Abstract
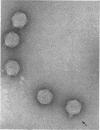
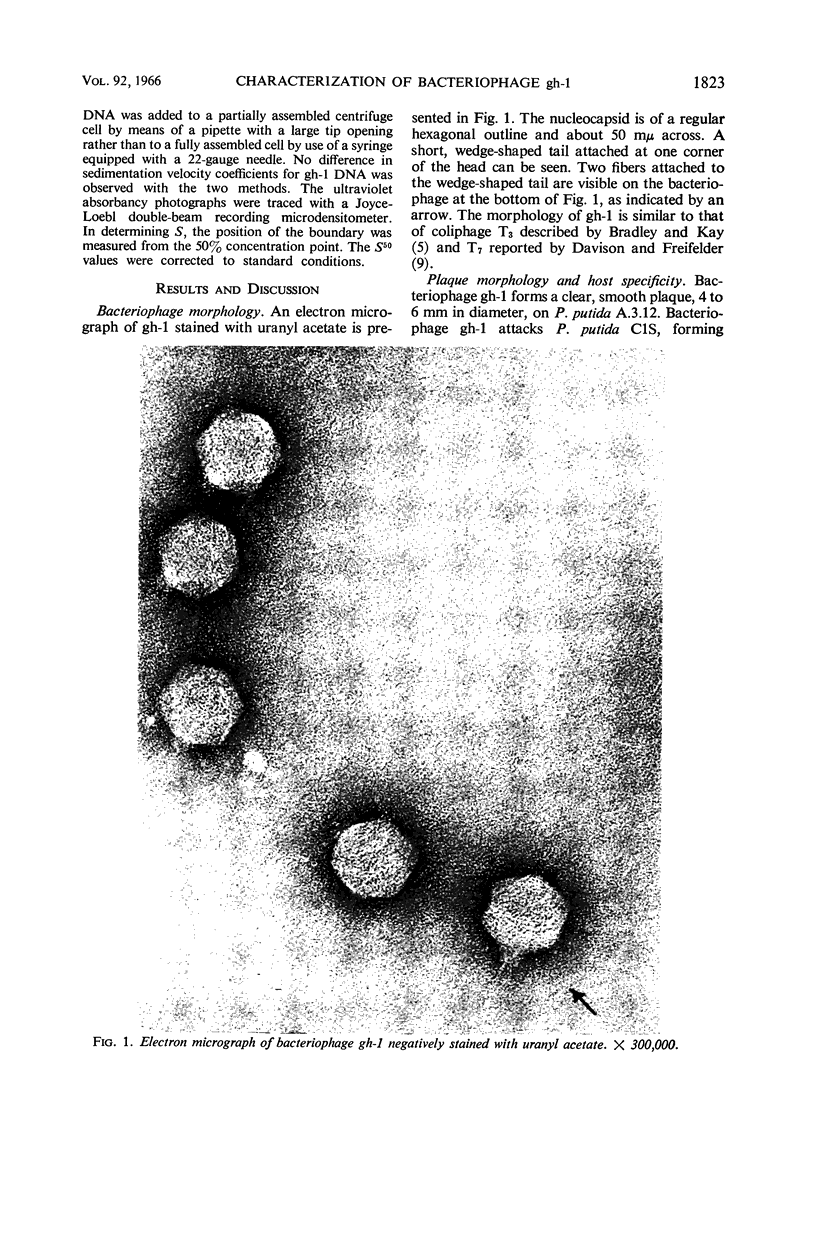
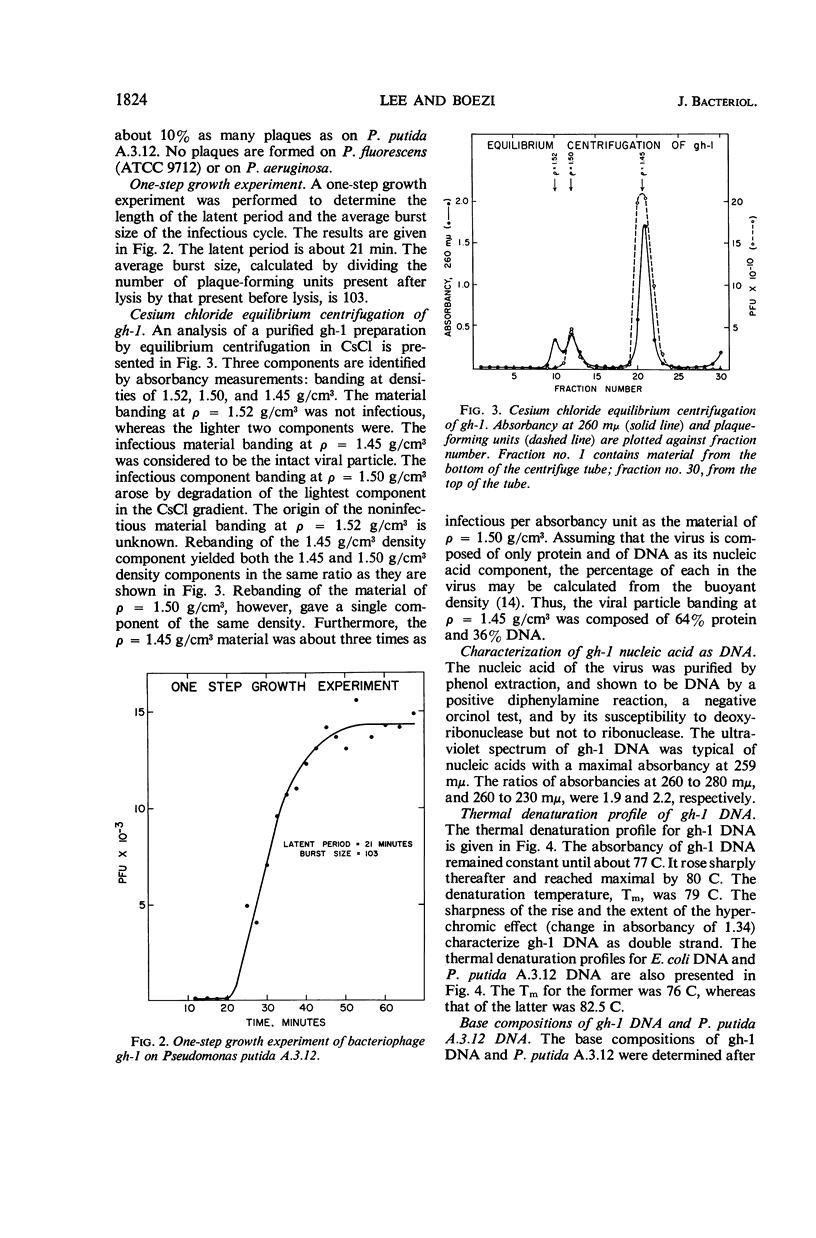
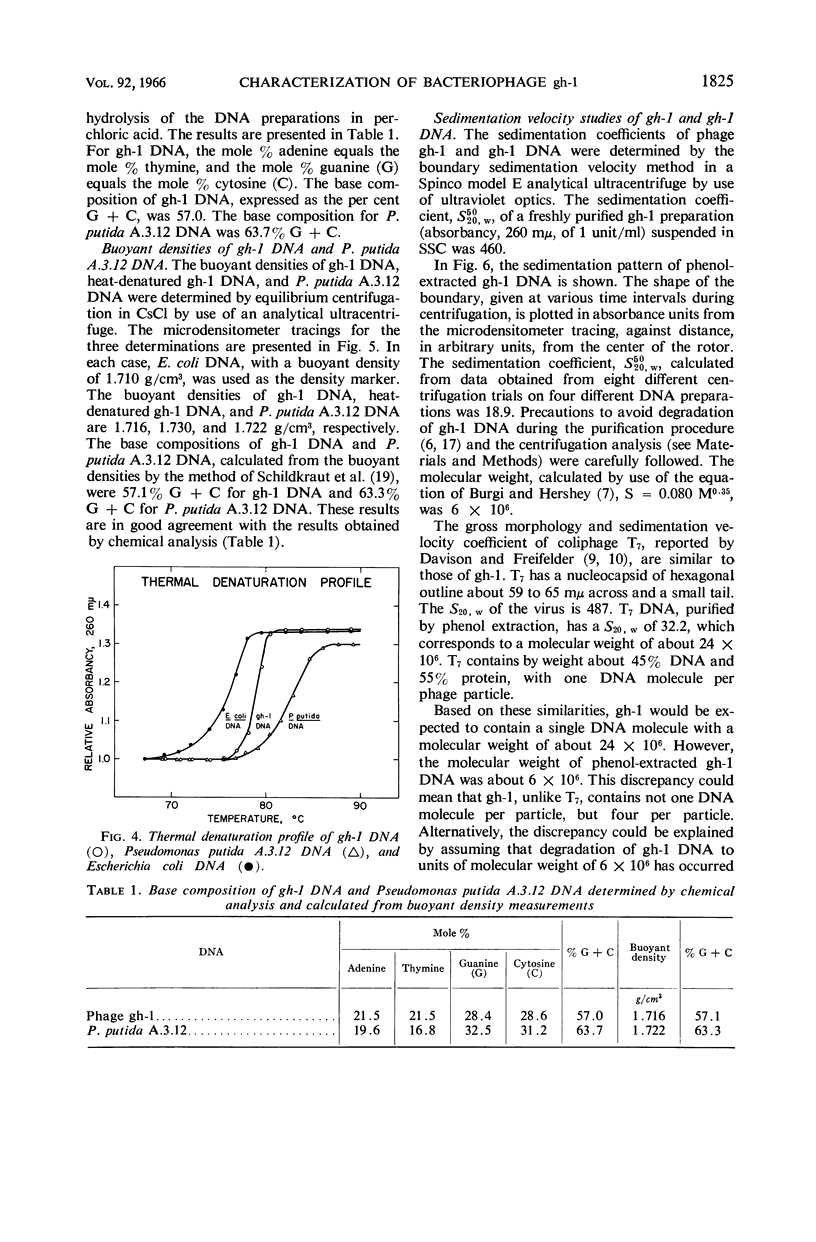
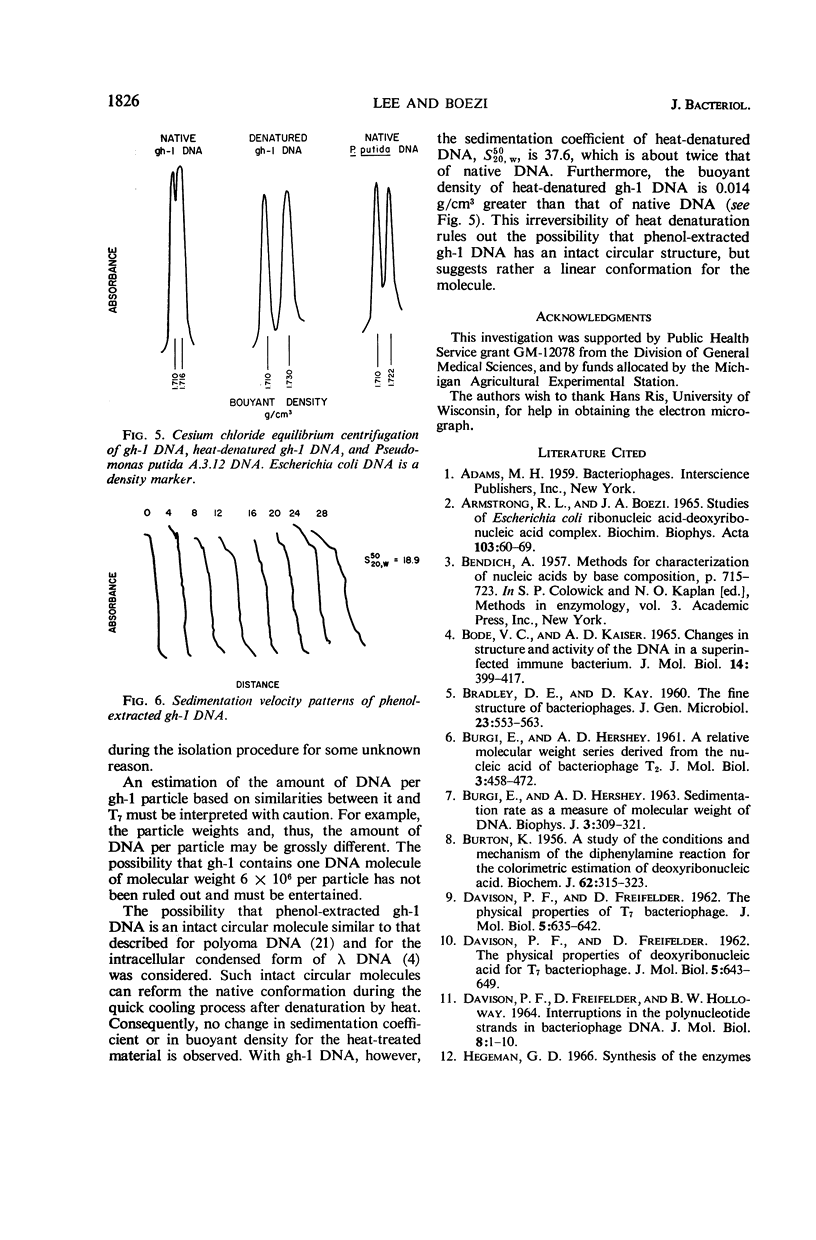
Lee, Lucy F. (Michigan State University, East Lansing), and J. A. Boezi. Characterization of bacteriophage gh-1 for Pseudomonas putida. J. Bacteriol. 92:1821–1827. 1966.—Bacteriophage gh-1 of Pseudomonas putida A.3.12 was isolated and purified by differential centrifugation and diethylaminoethyl (DEAE) cellulose chromatography. An electron micrograph of the phage stained with uranyl acetate revealed a regular hexagonal outline about 50 mμ across with a short wedge-shaped tail attached at one corner of the head. The phage formed 10% as many plaques on P. putida C1S as on P. putida A.3.12, the organism used in the isolation procedure. No plaques were formed on P. fluorescens (ATCC 9712) or P. aeruginosa. The latent period of the infectious cycle was 21 min, and the average burst size was 103. The nucleic acid component of gh-1 is double-stranded