cohorts (0.3 mg kg−1 and 1 mg kg−1) but not the higher-dose cohorts (3 mg kg−1, 6 mg kg−1 and 10 mg kg−1). Given the lack of a clear dose–response relationship, the development of a meaningful PK/PD model in healthy subjects using high-sensitivity CRP as the PD marker was not considered feasible. Nevertheless, as shown in Figure 4, these data show that high-sensitivity CRP concentrations could be sustainably suppressed after i.v. administration of sirukumab.
Figure 4.
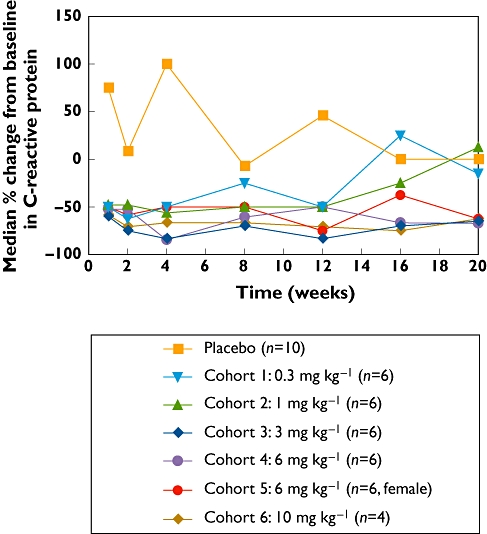
Percent change from baseline in C-reactive protein (CRP) serum concentration following a single intravenous administration of sirukumab in healthy subjects.
Immunogenicity
Antibodies to sirukumab were evaluated in all subjects prior to study agent administration and at weeks 6 and 20. None of the 31 subjects with appropriate samples was classified as positive for antibodies to sirukumab through week 20.
Discussion
The goals of this first-in-human study were to determine the single-dose safety, pharmacokinetics, pharmacodynamics and immunogenicity of sirukumab in healthy subjects.
The safety results from this study demonstrate that 10- to 15-min i.v. infusions of a single i.v. dose of sirukumab at doses ranging from 0.3 mg kg−1 to 10 mg kg−1 in 34 healthy subjects were well tolerated. No subject experienced anaphylaxis, severe allergic reaction or delayed hypersensitivity reactions. Among subjects receiving sirukumab, pharyngolaryngeal pain was the most commonly reported AE. The incidences of individual AEs were sporadic and showed no clear differences between sirukumab- and placebo-treated subjects. Observed AEs were generally transient and mild to moderate in intensity. No SAEs were observed in the sirukumab-treated subjects.
It has been reported that IL-6 can induce megakaryocyte maturation and thrombocytopoiesis [25]. In addition, IL-6 is known to demarginate neutrophils and the inhibition of IL-6 could be expected to marginate neutrophils [26]. Transient and moderate thrombocytopenia has been observed with treatment with other anti-IL-6 agents such as BE-8, siltuximab and tocilizumab in patients with HIV, lymphoma, multiple myeloma, Castleman's disease and rheumatoid arthritis [27–29]. In addition, changes in neutrophils, lipids and certain liver function tests have been reported with tocilizumab, a recently approved humanized anti-IL-6 receptor monoclonal antibody, in patients with active rheumatoid arthritis [14]. In this study, treatment with sirukumab led to a dose-dependent reduction in neutrophils and platelets, and three subjects who already started the study with low ANCs showed sustained reductions in ANCs up to week 20. Such dose-dependent decreases in neutrophil and platelet counts in sirukumab-treated subjects would be expected pharmacodynamic effects of anti-IL-6 treatment.
However, no substantial safety signals were observed in clinical chemistry, haematology, vital signs or electrocardiogram values in the healthy subjects who received i.v. sirukumab at doses ranging from 0.3 mg kg−1 to 10 mg kg−1. Importantly, the median neutrophil and platelet counts in the sirukumab-treated subjects remained within the normal range and were not associated with clinical manifestations of infections or bleeding. No dose-dependent elevations of liver transaminases or lipid concentrations were observed in the sirukumab-treated healthy subjects. Nevertheless, in future clinical studies with sirukumab, subjects should be monitored closely for AEs associated with anti-IL-6 treatment such as neutropenia and thrombocytopenia, elevated hepatic transaminases and changes of the lipid profile.
Exposure to sirukumab [Cmax and AUC(0,∞)] increased proportionally with dose. The clearance and half-life of sirukumab appeared to be independent of dose. Therefore, sirukumab exhibited linear pharmacokinetics over the dose range of 0.3 mg kg−1 to 10 mg kg−1. In contrast, tocilizumab showed non-linear pharmacokinetics [30]. These observations are in concordance with the known PK behaviours of two categories of monoclonal antibodies [31]: antibodies such as sirukumab against soluble antigens (e.g. cytokines) and antibodies such as tocilizumab against membrane-associated antigens (e.g. IL-6 receptor, human epidermal growth receptor 2, epidermal growth factor receptor). In addition, this study indicates that there are no apparent gender differences in the PK parameters of sirukumab.
