Abstract
AIM
To determine whether excessive IgE production by patients with atopic allergic asthma decreases with omalizumab therapy.
METHODS
Omalizumab, free and total IgE data were obtained from an epidemiological study and six randomized, double-blind, placebo-controlled trials in patients with allergic asthma. The binding between omalizumab and IgE together with the production and elimination of IgE were modelled as previously, except that, in order to explain why total IgE was decreasing over a period of 5 years, the expression of IgE was allowed to change.
RESULTS
The prior constant IgE production model failed to converge on the data once long-term observations were included, whereas models allowing IgE production to decrease fitted. A feedback model indicated that, on average, IgE production decreased by 54% per year. This model was further developed with covariate searches indicating clinically small but statistically significant effects of age, gender, body mass index and race on some parameters. Model predictions were checked internally and externally against 3–5 year data from paediatric and adult atopic asthmatic patients and externally against extensive total IgE data from a long-duration (>1 year) phase 1 study which was not used in the model building.
CONCLUSIONS
A pharmacokinetic–pharmacodynamic model incorporating omalizumab–IgE binding and feedback for control of IgE production indicates that omalizumab reduces production of IgE. This raises the possibility that indefinite treatment may not be required, only for perhaps a few years. After the initial accumulation, total IgE should provide a means to monitor IgE production and guide individual treatment decisions.
Keywords: asthma, IgE, omalizumab, pharmacodynamic, pharmacokinetic
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Omalizumab is a humanized anti-IgE monoclonal antibody that binds and captures circulating IgE, preventing interaction with receptors on mast cells and basophils, thereby interrupting the allergic cascade. It has a well-characterized efficacy and safety profile in patients with asthma. While omalizumab is known to reduce serum free IgE concentrations, effects on total IgE and IgE production are less well characterized.
WHAT THIS STUDY ADDS
(i) Confirmation of prior hypotheses that IgE production can decrease with time when patients are given anti-IgE therapy; (ii) guidance on a biomarker, total IgE, which can be used to ascertain whether individual patients experience a change in their IgE production; and (iii) a way to assess whether patients' IgE production has been sufficiently down-regulated such that they may consider stopping anti-IgE therapy.
Introduction
Immunoglobulin E (IgE) is the central mediator driving the allergic inflammatory cascade in patients with allergic (IgE-mediated) asthma [1, 2] and provides an attractive target for new treatment modalities. Omalizumab is a humanized anti-IgE monoclonal antibody that comprises a human immunoglobulin G (IgG) framework onto which is grafted the complementarity-determining region from a murine anti-IgE antibody [3–5]. The efficacy and tolerability of omalizumab has been established in clinical trials in patients with moderate to severe and severe persistent allergic asthma [5–8]. Omalizumab binds IgE, rapidly suppressing free IgE concentrations [7], preventing it from interacting with the high-affinity IgE receptor (FcεRI) on mast cells and basophils, thereby interrupting the allergic cascade [3, 4, 7].
Omalizumab has also been shown to down-regulate the expression of the high-affinity IgE receptor (FcεRI) on a variety of inflammatory cells, including basophils, mast and dendritic cells [9–12]. It has also been shown to cause concentration-dependent attenuation (by 30–75%) of the increase in low-affinity IgE receptor (FcεRII) expression in human bronchial smooth muscle cells exposed to atopic serum in vitro[13]. FcεRII plays a pivotal role in IgE homeostasis [14]. Changes in FcεRII expression on B cells in individuals with grass pollen allergy are closely related to changes in symptoms and medication requirement during the pollen season, indicating that FcεRII expression varies over time and affects B-cell reactivity [15]. The observation that omalizumab down-regulates expression of FcεRII and the established relationship between FcεRII and IgE production suggests one possible way in which treatment with omalizumab might reduce IgE production. Binding of anti-IgE antibodies to cell surface membrane IgE on B cells can alter B-cell function leading to changes in IgE production in vitro[16]. In vivo, down-regulation was demonstrated using the chimeric monoclonal anti-IgE CGP 51901, which reduced both circulating IgE and IgE expressing cells in a mouse model system [17].
The impact of long-term treatment with omalizumab on the IgE system is a key unanswered question. There is indirect evidence that omalizumab might modify the underlying IgE pathophysiology, based on observations of asthma symptoms after cessation of long-term therapy. In a study of patients who stopped treatment after approximately 6 years, 13 of 18 patients with allergic asthma had improved or remained stable 6–18 months after stopping omalizumab [18]. These benefits were sustained 3 years after cessation of omalizumab [19]. These findings may be explained by persistent down-regulation of basophil reactivity, although it should be noted that asthma symptoms have been shown to re-emerge after cessation of shorter-term treatment [20]. The current recommendation is that patients should continue to be treated at doses determined based on baseline total IgE and bodyweight using the dosing table included in the prescribing information [21]. However, some investigators have noted that after a period of time on the labelled regimen, the efficacy of omalizumab in allergic diseases such as asthma and urticaria could be maintained, even when extending the dosing interval beyond the original 2- or 4-weekly administrations [22]. This suggests that a fixed dosing rate may not be required in the long term.
