Abstract
AIMS
To determine the safety and tolerability of a novel selective CXCR2 antagonist and assess its pharmacodynamic effects using measures of neutrophil activation and function, including CD11b expression in whole blood and ozone-induced airway inflammation in healthy subjects.
METHODS
Flow cytometric determination of ex vivo CXCL1-induced CD11b expression on peripheral blood neutrophils was performed following single dose oral administration of SB-656933 (dose range 2–1100 mg). A subsequent randomized study (placebo, 50 mg and 150 mg) was performed to explore the dose–response for ozone-induced airway inflammation, as measured by sputum biomarkers.
RESULTS
Oral administration of SB-656933 resulted in significant inhibition of CXCL1-induced CD11b expression on peripheral blood neutrophils at single doses greater than or equal to 50 mg. Maximum inhibition (70%) relative to placebo was observed following administration of SB-656933 400 mg (95% CI 60%, 77%). This was sustained up to a dose of 1100 mg. Single doses of SB-656933 reduced ozone-induced airway inflammation in a dose-dependent manner. Relative to placebo, there were 55% (95% CI 20%, 75%) and 74% (95% CI 55%, 85%) fewer neutrophils in the sputum of subjects after a single dose of 50 mg or 150 mg, respectively. There was a corresponding reduction in myeloperoxidase concentrations in the sputum supernatant of 32.8% (95% CI 9.2, 50.3) and 50.5% (95% CI 33.3, 63.3). SB-656933 was safe and well-tolerated at all doses.
CONCLUSIONS
SB-656933 is a CXCR2 antagonist that demonstrates dose-dependent effects on neutrophil activation and recruitment within a well-tolerated dose range. These data suggest that SB-656933 may be an effective agent in neutrophil-predominant diseases.
Keywords: CD11b, chemokine, CXCR2, lung, neutrophils, ozone
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Receptor antagonists that block the binding of chemokines such as CXCL8 (IL-8) are effective in animals models of neutrophil-mediated inflammation.
It has been hypothesized that selective inhibition of neutrophil trafficking and activation may be a useful adjunct for the treatment of inflammatory airway diseases such as chronic obstructive pulmonary disease or cystic fibrosis. A CXCR1/2 receptor antagonist has shown activity in an ozone challenge model in humans.
WHAT THIS STUDY ADDS
SB-656933, a selective CXCR2 antagonist, is safe and well-tolerated at single doses and is shown to inhibit agonist (CXCL1)-mediated expression of the CD11b on peripheral blood neutrophils as well as ozone-induced airway neutrophilia in healthy subjects.
Introduction
Neutrophils are key effector cells in a number of acute and chronic respiratory diseases including chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), acute lung injury and bronchiolitis obliterans syndrome [1, 2]. During a normal immune response, neutrophils are the first haematopoietic cells that migrate into inflamed or infected tissues, in order to eliminate invading micro-organisms (e.g. viruses and bacteria). In response to chemokine signals generated by resident cells at the site of injury, neutrophils up-regulate expression of the cell surface integrin CD11b that subsequently binds to the adhesion molecule ICAM-1 on endothelial cells, enabling neutrophil transmigration through the endothelial cell lining into the underlying parenchyma [3]. Recruitment of neutrophils to sites of inflammation, if followed by inadequate removal of them from the injured site, results in the subsequent and persistent release of a number of inflammatory mediators and proteinases from neutrophils, including neutrophil elastase and matrix metalloproteinases that contribute to the progressive fibrosis, airway stenosis and destruction of the lung parenchyma.
The chemokine receptor CXCR2 is a member of the G protein-coupled receptor superfamily and is expressed on the surface of neutrophils in mammals [4]. In humans, CXCR2 mediates neutrophil chemotaxis in response to tissue injury and many types of infections (reviewed in [5]). However, there are also pathways for neutrophil recruitment that are CXCR2-independent [6]. CXCR2 chemokine ligands, such as CXCL8 (IL-8) or CXCL5 (ENA-78), are elevated in bronchoalveolar lavage (BAL) fluid and sputum of COPD and CF patients [7, 8]. Other peptides, such as PGPα and LL-37, have also been shown to bind CXCR2 on neutrophils [9, 10] and may act as chemoattractants. Selective antagonism of the interaction between CXCR2 and its various ligands therefore provides a potential strategy for impacting neutrophil chemotaxis to sites of airway injury and hence reduces the underlying inflammation that contributes to the progression of COPD and other respiratory diseases [11]. In preclinical studies, administration of a selective CXCR2 antagonist was shown to inhibit CXCL1-induced neutrophil chemotaxis in vitro and LPS-induced airway neutrophilia in vivo[12]. Recent clinical studies have demonstrated that a potent antagonist of both CXCR1 and CXCR2 inhibits ozone-induced airway neutrophilia [13, 14].
SB-656933 is a novel, selective, competitive and reversible CXCR2 antagonist in development for the treatment of CF and COPD [15]. In preclinical studies, the compound was shown to inhibit CXCL1-induced CD11b up-regulation on PMNs in an in vitro whole blood assay and to be active in in vivo rodent inhalation challenge models of airway inflammation that used endotoxin and ozone to induce airway neutrophilia [16, 17]. This same assay (with minor modifications for a clinical setting) demonstrated that the compound inhibited neutrophil CD11b up-regulation in whole blood cells from patients with COPD [18], suggesting that the assay might be a useful adjunct for monitoring neutrophil activation in disease and may facilitate clinical dose selection for studies in patients.
In this report, we describe the results of two studies with SB-656933 designed to test the hypothesis that the CD11b pharmacodynamic assay provides a means to monitor the potential effects of SB-656933 on parameters of acute lung inflammation. These studies assessed the safety, pharmacokinetics and pharmacodynamics of single escalating doses of SB-656933 in healthy subjects during basal conditions (FTIH), as well as examined proof of mechanism (PoM) of CXCR2-selective antagonism on airway inflammation in healthy humans using an inhalation challenge model of ozone-induced airway inflammation. In particular, the CD11b data collected from the FTIH study were used to select the doses that were tested in the PoM study. Together, the two studies provide a comprehensive overview of the pharmacodynamics of SB-656933, using both whole blood CD11b expression and ozone challenge, and can be used to support dose selection for clinical efficacy studies.
Methods
First time in humans (FTIH) study (GSK Protocol CR2100595)
The FTIH study was designed as a randomized, single blind, placebo controlled dose escalation study and was conducted at a single centre in the United Kingdom between June–September 2004. Two cohorts consisting of a total of 20 healthy non-smoking male subjects between the ages of 18 and 50 years were randomized to receive single, escalating oral doses of SB-656933. Subjects in cohort 1 received doses of 2–150 mg and subjects in cohort 2 received doses of 150–1100 mg [2 mg (n = 10), 10 mg (n = 10), 50 mg (n = 10), 150 mg (n = 20), 400 mg (n = 10), 800 mg (n = 10), 1100 mg (n = 10)], as well as placebo (n = 18). A third cohort consisting of 12 healthy non-smoking male subjects between the ages of 18 and 50 years was randomized to receive placebo, 150 mg and 1100 mg SB-656933 in a three-way crossover study, designed to provide more comprehensive pharmacodynamic data.