deoxyribonucleic acid (DNA), with a base composition of 57.0% guanine plus cytosine (G + C) as determined by chemical analysis. The per cent G + C of P. putida A.3.12 DNA measured in a similar manner was 63.7%. The buoyant density of phage gh-1 measured by cesium chloride equilibrium centrifugation was 1.45 g/cm3, whereas that of gh-1 DNA, heat-denatured gh-1 DNA, and P. putida A.3.12 DNA was 1.716, 1.730, and 1.722 g/cm3, respectively. The per cent G + C of gh-1 DNA and P. putida A.3.12 DNA calculated from the buoyant densities was 57.1 and 63.3%, respectively. The sedimentation coefficients, S5020,w, of gh-1 and the phenol-extracted gh-1 DNA, measured by the boundary sedimentation velocity method, were 460 and 18.9, respectively. The molecular weight of phenol-extracted gh-1 DNA, calculated by use of the equation of Burgi and Hershey, is 6 × 106.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARMSTRONG R. L., BOEZI J. A. STUDIES OF ESCHERICHIA COLI RIBONUCLEIC ACID-DEOXYRIBONUCLEIC ACID COMPLEX. Biochim Biophys Acta. 1965 May 11;103:60–69. doi: 10.1016/0005-2787(65)90541-1. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. A relative molecular weight series derived from the nucleic acid of bacteriophage T2. J Mol Biol. 1961 Aug;3:458–472. doi: 10.1016/s0022-2836(61)80058-2. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode V. C., Kaiser A. D. Changes in the structure and activity of lambda DNA in a superinfected immune bacterium. J Mol Biol. 1965 Dec;14(2):399–417. doi: 10.1016/s0022-2836(65)80190-5. [DOI] [PubMed] [Google Scholar]
- DAVISON P. F., FREIFELDER D., HOLLOWAY B. W. INTERRUPTIONS IN THE POLYNUCLEOTIDE STRANDS IN BACTERIOPHAGE DNA. J Mol Biol. 1964 Jan;8:1–10. doi: 10.1016/s0022-2836(64)80142-x. [DOI] [PubMed] [Google Scholar]
- DAVISON P. F., FREIFELDER D. The physical properties of T7 bacteriophage. J Mol Biol. 1962 Dec;5:635–642. doi: 10.1016/s0022-2836(62)80091-6. [DOI] [PubMed] [Google Scholar]
- DAVISON P. F., FREIFELDER D. The physical properties of the deoxyribonucleic acid from T7 bacteriophage. J Mol Biol. 1962 Dec;5:643–649. doi: 10.1016/s0022-2836(62)80092-8. [DOI] [PubMed] [Google Scholar]