A two-compartment linear model with zero-order input and first-order elimination was chosen to characterize the PK profile of sirukumab following i.v. administration. Similar two-compartment linear models have been used to describe the PK of other monoclonal antibodies, such as infliximab [32], golimumab [33], rituximab [34] and pertuzumab [35]. The volume of distribution at steady state for sirukumab in a subject weighing 70 kg was 8.3 l. This volume of distribution corresponds to approximately two times the plasma volume, suggesting that sirukumab is primarily located in the circulatory system with limited extravascular tissue distribution. The inter-individual variability for CL, V1, Q and V2 were quantified as 24.3%, 19.3%, 53.4% and 28.3%, respectively. These variability estimates were consistent with those for other monoclonal antibodies following i.v. administration [32–35].
In the literature, a strong correlation between CRP and IL-6 has been described in healthy subjects [36]. A sustained decrease from baseline in CRP concentrations was observed in sirukumab-treated subjects compared with placebo-treated subjects in this study. This finding is consistent with the anticipated biological activity of sirukumab. However, a clear dose–response relationship was not observed with CRP suppression by sirukumab, so a meaningful PK/PD relationship could not be developed. A possible explanation for the lack of a dose–response relationship for CRP suppression is that most healthy subjects had a large intra-subject variability in CRP concentrations and did not have sufficiently elevated CRP concentrations at baseline.
Antibodies to sirukumab were evaluated in all subjects prior to study agent administration and at weeks 6 and after a long washout at week 20. Therefore, potential interference with the detection of anti-drug antibodies by the study drug in the serum sample would have been minimized. None of the sirukumab-treated subjects tested positive for anti-sirukumab antibodies.
In summary, this clinical study provides initial information on the safety, pharmacokinetics and pharmacodynamics of sirukumab administered i.v. in healthy subjects. Intravenous administration of sirukumab at doses of 0.3 mg kg−1 to 10 mg kg−1 was generally safe and well tolerated. AEs were short in duration, mild or moderate in intensity and non-dose-dependent. No significant safety concerns were observed over the 20-week follow-up. Sirukumab exhibited linear pharmacokinetics without apparent gender differences. A two-compartment population PK model has been developed to describe adequately the PK behaviour of sirukumab. The safety and PK profiles observed suggest that sirukumab may be a valuable therapeutic agent for further development in patients who would potentially benefit from anti-IL-6 treatment. With the developed population PK model, serum concentration–time profiles can be readily predicted for different dosing regimens of sirukumab in future clinical trials.
Acknowledgments
The authors would like to acknowledge Ulrike Lorch, MD and the study personnel at Richmond Pharmacology Ltd, Mayday Hospital, Croydon, UK and Qingmin Wang, MD, PhD of Centocor Research & Development, Inc. for conducting the study. The authors thank Chuanpu Hu, PhD, Hong Yan, MD, MPH, Xiang Zong, MS and Kenneth Graham, PhD of Centocor Research & Development, Inc. for their input during data analysis and manuscript review and John (Jay) Getsy, DMD, DO formerly of Centocor Research & Development, Inc. for his significant contribution during the concept and design phase of the study. The authors also thank Jennifer Han and Robert Achenbach of Centocor Ortho Biotech Services, LLC. for their assistance in preparing the manuscript.
Competing Interests
This study was funded by Centocor Research & Development, Inc., a subsidiary of Johnson & Johnson and the manufacturer of sirukumab. All authors are employees of Centocor Research & Development, Inc. and own stock in Johnson & Johnson.