Ozone challenge study (GSK Protocol CR2100597; NCT00551811)
The ozone challenge study was a randomized, double-blind, placebo controlled three-way crossover trial that was conducted at a single centre in Germany between October 2007 and July 2008. Subjects were required to have an FEV1 of ≥80% predicted and a documented response to ozone (>10 percentage point increase from baseline) in sputum neutrophils [19]. Twenty-four healthy non-smoking male subjects were randomized to receive a single dose of 50 mg, 150 mg or placebo 1 h prior to ozone challenge.
Subjects were exposed to 250 ppb ozone over a 3 h period in each of the challenge sessions (Figure 1). During exposure, subjects alternated between 15 min of light exercise on a bicycle ergometer and 15 min of rest. The minute ventilation (VE) was measured during the last 2 min of each exercise period and exercise intensity adjusted to maintain the target minute ventilation of approximately 12.5 l min−1m−2 BSA (∼25 l min−1). The dose of ozone and design of the ozone-exercise challenge selected for this study was based on previous studies demonstrating poor response at ozone exposures below 200 ppb, adequate response at 250–270 ppb and maximum responses at 400 ppb [19–23]. This protocol has been shown to induce reproducible mild transient neutrophilic airway inflammation that resolves back to pre-exposure levels by 24 h [20]. Induced sputum was collected at 6 h following the initiation of ozone challenge [24]. The sputum neutrophil response following ozone exposure for the two active treatment periods was compared with the response following placebo.
Figure 1.
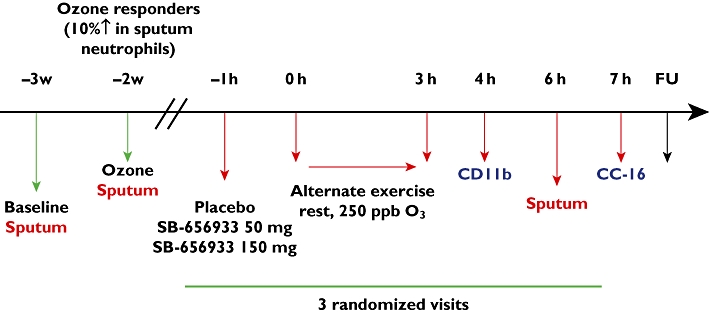
Study design for the single dose human healthy volunteer ozone challenge
Sputum induction and processing
Sputum induction and processing were performed as previously described [24]. Differential cell counts were performed on 400 non-squamous cells by two independent readers. Sputum supernatant was analyzed for total protein content (Pierce) and myeloperoxidase (MPO) (Immunodiagnostik) by ELISA [24–26], according to the manufacturer's instructions. Dithiothreitol was used as a reducing agent in the processing of the sputum samples but previous validation experiments have shown it to have negligible impact on the fluid phase components measured in this report. Results of the MPO assay were normalized to total protein in the sputum supernatant. Serum Clara cell protein-16 (CC-16) was analyzed by ELISA, according to the manufacturer's instructions (BioVendor).
Flow cytometric analysis of CD11b expression on neutrophils
CXCL1-induced CD11b expression on peripheral blood neutrophils was determined using a whole blood assay as previously described [18] with minor modifications for a clinical site setting at varying times post-dosing. Briefly, whole blood was incubated at 37°C for 10 min with either CXCl-1 or buffer. The samples were placed on ice and ice-cold fixative added. After 1 min, an aliquot was removed and incubated with CD11b FITC (Beckman Coulter) and CD16 PE (Dako). A control sample containing mouse IgG2a FITC (Beckman Coulter) and CD16 was also prepared. The tubes were gently mixed and left on ice in the dark for 20 min. After staining with antibody, the sample was added to cold DPBS (0.5 ml) containing LDS-751 solution (Cambridge Bioscience). The samples were mixed again and kept on ice in the dark for 10 min prior to analysis by flow cytometry (FACSCalibur, BD Biosciences).
Safety
Safety was monitored by the measurement of ECG, vital signs and assessment of adverse events (AEs). In addition, blood was collected for haematology and clinical chemistry determinations. In the ozone challenge study, pulmonary function tests (FVC, PEFR, FEV1) were collected at baseline and hourly between 0–8 h following the initiation of the ozone challenge.
Ethics
The study protocol, any amendments, the informed consent, and other information that required pre-approval were reviewed and approved by a national, regional or investigational centre ethics committee. Each study was conducted in accordance with good clinical practice (GCP) and all regulatory requirements, including, where applicable, those originating from the Declaration of Helsinki. All subjects provided written informed consent before treatment.
Dose selection for FTIH and ozone challenge
The pharmacokinetics (PK) of SB-656933 in humans were predicted by allometric scaling (data not shown). This information, combined with preclinical pharmacology and safety, informed the FTIH doses which reflected the exposure range between minimal anticipated biologically effective level (MABEL) and maximal no observed adverse effect level (NOAEL). On this basis a 2 mg dose was selected as the starting dose.
The dose selection for the single dose ozone challenge was made on the basis of safety, PK and PD data obtained from the FTIH study. CXCL1-induced CD11b inhibition data collected in the FTIH study were subject to population PK/PD modelling. Doses of 50 and 150 mg were subsequently selected to achieve CD11b expression levels corresponding to clear anti-neutrophil effects in pre-clinical animal models.
Bioanalytical method and pharmacokinetic assessment
Blood samples for the determination of SB-656933 plasma concentrations were taken in the FTIH study at pre-dose and at the nominal times of 10 min and 0.5, 1, 2, 3, 4, 6, 8, 12, 18, 24, 32, 48 and 72 h post-dose. Samples were collected into tubes containing EDTA and immediately chilled on crushed ice. Plasma was separated by centrifugation at approximately 4°C, at 1500 g for 10 min and transferred to polypropylene specimen containers. Plasma specimens were immediately frozen and stored at approximately −70°C (or colder). Plasma samples were analyzed for SB-656933 by GSK using a validated analytical method based on protein precipitation, followed by HPLC/MS/MS analysis. For the initial 2 mg dose group, 50 µl aliquots of EDTA human plasma were used. The lower limit of quantification (LLQ) for SB-656933 was 1 ng ml−1 and the upper limit of quantification (HLQ) was 1000 ng ml−1. For subsequent dose groups, the same method with a higher quantification range (10–10 000 ng ml−1) and 25 µl aliquots of EDTA human plasma was used.
Pharmacokinetic assessment of individual plasma SB-656933 concentration–time profiles was conducted by non-compartment analysis using the Model 200 (for extravascular administration) of WinNonlin Professional (Pharsight Corporation, Mountain View, CA). Actual elapsed time from dosing was used to estimate all individual plasma PK parameters. The maximum observed plasma concentration (Cmax) and the first time to reach Cmax (tmax), were the actual observed values. The terminal plasma elimination rate constant (λz) was estimated from log-linear regression analysis of the terminal phase of the plasma concentration–time profile. The associated apparent terminal elimination half-life (t1/2) was calculated as t1/2 = ln2/λz. The area under the plasma concentration–time curve from time zero extrapolated to infinity [AUC(0,∞)] was calculated by the linear up/logarithmic down trapezoidal method. The apparent oral clearance (CL/F) was calculated as Dose/AUC(0,∞). PK parameters were summarized by dose using descriptive statistics.