- HOLLOWAY B. W., MONK M., HODGINS L., FARGIE B. Effects of radiation on transduction on Pseudomonas aeruginosa. Virology. 1962 Sep;18:89–94. doi: 10.1016/0042-6822(62)90180-0. [DOI] [PubMed] [Google Scholar]
- HUXLEY H. E., ZUBAY G. Preferential staining of nucleic acid-containing structures for electron microscopy. J Biophys Biochem Cytol. 1961 Nov;11:273–296. doi: 10.1083/jcb.11.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R. C., Bonner J. Histone-bound RNA, a component of native nucleohistone. Proc Natl Acad Sci U S A. 1965 Sep;54(3):960–967. doi: 10.1073/pnas.54.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- SLAYTER H. S., HOLLOWAY B. W., HALL C. E. THE STRUCTURE OF PSEUDOMONAS AERUGINOSA PHAGES B3, E79, AND F116. J Ultrastruct Res. 1964 Oct;11:274–281. doi: 10.1016/s0022-5320(64)90032-2. [DOI] [PubMed] [Google Scholar]
- WEIL R., VINOGRAD J. THE CYCLIC HELIX AND CYCLIC COIL FORMS OF POLYOMA VIRAL DNA. Proc Natl Acad Sci U S A. 1963 Oct;50:730–738. doi: 10.1073/pnas.50.4.730. [DOI] [PMC free article] [PubMed] [Google Scholar]