REFERENCES
- 1.Kishimoto T. Interleukin-6: from basic science to medicine – 40 years in immunology. Annu Rev Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 2.Liles WC, Van Voorhis WC. Review: nomenclature and biologic significance of cytokines involved in inflammation and the host immune response. J Infect Dis. 1995;172:1573–80. doi: 10.1093/infdis/172.6.1573. [DOI] [PubMed] [Google Scholar]
- 3.Nicola NA, Matsumoto M, Metcalf D, Johnson GR. Molecular properties of a factor inducing differentiation in murine myelomonocytic leukemic cells. Haematol Blood Transfus. 1983;28:345–7. doi: 10.1007/978-3-642-68761-7_65. [DOI] [PubMed] [Google Scholar]
- 4.Hirano T, Matsuda T, Nakajima K. Signal transduction through gp130 that is shared among the receptors for the interleukin 6-related cytokine subfamily. Stem Cells. 1994;12:262–77. doi: 10.1002/stem.5530120303. [DOI] [PubMed] [Google Scholar]
- 5.Pittas AG, Joseph NA, Greenberg AS. Adipocytokines and insulin resistance. J Clin Endocrinol Metab. 2004;89:447–52. doi: 10.1210/jc.2003-031005. [DOI] [PubMed] [Google Scholar]
- 6.Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Kohler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–42. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 7.Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, Mucke L. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci USA. 1993;90:10061–5. doi: 10.1073/pnas.90.21.10061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liang B, Song Z, Wu B, Gardner D, Shealy D, Song XY, Wooley PH. Evaluation of anti-IL-6 monoclonal antibody therapy using murine type II collagen-induced arthritis. J Inflamm (Lond) 2009;6:10. doi: 10.1186/1476-9255-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linker-Israeli M, Deans RJ, Wallace DJ, Prehn J, Ozeri-Chen T, Klinenberg JR. Elevated levels of endogenous IL-6 in systemic lupus erythematosus. A putative role in pathogenesis. J Immunol. 1991;147:117–23. [PubMed] [Google Scholar]
- 10.Madhok R, Crilly A, Watson J, Capell HA. Serum interleukin 6 levels in rheumatoid arthritis: correlations with clinical and laboratory indices of disease activity. Ann Rheum Dis. 1993;52:232–4. doi: 10.1136/ard.52.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jongen-Lavrencic M, Peeters HR, Wognum A, Vreugdenhil G, Breedveld FC, Swaak AJ. Elevated levels of inflammatory cytokines in bone marrow of patients with rheumatoid arthritis and anemia of chronic disease. J Rheumatol. 1997;24:1504–9. [PubMed] [Google Scholar]
- 12.Fernandez-Real JM, Vayreda M, Richart C, Gutierrez C, Broch M, Vendrell J, Ricart W. Circulating interleukin 6 levels, blood pressure, and insulin sensitivity in apparently healthy men and women. J Clin Endocrinol Metab. 2001;86:1154–9. doi: 10.1210/jcem.86.3.7305. [DOI] [PubMed] [Google Scholar]
- 13.Akira S, Kishimoto T. The evidence for interleukin-6 as an autocrine growth factor in malignancy. Semin Cancer Biol. 1992;3:17–26. [PubMed] [Google Scholar]
- 14.Actemra (Package Insert) San Francisco, CA: Genentech, Inc.; 2010. [Google Scholar]
- 15.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–12. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Ljungberg B, Grankvist K, Rasmuson T. Serum interleukin-6 in relation to acute-phase reactants and survival in patients with renal cell carcinoma. Eur J Cancer. 1997;33:1794–8. doi: 10.1016/s0959-8049(97)00179-2. [DOI] [PubMed] [Google Scholar]
- 17.Mahmoud FA, Rivera NI. The role of C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep. 2002;4:250–5. doi: 10.1007/s11912-002-0023-1. [DOI] [PubMed] [Google Scholar]
- 18.Puchalski T, Prabhakar U, Jiao Q, Berns B, Davis HM. Pharmacokinetic and pharmacodynamic modeling of an anti-interleukin-6 chimeric monoclonal antibody (siltuximab) in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2010;16:1652–61. doi: 10.1158/1078-0432.CCR-09-2581. [DOI] [PubMed] [Google Scholar]
- 19.Voulgari PV, Katsimbri P, Alamanos Y, Drosos AA. Gender and age differences in systemic lupus erythematosus. A study of 489 Greek patients with a review of the literature. Lupus. 2002;11:722–9. doi: 10.1191/0961203302lu253oa. [DOI] [PubMed] [Google Scholar]