PK/PD analysis
Population PK/PD analysis of the FTIH study was performed using non-linear mixed effects modelling utilizing NONMEM V on a PC-based platform. A two-step sequential PK/PD methodology was employed. First, a compartmental pharmacokinetic model was fitted to the PK data in order to estimate concentrations for each subject at the time points of CD11b measurements. Second, a physiological, competitive inhibition pharmacodynamic model was fitted to the ex vivo stimulated whole blood CD11b expression data, accounting for relationship between stimulating ligand (CXCL1), drug concentration and CD11b expression.
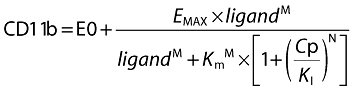 |
where CD11b is the absolute CD11b expression, E0 is the baseline or unstimulated level of CD11b expression, ligand is the concentration of CXCL, Cp is the plasma concentration of SB-656933, Emax is the maximum stimulated CD11b expression, Km is concentration of CXCL1 required to achieve half the maximum stimulated CD11b expression, KI is the inhibition constant for the inhibition of stimulated CD11b expression by SB-656933, M is the hill coefficient for CXCL1 concentration and N is the Hill coefficient for SB-656933 concentration.
Statistical analysis
For the FTIH study, the sample size was primarily based on balancing the need to obtain adequate safety data and logistical considerations, although the following statistical considerations were applied. It was planned that data from overlapping doses in cohorts I and II would be pooled resulting in a maximum of 32 subjects on the overlapping dose and would give at least 90% power to detect an inhibition of 50%, or more, on neutrophil CD11b surface expression. The lowest dose level in cohort II matched the highest dose level in cohort I.
For the ozone study, the planned sample size arose primarily from logistic feasibility and not statistical considerations. Estimates of the within subject variation for total sputum neutrophil count following challenge with inhaled ozone and intermittent exercise in healthy volunteers, relative to placebo were based on the data of Alexis et al. [25]. Based on the estimate of a within subject standard deviation of 0.65, with a sample size of 24 subjects, the lower and upper bounds of the 95% confidence interval for the total neutrophil count active vs. placebo was calculated to be 46.9% of the point estimate.
The primary endpoint, total number of neutrophils in induced sputum post-ozone challenge, was analyzed following a natural logarithmic transformation using a mixed effects model, with period and treatment fitted as fixed effects and subject as a random effect. The suitability of the transformation was assessed by examining the model residuals. Treatment effects were evaluated in terms of treatment ratios and were calculated as the anti-log for the differences between the LS means and 95% confidence intervals were determined using pooled estimates of variance for the LS means difference and then anti-logged. A similar mixed effects model was fitted for the untransformed percent neutrophils/ml post-ozone challenge. Adjusted means for each treatment group were calculated, along with corresponding 95% confidence intervals. The differences between each SB-656933 dose and placebo were calculated along with the corresponding 95% confidence intervals of these differences. Similar calculations were made for the analysis of total leucocytes, macrophages, lymphocytes and eosinophils.
The concentration of inflammatory mediators in sputum (ratio of MPO : total protein) and serum (CC-16) was analyzed following a loge transformation using a similar mixed effects model. The suitability of transformation of the data was assessed by examining the model residuals. Assumptions of normality and homoscedasticity were examined using residual plots. Where necessary, an appropriate transformation of the response variable was be used and the analysis was performed on the transformed scale.
The mean fluorescence signal for CD11b surface expression on neutrophils following exposure to the 30 nm CXCL1 was adjusted to baseline levels to account for variation in sampling processing and assay sensitivity. The adjusted endpoint was analyzed following a loge transformation. Subject and period baselines, period, treatment, time, baseline by time and treatment × time interactions were fitted as fixed effects, with subject as a random effect and time as a repeated effect. Adjusted geometric means for each treatment and ratios in adjusted geometric means for comparisons of each dose vs. placebo were determined. Assumptions of normality and homoscedasticity were examined using residual plots.
Results
Subject disposition
Thirty-two subjects entered the FTIH study and received at least one dose of study medication. Two subjects withdrew from the study, one due to a protocol violation (failed drugs of abuse screen) and the other because he was unable to attend the second study session.
One hundred forty-eight subjects were screened to enrol 24 in the ozone challenge study. There was a high screen failure rate due to either inability to produce sputum or non-responsiveness to ozone. All subjects completed all assessments and there were no withdrawals.
The demographics and baseline characteristics for these subjects are shown in Table 1.
Table 1.
Demographics
| FTIH | Ozone challenge | |
|---|---|---|
| Mean (range) | Mean (range) | |
| Age (years) | 25.6 (19, 38) | 35.5 (27–50) |
| Sex (M/F) | 32/0 | 24/0 |
| Race (White/Asian/Other) | 30/1/1 | 22/0/2 |
| Height (cm) | 178.4 (168, 190) | 179.9 (169, 194) |
| Weight (kg) | 76.3 (56, 95) | 84.7 (66.2, 104.8) |
| BMI (kg m−2) | 23.9 (18.9, 28.7) | 26.1 (21.3, 29.8) |
| FEV1 (l) | 4.2 (1.9, 5.5) | 4.51 (3.63, 6.26) |
FEV1: forced expiratory volume in one second.
Pharmacokinetics
Plasma pharmacokinetic concentration–time profiles over 96 h for single doses 2–1100 mg are shown in Figure 2. Following ascending dose administration, systemic exposure to SB-656933 increased with dose. The concentration–time profiles depicted an apparent multi-exponential decline following the peak, with concentrations of SB-656933 measurable at all time points in the majority of subjects for each treatment (i.e. median values for SB-656933 were above the lower limit of quantitation (LLQ) up to 72 h), with the exception of 2 mg, where median concentrations were above the LLQ only up to 32h.
Figure 2.
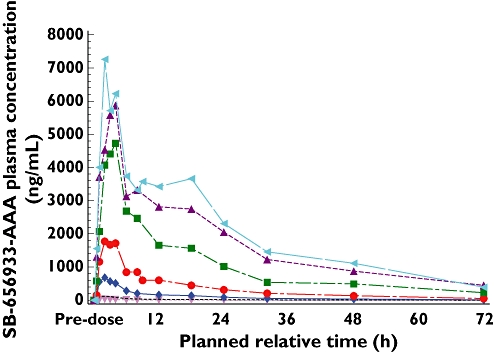
SB-656933 concentration–time profiles after administration of single doses in human healthy volunteers. 933 2 mg (▾); 933 10 mg (-------); 933 50 mg (♦); 933 150 mg (•); 933 400 mg (▪); 933 800 mg (▴); 933 1100 mg (◂)
The time to maximum concentration (tmax) was between 2–3 h for all doses ranging from 2 mg–1100 mg. Based on visual inspection of within-individual data (Figure 3), the maximum plasma drug concentration (Cmax) and area under curve (AUC(0,∞)) appeared dose proportional following single doses up to 400 mg, with less than dose proportional increases at higher doses. The t1/2 was approximately 14–20 h, and with the exception of the 2 mg data where low, variable exposure combined with assay limitations likely contributed to estimation of a shorter median apparent t1/2, t1/2 appeared independent of dose. These empirical observations suggested some absorption limitations of SB-6565933 with the tested formulation as the dose increased.
Figure 3.

Individual dose-normalized Cmax and AUC(0,∞) vs. dose following administration of single doses of SB-656933
CD11b surface expression on neutrophils
Human peripheral blood neutrophils express both CXCR1 and CXCR2 [27]. In order to assess neutrophil function in response to dose escalation of a CXCR2 antagonist, we measured CD11b expression induced by the CXCR2-specific ligand, CXCL1. Baseline expression of CD11b was low in all subjects; stimulation of cells with 30 nm CXCL1 resulted in an approximate three-fold induction in CD11b expression on neutrophils (data not shown). The dose–response for agonist-induced CD11b following dosing with SB-656933 is demonstrated in Figure 4. The plot shows the ratio of peak mean fluorescent signal (MFS) representing CXCL1-induced CD11b expression on neutrophils relative to placebo over a period of 0–9 h. A dose-dependent inhibition of CD11b was observed at doses up to 400 mg, at which point a maximum level of inhibition of approximately 70% (95% CI 60%, 77%) was achieved and sustained up to the dose level of 1100 mg.
Figure 4.
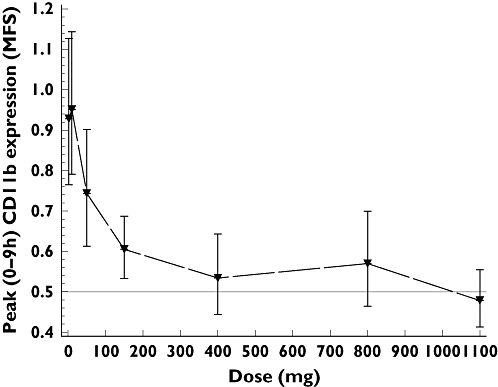
Peak CD11b expression (measured as mean fluorescence signal, MSF 0–9 h) relative to placebo adjusted for unstimulated control (±95% CI), on peripheral blood neutrophils following stimulation of whole blood with 30 nm CXCL1
The time course of inhibition of CD11b expression by SB-656933 is illustrated in Figure 5. For doses between 50 mg and 1100 mg, the level of CXCL1-induced CD11b expression declined between 0–6 h following dosing. An extended pharmacodynamic profile over 48 h was obtained in subjects receiving doses of 150 mg and 1100 mg, demonstrating sustained inhibition of CD11b expression for at least 24 h compared with placebo.
Figure 5.
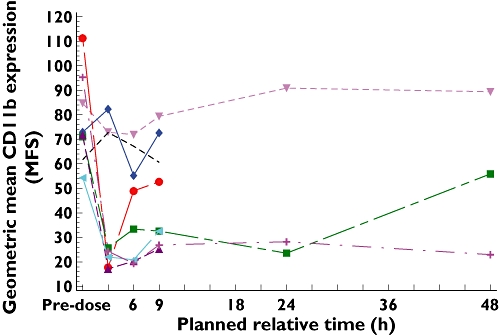
The period adjusted geometric mean for the change in CD11b expression (MSF) relative to control (30 nm–0 nm CXCL1) on peripheral blood neutrophils over time by treatment. Plocebo (▾); 933 2 mg (-------); 933 10 mg (♦); 933 50 mg (•); 933 150 mg (▪); 933 400 mg (▴); 933 800 mg (◂); 933 1100 mg (+)
PK/PD analysis
PK and CD11b expression data from all 32 subjects were included in the population PK/PD analysis. The model give a reasonable fit to the observed data and population PD parameters were estimated with reasonable precision (SEM <50% for all parameters). Based on pre-clinical rodent models of acute lung inflammation, a target range of 50–70% inhibition of CXCL1-induced CD11b expression was established, which corresponded to reductions in airway neutrophils of up to 75% relative to controls. Maximum inhibition of CD11b expression was not required to achieve maximal reductions in neutrophils in these models. Mean CD11b response in the ozone challenge study was predicted by simulation using the PK/PD model developed in the FTIH study. Based on these simulations, 50 mg and 150 mg were predicted to achieve peak population predicted CD11b expression inhibition close to 50–70% range established pre-clinically (Figure 6). For the 150 mg dose, inhibition was predicted to be maintained above the lower 50% range for the entire duration of the ozone challenge.
Figure 6.
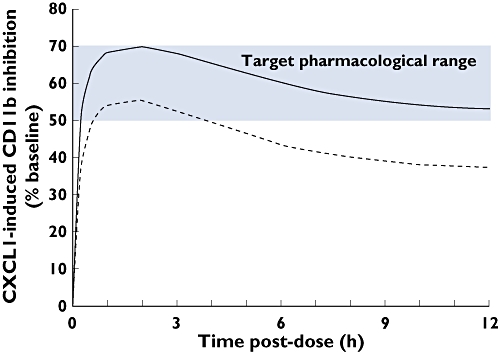
Time course of population predicted CXCl-1 induced CD11b inhibition, expressed as % baseline, for 30 nm concentration of CXCL1, simulated for proposed doses 50 mg (-------) and 150 mg (—) SB-656933 in the single dose ozone challenge study, overlaid with the target pharmacological range obtained from pre-clinical acute lung inflammation models
Reduction in ozone-induced airway inflammation
Sputum neutrophils
Having demonstrated that SB-656933 was active in an ex vivo assay, we then tested whether SB-656933 would be active in an in vivo ozone challenge model. Following ozone challenge, the adjusted geometric mean total number of neutrophils in induced sputum from subjects who received placebo was 74 (95% CI: 45.8, 118.8) × 104 cells ml−1 (Figure 7). The increase in sputum neutrophils following ozone challenge in patients receiving placebo was similar to that seen at baseline ozone screening and suggests that the subjects remained ozone responsive (data not shown).
Figure 7.