- 20.Kay J, Matteson EL, Dasgupta B, Nash P, Durez P, Hall S, Hsia EC, Han J, Wagner C, Xu Z, Visvanathan S, Rahman MU. Golimumab in patients with active rheumatoid arthritis despite treatment with methotrexate: a randomized, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2008;58:964–75. doi: 10.1002/art.23383. [DOI] [PubMed] [Google Scholar]
- 21.Wang DD, Zhang S, Zhao H, Men AY, Parivar K. Fixed dosing versus body size-based dosing of monoclonal antibodies in adult clinical trials. J Clin Pharmacol. 2009;49:1012–24. doi: 10.1177/0091270009337512. [DOI] [PubMed] [Google Scholar]
- 22.Ette EI, Williams PJ, Kim YH, Lane JR, Liu MJ, Capparelli EV. Model appropriateness and population pharmacokinetic modeling. J Clin Pharmacol. 2003;43:610–23. [PubMed] [Google Scholar]
- 23.Parke J, Holford NH, Charles BG. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed. 1999;59:19–29. doi: 10.1016/s0169-2607(98)00098-4. [DOI] [PubMed] [Google Scholar]
- 24.Post TM, Freijer JI, Ploeger BA, Danhof M. Extensions to the visual predictive check to facilitate model performance evaluation. J Pharmacokinet Pharmacodyn. 2008;35:185–202. doi: 10.1007/s10928-007-9081-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wickenhauser C, Lorenzen J, Thiele J, Hillienhof A, Jungheim K, Schmitz B, Hansmann ML, Fischer R. Secretion of cytokines (interleukins-1 alpha, -3, and -6 granulocyte-macrophage colony-stimulating factor) by normal human bone marrow megakaryocytes. Blood. 1995;85:685–91. [PubMed] [Google Scholar]
- 26.Suwa T, Hogg JC, English D, Van Eeden SF. Interleukin-6 induces demargination of intravascular neutrophils and shortens their transit in marrow. Am J Physiol Heart Circ Physiol. 2000;279:H2954–60. doi: 10.1152/ajpheart.2000.279.6.H2954. [DOI] [PubMed] [Google Scholar]
- 27.Emilie D, Wijdenes J, Gisselbrecht C, Jarrousse B, Billaud E, Blay JY, Gabarre J, Gaillard JP, Brochier J, Raphael M, Boue F, Galanaud P. Administration of an anti-interleukin-6 monoclonal antibody to patients with acquired immunodeficiency syndrome and lymphoma: effect on lymphoma growth and on B clinical symptoms. Blood. 1994;84:2472–9. [PubMed] [Google Scholar]
- 28.Nishimoto N, Sasai M, Shima Y, Nakagawa M, Matsumoto T, Shirai T, Kishimoto T, Yoshizaki K. Improvement in Castleman's disease by humanized anti-interleukin-6 receptor antibody therapy. Blood. 2000;95:56–61. [PubMed] [Google Scholar]
- 29.van Zaanen HC, Lokhorst HM, Aarden LA, Rensink HJ, Warnaar SO, van der Lelie J, van Oers MH. Chimaeric anti-interleukin 6 monoclonal antibodies in the treatment of advanced multiple myeloma: a phase I dose-escalating study. Br J Haematol. 1998;102:783–90. doi: 10.1046/j.1365-2141.1998.00835.x. [DOI] [PubMed] [Google Scholar]
- 30.Frey N, Grange S, Woodworth T. Population pharmacokinetic analysis of tocilizumab in patients with rheumatoid arthritis. J Clin Pharmacol. 2010;50:754–66. doi: 10.1177/0091270009350623. [DOI] [PubMed] [Google Scholar]
- 31.Tabrizi MA, Tseng CM, Roskos LK. Elimination mechanisms of therapeutic monoclonal antibodies. Drug Discov Today. 2006;11:81–8. doi: 10.1016/S1359-6446(05)03638-X. [DOI] [PubMed] [Google Scholar]
- 32.Xu Z, Seitz K, Fasanmade A, Ford J, Williamson P, Xu W, Davis HM, Zhou H. Population pharmacokinetics of infliximab in patients with ankylosing spondylitis. J Clin Pharmacol. 2008;48:681–95. doi: 10.1177/0091270008316886. [DOI] [PubMed] [Google Scholar]
- 33.Zhou H, Jang H, Fleischmann RM, Bouman-Thio E, Xu Z, Marini JC, Pendley C, Jiao Q, Shankar G, Marciniak SJ, Cohen SB, Rahman MU, Baker D, Mascelli MA, Davis HM, Everitt DE. Pharmacokinetics and safety of golimumab, a fully human anti-TNF-alpha monoclonal antibody, in subjects with rheumatoid arthritis. J Clin Pharmacol. 2007;47:383–96. doi: 10.1177/0091270006298188. [DOI] [PubMed] [Google Scholar]
- 34.Ng CM, Bruno R, Combs D, Davies B. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol. 2005;45:792–801. doi: 10.1177/0091270005277075. [DOI] [PubMed] [Google Scholar]
- 35.Ng CM, Lum BL, Gimenez V, Kelsey S, Allison D. Rationale for fixed dosing of pertuzumab in cancer patients based on population pharmacokinetic analysis. Pharm Res. 2006;23:1275–84. doi: 10.1007/s11095-006-0205-x. [DOI] [PubMed] [Google Scholar]
- 36.Williams RC, Harmon ME, Burlingame R, Du Clos TW. Studies of serum C-reactive protein in systemic lupus erythematosus. J Rheumatol. 2005;32:454–61. [PubMed] [Google Scholar]