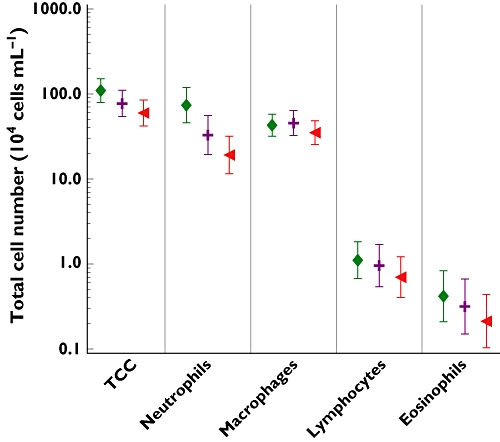
The adjusted geometric mean (±95% CI) for leucocyte counts in sputum following ozone challenge. Plocobo (♦); 933 50 mg (+); 933 150 mg (◂)
For subjects receiving single doses of SB-656933 50 mg and 150 mg, the mean total numbers of neutrophils were reduced to 33 × 104 cells ml−1 (95% CI 19.3, 55.7) and 19 × 104 cells ml−1 (95% CI 11.6, 31.8), respectively. Relative to placebo, estimates adjusted for period indicated that on average there were 55% (P = 0.0084) fewer neutrophils in the sputum of subjects after a single 50 mg dose of SB-656933 and 74% (P < 0.0001) fewer neutrophils in the sputum of subjects after a single 150 mg dose of SB-656933, 7 h post-dose. The corresponding reduction in CXCL1-induced CD11b expression on peripheral blood neutrophils 5 h post-dose was 42.8% (P = 0.0119) and 56.5% (P = 0.0002), respectively (Table 2).
Table 2.
Change from control of CXCL1-induced expression of CD11b on peripheral blood neutrophils following ozone challenge
| Treatment group | Adjusted geometric mean CD11b (30 nm CXCL1) MFS (95% CI) | Inhibition (%) relative to placebo (95% CI) |
|---|---|---|
| Placebo | 252.087 (186.742, 340.297) | |
| SB-656933 50 mg | 144.076 (102.843, 201.840) | 42.8 (12.1, 62.9) |
| SB-656933 150 mg | 109.710 (79.439, 151.516) | 56.5 (34.0, 71.3) |
CD11b MFS (30 nm CXCL1) has been adjusted for isotype and 0 nm CXCL1. MFS, mean fluorescent signal.
Similarly, the mean percentage of neutrophils in induced sputum following ozone challenge was 60.44% for subjects receiving placebo (Table 3). For subjects receiving single doses of SB-656933 50 mg and 150 mg, the mean percentage of neutrophils was 42.3% and 36.3%, respectively. This represents a difference of 18.10% (P < 0.0001) and 24.09% (P < 0.0001), compared with placebo.
Table 3.
Sputum cell differential following ozone challenge
| Treatment group | Adjusted mean (95% CI) | Difference relative to placebo (95% CI) |
|---|---|---|
| Neutrophils (%) | ||
| Placebo | 60.44 (52.21, 68.67) | – |
| SB-656933 50 mg | 42.34 (33.53, 51.15) | −18.10 (−26.23, −9.98) |
| SB-656933 150 mg | 36.35 (27.81, 44.89) | −24.09 (−31.51, −16.68) |
| Macrophages (%) | ||
| Placebo | 36.96 (29.22, 44.70) | – |
| SB-656933 50 mg | 52.59 (44.27, 60.92) | 15.63 (7.76, 23.51) |
| SB-656933 150 mg | 57.75 (49.70, 65.81) | 20.80 (13.60, 28.00) |
| Lymphocytes (%) | ||
| Placebo | 1.20 (0.70, 1.71) | – |
| SB-656933 50 mg | 1.31 (0.75, 1.87) | 0.11 (−0.52, 0.73) |
| SB-656933 150 mg | 1.71 (1.17, 2.25) | 0.50 (−0.08, 1.08) |
| Eosinophils (%) | ||
| Placebo | 0.30 (−0.70, 1.31) | – |
| SB-656933 50 mg | 0.65 (−0.45, 1.74) | 0.34 (−0.80, 1.48) |
| SB-656933 150 mg | 1.37 (0.31, 2.42) | 1.06 (0.02, 2.11) |
Other sputum leucocytes
The mean total number of leucocytes in induced sputum from subjects who received placebo was 109.5 × 104 cells ml−1 (95% CI 79.5, 150.7) (Figure 7). For subjects receiving single doses of SB-656933 50 mg and 150 mg, the mean total number of leucocytes was 77.1 × 104 cells ml−1 (95% CI 54.0, 110.0) and 59.8 × 104 cells ml−1 (95% CI 42.0, 85.0), respectively. There was a trend for a reduction in total leucocytes ml−1 following a single dose of SB-656933 50 mg, though this did not reach statistical significance. In contrast, a single dose of SB-656933 150 mg reduced sputum leucocytes by approximately 45% compared with placebo (P = 0.0022) (Table 3).
There was no difference in macrophage numbers between the active treatments and placebo (Figure 7). Conversely, analysis of the percent macrophages following ozone challenge demonstrated an increase in levels for both active treatments (SB-656933 50 mg and 150 mg) relative to placebo. Estimates indicated that on average there was a 15.6% increase relative to placebo (P = 0.0003) in the percentage of macrophages in the sputum of subjects following a single dose of SB-656933 50 mg and a 20.8% increase (P < 0.0001) in subjects receiving 150 mg (Table 3). There were no significant differences in the number or percent of eosinophils or lymphocytes in the sputum of subjects receiving active treatment compared with placebo.
Myeloperoxidase
Following ozone challenge, there was a significant decrease in the levels of sputum MPO following single doses of both SB-65693 50 mg and 150 mg. Relative to placebo, there was a 32.8% reduction in the sputum of subjects dosed with SB-656933 50 mg (P = 0.0109) and a 50.5% reduction in subjects dosed with SB-656933 150 mg (P < 0.0001) (Figure 8). In contrast, while ozone challenge significantly increased the expression of the airway-derived serum biomarker CC-16 in all groups, there was no effect on serum concentrations following single doses of SB-656933 (data not shown).
Figure 8.
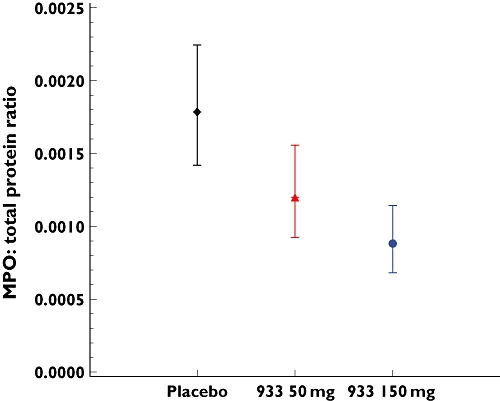
The adjusted geometric mean (±95% CI) for myeloperoxidase (MPO) in sputum supernatant following ozone challenge
Safety
For the FTIH study, SB-656933 was generally well tolerated when administered as a single dose (2–1100 mg) and the proportion of subjects with adverse events (AEs) was similar across all dose groups. All AEs were considered mild to moderate and no subjects discontinued because of AEs. The most frequently experienced AE was headache, reported in one subject each on placebo, 50 mg, 150 mg and 400 mg SB-656933. Three subjects receiving SB-656933 1100 mg reported headache.
For the ozone challenge study, SB-656933 was well tolerated and the numbers of clinical AEs reported across the three treatment sequences were similar. The most frequently experienced AE in all treatment sequences was headache, reported in four subjects on placebo and 150 mg SB-656933 and in six subjects receiving 50 mg SB-656933.
One subject was diagnosed with Type 1 diabetes mellitus following completion of the ozone challenge study; this event was reported as an SAE. Examination of blood samples taken at screening revealed the presence of antibodies against islet cells and glutamate decarboxylase. Given these results, the investigator deemed the diabetes mellitus as being unrelated to study medication.
A transient reduction in blood neutrophils was noted in some subjects dosed with ≥400 mg SB-656933 (Figure 9). The maximum effect was a mean reduction of approximately 50% observed 12 h following dosing, which was when the first sample was taken. Neutrophil counts recovered fully without additional treatment at 48 h after dosing in the 400 mg dose group and by 72 h in the 800 mg and 1100 mg groups. In the ozone study, four subjects were noted to have a neutrophil count below 2.05 GI l−1 after receiving a single dose of SB-656933 150 mg, though no subject was neutropenic. All counts returned to the normal range on repeat testing.
Figure 9.
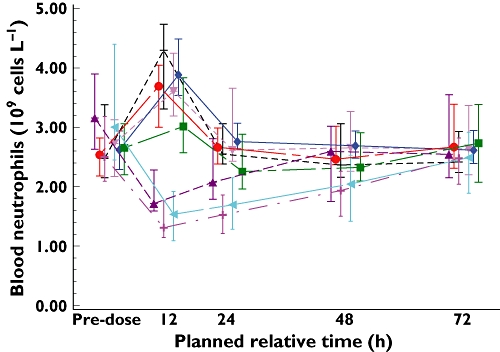
Median (IQR) peripheral blood neutrophil counts over time in the FTIH study over the dose range 2–1100 mg of SB-656933. IQR: interquartile range. Plocebo (▾); 933 2 mg (-------); 933 10 mg (♦); 933 50 mg (•); 933 150 mg (▪); 933 400 mg (▴); 933 800 mg (◂); 933 1100 mg (+)
Discussion
A challenge in the design of clinical trials is the need to demonstrate proof of mechanism in humans within an acceptable dose range, thereby selecting the most suitable compounds for further development. The clinical doses for SB-656933 were selected by linking inhibition of agonist-induced CD11b expression on peripheral blood cells to inhibition of airway neutrophilia in pre-clinical species, and thus demonstrating an acceptable therapeutic index. The CD11b assay was also implemented in the single dose escalation study in healthy subjects presented here, similar to the approach used in the testing of other chemokine antagonists [28, 29]. SB-656933 was then assessed in an ozone challenge model, where single doses of SB-656933 resulted in a dose-dependent inhibition of ozone-induced airways neutrophilia at a magnitude predicted by the ex vivo CD11b data. These results demonstrate how bridging between pre-clinical species and humans is critical for simplifying early clinical development of novel therapies.
The PK/PD modelling of the FTIH data effectively informed dose selection of the ozone-challenge study with confidence. The advantage of using a model-based PK/PD analysis, particularly using a mechanistic population approach to assess CD11b expression, was that all data were combined and integrated in the model, providing a more robust and certain interpolation and extrapolation across the dose range rather than using the raw data in isolation. This was especially useful with the type of crossover study conducted for the FTIH study, where the same subjects received different treatments on multiple occasions, and where pharmacodynamic measurements at low doses were variable. One limitation of the ozone challenge study was that pharmacokinetic parameters could not be determined by population analysis due to limited sampling, thus precluding assessment of a definitive PK–PD relationship.
In both studies, SB-656933 was well-tolerated. The observed fall in neutrophil counts after the higher doses of SB-656933 in the FTIH study must be assumed to be caused by the drug until proven otherwise, because of (i) the relationship with size of dose and (ii) the time course of recovery after dosing. The reduction in neutrophils is unlikely to have been due to bone marrow suppression. Although mature neutrophils are not long-lived, they are not so evanescent that cessation of production would cause their circulating numbers to fall by 50% within 12 h of drug administration. A more likely explanation is that SB-656933 caused a shift in distribution of neutrophils from the circulating pool to the marginated pool. Significant and more prolonged reductions in peripheral blood neutrophils had been seen with other CXCR1/CXCR2 antagonists [13, 30] with activity in the ozone challenge model. Initially, it was not clear whether this reduction in blood neutrophils was necessary or contributed to the pharmacological effect. Our own data demonstrating a reduction in blood neutrophils at doses of 400 mg and above, led us towards investigating lower doses of SB-656933 in the ozone challenge study. In addition, the PK/PD modelling results showed that doses below 400 mg would be sufficient to achieve the target pharmacology range informed by rodent models. The results of the current study clearly demonstrate that a reduction in peripheral blood neutrophils is not a requirement for inhibiting airway neutrophilia under challenge conditions for this mechanism.
A reduction in neutrophil recruitment and activation has several potential benefits in COPD and cystic fibrosis. Neutrophil secretory products such as elastases and matrix metalloproteinases are hypothesized to destroy lung tissue and promote mucus hypersecretion. In addition, elastase-mediated cleavage of CXCR1 is one proposed mechanism for the reduced bacteriocidal capacity of neutrophils in patients with CF [31]. However, neutrophil serine proteases such as cathepsin G and neutrophil elastase are also required to kill phagocytosed bacteria and fungi [32, 33]. Since patients with COPD have decreased macrophage phagocytic capacity, the ability to clear apoptotic neutrophils is reduced promoting a resting burden of neutrophils in the airways and neutrophil-mediated tissue damage [34]. Reduced neutrophil recruitment with CXCR2 therapy therefore, may allow macrophage phagocytic function to keep pace with the neutrophil burden in the airways of patients with COPD and CF. Recent data suggest that CXCR2 also mediates the formation of neutrophil extracellular traps (NETs), DNA fibres coated with antimicrobial proteins that serve an important function in host defense [35]. Excessive deposition of NETs in the airway of patients with cystic fibrosis correlated with poorer lung function. In a murine model of cystic fibrosis, treatment with a CXCR2 antagonist decreased NET formation and improved lung function [35].
SB-656933 may represent a novel therapeutic option for patients with cystic fibrosis or other neutrophil-predominant inflammatory respiratory diseases such as COPD. The key challenge for this mechanism will be the balance between inhibiting pro-inflammatory neutrophil functions and maintaining host defense effector functions, particularly in patients who may be chronically colonized with bacteria. Previous neutrophil inhibitors have failed in clinical trials in cystic fibrosis due to an increased risk of pneumonia and exacerbation [36]. Recent studies with a CXCR1/R2 antagonist have demonstrated almost complete inhibition of ozone-induced neutrophilia [13] and a significant reduction in airway neutrophils in patients with neutrophilic asthma [37] and COPD [38]. It has yet to be determined whether inhibition of neutrophilia in a challenge model such as ozone will translate into clinical benefits for patients with COPD or CF, or other neutrophil-predominant lung diseases. Encouraging data on ACQ scores have been obtained in severe asthma [37].
In summary, these studies demonstrate that the selective CXCR2 antagonist SB-656933 inhibits agonist-induced up-regulation of CD11b on peripheral blood neutrophils, an effect that correlates with inhibition of ozone-induced neutrophilia, at doses that are safe and well-tolerated. Large scale studies are needed to determine whether inhibition of neutrophilic inflammation will impact on long term outcomes in CF or COPD.
Acknowledgments
The authors would like to thank the staff of Hammersmith Medicines Research (London, UK), PAREXEL International GmbH (Berlin, Germany), and most importantly, the volunteers for their participation in these studies.
All studies were funded by GlaxoSmithKline.
Competing Interests
All authors, with the exception of Neil Alexis, are employees of GlaxoSmithKline and receive stocks and shares from the company. Neil Alexis receives consulting fees from GlaxoSmithKline.
REFERENCES
- 1.Quint JK, Wedzicha JA. The neutrophil in chronic obstructive pulmonary disease. J Allergy Clin Immunol. 2007;119:1065–71. doi: 10.1016/j.jaci.2006.12.640. [DOI] [PubMed] [Google Scholar]
- 2.Tirouvanziam R. Neutrophilic inflammation as a major determinant in the progression of cystic fibrosis. Drug News Perspect. 2006;19:609–14. doi: 10.1358/dnp.2006.19.10.1068008. [DOI] [PubMed] [Google Scholar]
- 3.Wang Q, Doerschuk CM, Mizgerd JP. Neutrophils in innate immunity. Semin Respir Crit Care Med. 2004;25:33–41. doi: 10.1055/s-2004-822303. [DOI] [PubMed] [Google Scholar]
- 4.Tsai HH, Frost E, To V, Robinson S, ffrench-Constant C, Geertman R, Ransohoff RM, Miller RH. The chemokine receptor CXCR2 controls positioning of oligodendrocyte precursors in developing spinal cord by arresting their migration. Cell. 2002;110:373–83. doi: 10.1016/s0092-8674(02)00838-3. [DOI] [PubMed] [Google Scholar]
- 5.Stillie R, Farooq SM, Gordon JR, Stadnyk AW. The functional significance behind expressing two IL-8 receptor types on PMN. J Leukoc Biol. 2009;86:529–43. doi: 10.1189/jlb.0208125. [DOI] [PubMed] [Google Scholar]
- 6.Ajuebor MN, Zagorski J, Kunkel SL, Strieter RM, Hogaboam CM. Contrasting roles for CXCR2 during experimental colitis. Exp Mol Path. 2004;76:1–8. doi: 10.1016/j.yexmp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Mackerness KJ, Jenkins GR, Bush A, Jose PJ. Characterisation of the range of neutrophil stimulating mediators in cystic fibrosis sputum. Thorax. 2008;63:614–20. doi: 10.1136/thx.2007.089359. [DOI] [PubMed] [Google Scholar]
- 8.Mukaida N, Harada A, Matsushima K. A novel leukocyte chemotactic and activating cytokine, interleukin-8 (IL-8) Canc Res. 1995;80:261–86. doi: 10.1007/978-1-4613-1241-3_10. [DOI] [PubMed] [Google Scholar]
- 9.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med. 2006;12:317–23. doi: 10.1038/nm1361. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Cherryholmes G, Chang F, Rose DM, Schraufstatter I, Shively JE. Evidence that cathelicidin peptide LL-37 may act as a functional ligand for CXCR2 on human neutrophils. Eur J Immonol. 2009;39:3181–94. doi: 10.1002/eji.200939496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chapman RW, Phillips JE, Hipkin RW, Curran AK, Lundell D, Fine JS. CXCR2 antagonists for the treatment of pulmonary disease. Pharmacol Ther. 2009;121:55–68. doi: 10.1016/j.pharmthera.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 12.Sarau HM, Widdowson KL, Palovich MR, White JR, Underwood DC, Griswold DE. Interleukin-8 receptor (CXCR2) antagonists. In: Hansel TT, Barnes PJ, editors. New Drugs for Asthma, Allergy and COPD: Progress in Respiratory Research. 31 edn. Basel: Kargel; 2001. pp. 293–6. [Google Scholar]
- 13.Holz O, Khalilieh S, Ludwig-Sengpiel A, Watz H, Stryszak P, Soni P, Tsai M, Sadeh J, Magnussen H. SCH527123, a novel CXCR2 antagonist, inhibits ozone-induced neutrophilia in healthy subjects. Eur Resp J. 2010;35:564–70. doi: 10.1183/09031936.00048509. [DOI] [PubMed] [Google Scholar]
- 14.Dwyer MP, Yu Y, Chao J, Aki C, Chao J, Biju P, Girijavallabhan V, Rindgen D, Bond R, Mayer-Ezel R, Jakway J, Hipkin RW, Fossetta J, Gonsiorek W, Bian H, Fan X, Terminelli C, Fine J, Lundell D, Merritt JR, Rokosz LL, Kaiser B, Li G, Wang W, Stauffer T, Ozgur L, Baldwin J, Taveras AG. Discovery of 2-Hydroxy-N,N-dimethyl-3-{2-[[(R)-1-(5- methylfuran-2-yl)propyl]amino]-3,4-dioxocyclobut-1-enylamino}benzamide (SCH 527123): a potent, orally bioavailable CXCR2/CXCR1 receptor antagonist. J Med Chem. 2006;49:7603–6. doi: 10.1021/jm0609622. [DOI] [PubMed] [Google Scholar]
- 15.Busch-Petersen J. Small molecule antagonists of the CXCR2 and CXCR1 chemokine receptors as therapeutic agents for the treatment of inflammatory diseases. Curr Top Med Chem. 2006;6:1345–52. doi: 10.2174/15680266106061345. [DOI] [PubMed] [Google Scholar]
- 16.Carpenter DC, Rumsey WL, Busch-Petersen J, Sarau HM, Salmon M. The selective CXCR2 receptor antagonist SB-656933 inhibits CXCL1-induced neutrophil CD11b expression in human whole blood. Eur Resp J. 2004;24:218s. [Google Scholar]
- 17.Salmon M, Carpenter DC, Dehaas C, Tal-Singer R, Sarau HM, Underwood DC. Inhibition of LPS-induced neutrophil recruitment in the lungs correlates with modulation of neutrophil CD11b expression using the selective CXCR2 receptor antagonist SB-656933. Eur Resp J. 2004;24:218s. [Google Scholar]
- 18.Nicholson GC, Tennant RC, Carpenter DC, Sarau HM, Kon OM, Barnes PJ, Salmon M, Vessey RS, Tal-Singer R, Hansel TT. A novel flow cytometric assay of human whole blood neutrophil and monocyte CD11b levels: upregulation by chemokines is related to receptor expression, comparison with neutrophil shape change, and effects of a chemokine receptor (CXCR2) antagonist. Pulm Pharm Ther. 2007;20:52–9. doi: 10.1016/j.pupt.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Holz O, Jorres RA, Timm P, Mucke M, Richter K, Koschyk S, Magnussen H. Ozone-induced airway inflammatory changes differ between individuals and are reproducible. Am J Respir Crit Care Med. 1999;159:776–84. doi: 10.1164/ajrccm.159.3.9806098. [DOI] [PubMed] [Google Scholar]
- 20.McDonnell WF, Horstman DH, Abdul-Salaam S, Raggio LJ, Green JA. The respiratory responses of subjects with allergic rhinitis to ozone exposure and their relationship to nonspecific airway reactivity. Toxicol Ind Health. 1987;4:507–17. doi: 10.1177/074823378700300405. [DOI] [PubMed] [Google Scholar]
- 21.Nightingale JA, Rogers DF, Barnes PJ. Effect of inhaled ozone on exhaled nitric oxide, pulmonary function, and induced sputum in normal and asthmatic subjects. Thorax. 1999;54:1061–9. doi: 10.1136/thx.54.12.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vagaggini B, Carnevali S, Macchioni P, Taccola M, Fornai E, Bacci E, Bartoli ML, Cianchetti S, Dente FL, Di Franco A, Giannini D, Paggiaro PL. Airway inflammatory response to ozone in subjects with different asthma severity. Eur Resp J. 1999;13:274–80. doi: 10.1034/j.1399-3003.1999.13b09.x. [DOI] [PubMed] [Google Scholar]
- 23.Vagaggini B, Taccola M, Conti I, Carnevali S, Cianchetti S, Bartoli ML, Bacci E, Dente FL, Di Franco A, Giannini D, Paggiaro PL. Budesonide reduces neutrophilic but not functional airway response to ozone in mild asthmatics. Am J Respir Crit Care Med. 2001;164:2172–6. doi: 10.1164/ajrccm.164.12.2009090. [DOI] [PubMed] [Google Scholar]
- 24.Schelegle ES, Siefkin AD, McDonald RJ. Time course of ozone-induced neutrophilia in normal humans. Am Rev Resp Dis. 1991;143:1353–135. doi: 10.1164/ajrccm/143.6.1353. [DOI] [PubMed] [Google Scholar]
- 25.Alexis NE, Lay JC, Haczku A, Gong H, Linn W, Hazucha MJ, Harris B, Tal-Singer R, Peden DB. Fluticasone propionate protects against ozone-induced airway inflammation and modified immune cell activation markers in healthy volunteers. Environ Health Perspect. 2008;116:799–805. doi: 10.1289/ehp.10981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holz O, Tal-Singer R, Kanniess F, Simpson KJ, Gibson A, Vessey RSJ, Janicki S, Magnussen H, Jörres RA, Richter K. Validation of the human ozone challenge model as a tool for assessing anti-inflammatory drugs in early development. J Clin Pharm. 2005;45:498–503. doi: 10.1177/0091270004273527. [DOI] [PubMed] [Google Scholar]
- 27.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Ann Rev Immunol. 2003;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 28.Liang M, Mallari C, Rosser M, Ng HP, May K, Monahan S, Bauman JG, Islam I, Ghannam A, Buckman B, Shaw K, Wei GP, Xu W, Zhao Z, Ho E, Shen J, Oanh H, Subramanyam B, Vergona R, Taub D, Dunning L, Harvey S, Snider RM, Hesselgesser J, Morrissey MM, Perez HD, Horuk R. Identification and characterization of a potent, selective, and orally active antagonist of the CC chemokine receptor-1. J Biol Chem. 2000;275:19000–8. doi: 10.1074/jbc.M001222200. [DOI] [PubMed] [Google Scholar]
- 29.Liston TE, Conklyn MJ, Houser J, Wilner KD, Johnson A, Apseloff G, Whitacre C, Showell HJ. Pharmacokinetics and pharmacodynamics of the leukotriene B4 receptor antagonist CP-105,696 in man following single oral administration. Br J Clin Pharmacol. 1998;45:115–21. doi: 10.1046/j.1365-2125.1998.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khalilieh S, Tsai M, de Vries D, Kraan M. Rising single-dose safety and pharmacokinetics of oral SCH 527123, a novel antagonist of CXCR2, in healthy volunteers. Eur Resp J. 2007;30:613S. [Google Scholar]
- 31.Hartl D, Latzin P, Hordijk P, Marcos V, Rudolph C, Woischnik M, Krauss-Etschmann S, Koller B, Reinhardt D, Roscher AA, Roos D, Griese M. Cleavage of CXCR1 on neutrophils disables bacterial killing in cystic fibrosis lung disease. Nat Med. 2007;13:1423–30. doi: 10.1038/nm1690. [DOI] [PubMed] [Google Scholar]
- 32.Belaaouaj A, McCarthy R, Baumann M, Gao Z, Ley TJ, Abraham SN, Shapiro SD. Mice lacking neutrophil elastase reveal impaired host defense against gram negative bacterial sepsis. Nat Med. 1998;4:615–8. doi: 10.1038/nm0598-615. [DOI] [PubMed] [Google Scholar]
- 33.Tkalcevic J, Novelli M, Phylactides M, Iredale JP, Segal AW, Roes J. Impaired immunity and enhanced resistance to endotoxin in the absence of neutrophil elastase and cathepsin G. Immunity. 2000;12:201–10. doi: 10.1016/s1074-7613(00)80173-9. [DOI] [PubMed] [Google Scholar]
- 34.Kirkham PA, Spooner G, Rahman I, Rossi AG. Macrophage phagocytosis of apoptotic neutrophils is compromised by matrix proteins modified by cigarette smoke and lipid peroxidation products. Biochem Biophys Res Comm. 2004;318:32–7. doi: 10.1016/j.bbrc.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Marcos V, Zhou Z, Yildirim AO, Bohla A, Hector A, Vitkov L, Wiedenbauer EM, Krautgartner WD, Stoiber W, Belohradsky BH, Rieber N, Kormann M, Koller B, Roscher A, Roos D, Griese M, Eickelberg O, Doring G, Mall MA, Hartl D. CXCR2 mediates NADPH oxidase-independent neutrophil extracellular trap formation in cystic fibrosis airway inflammation. Nat Med. 2010;16:1018–23. doi: 10.1038/nm.2209. [DOI] [PubMed] [Google Scholar]
- 36.Konstan MW, Doring G, Lands LC, Hilliard KA, Koker P, Bhattacharya S, Staab A, Hamilton AL. Results of a Phase II clinical trial BIIL 248 BS (an LTB4 receptor antagonist)for the treatment of CF lung disease. Ped Pulm. 2005;40:125–6. [Google Scholar]
- 37.Gaga M, Nair PK, Hargreave F, Sadeh J, Chanez P. SCH527123, a novel treatment option for severe neutrophilic asthma. Am J Respir Crit Care Med. 2010;181:A6762. [Google Scholar]
- 38.Magnussen H, Holz O, Watz H, Sauer M, Khanskaya I, Gann L, Stryszak P, Staudinger H, Sadeh J. Safety and efficacy of SCH527123, a novel CXCR2 antagonist, in patients with COPD. Eur Resp J. 2010;36:38s. doi: 10.1183/09031936.00048509. [DOI] [PubMed] [Google Scholar]