Regarding the search for indicators or means to measure IgE production, in a study of 19 patients with allergic asthma, omalizumab significantly inhibited the stimulated release of IgE as well as reducing B-lymphocyte counts [23]. In another study of nine patients with severe asthma, treatment with omalizumab significantly reduced total IgE concentrations, which it was suggested might provide a tool for monitoring therapeutic responses and determining the appropriate dose of omalizumab [24]. However, the ADVIA Centaur-specific IgE assay used in that study binds to the same epitope as omalizumab and would therefore not measure omalizumab-bound IgE [21]. Consequently, the assay may have been measuring free rather than total IgE. In a study of paediatric patients, total IgE increased from baseline shortly after commencing therapy (as expected due to formation of more slowly eliminating omalizumab–IgE complexes), with a 431% increase over baseline at 16 weeks that decreased to 281% at 48 weeks, even though patients continued to receive omalizumab every 4 weeks [25]. Even larger relative changes in total IgE, 168% down to 74%, were noted among patients with higher baseline IgE who required omalizumab 375 mg every 2 weeks. Lanier suggested that omalizumab would be expected to eliminate or down-regulate IgE expressing lymphoblasts and memory cells resulting in decreases in total IgE that would only become apparent after several weeks to several months due to the longevity of IgE secreting cells [26].
The objective of this analysis was to ascertain whether the excessive production of IgE by patients with atopic allergic asthma remains constant over time, or decreases with omalizumab therapy. This was achieved using a model-based pharmacokinetic–pharmacodynamic analysis of omalizumab, free and total IgE, contrasting (i) a published model where IgE production was assumed to be constant [27–29]; and (ii) models where IgE production can change over time.
Methods
Study design and conduct
Data for the present analyses were obtained from clinical studies of omalizumab in patients with asthma, including long-term adult data from the EXCELS study (Q2948g) and children from study 10 and its extension (Table 1). EXCELS is an ongoing epidemiological study initiated in June 2004 to evaluate the clinical effectiveness and long-term safety of omalizumab in patients with moderate to severe asthma [30]. A substudy of EXCELS was designed to provide a longitudinal assessment of serum IgE concentrations in omalizumab- and non-omalizumab-treated patients. Data as of April 2009 were utilized in the analysis reported here. Study 10 was a phase III, 7-month double-blind, randomized, placebo-controlled trial [31] with a 5-month open-label extension period [25] to assess safety and efficacy of omalizumab in children (6–12 years) with allergic asthma requiring daily treatment with inhaled corticosteroids. The extension protocol (10E1) provided continued treatment with omalizumab for up to three further years [25].
Table 1.
Patient numbers and baseline demographic data for studies contributing to the population PK/PD model analysis
| Patient numbers | Demographic data, mean ± SD (range) | ||||||
|---|---|---|---|---|---|---|---|
| Study | Treatment | Active, placebo | Treated | Used in analysis | Age (years) | Bodyweight (kg) | Baseline IgE (ng ml−1) |
| 10 | 1 year, washout, 3-year extension then washout | A | 225 | 225 | 9 ± 2 (5–12) | 39 ± 13 (20–79) | 841 ± 645 (48–3 071) |
| P | 108 | 80 | 10 ± 2 (6–12) | 39 ± 14 (20–78) | 853 ± 710 (75–2 933) | ||
| 10E1* | A | 189 | 189 | 9 ± 2 (5–12) | 38 ± 14 (20–79) | 808 ± 624 (48–3 071) | |
| EXCELS† | Up to 5 years | A | 127 | 81 | 51 ± 14 (12–76) | 85 ± 21 (46–143) | 907 ± 2 281 (44–15 799) |
| IA05 | 1 year plus washout | A | 421 | 373 | 9 ± 2 (6–11) | 34 ± 11 (19–92) | 1 155 ± 846 (65–3 318) |
| P | 207 | 181 | 8 ± 2 (6–11) | 34 ± 12 (20–78) | 1 116 ± 795 (70–3 027) | ||
| 8 | 1 year | A | 268 | 268 | 39 ± 13 (12–73) | 80 ± 20 (39–150) | 417 ± 341 (48–2 081) |
| P | 257 | 257 | 39 ± 14 (12–74) | 78 ± 19 (39–136) | 451 ± 345 (51–1 699) | ||
| 9 | 1 year | A | 274 | 271 | 40 ± 15 (12–76) | 77 ± 17 (46–136) | 541 ± 411 (51–1 900) |
| P | 272 | 266 | 39 ± 14 (12–72) | 78 ± 18 (40–148) | 501 ± 391 (53–1 970) | ||
| 11 | 32 weeks | A | 175 | 144 | 43 ± 14 (12–73) | 76 ± 18 (41–135) | 578 ± 461 (63–2 553) |
| P | 164 | 130 | 43 ± 14 (12–74) | 74 ± 14 (41–115) | 613 ± 450 (46–1 902) | ||
| 2306 | 28 weeks plus washout | A | 245 | 226 | 42 ± 14 (12–79) | 79 ± 20 (45–148) | 509 ± 375 (51–1 692) |
| P | 232 | 214 | 43 ± 13 (14–74) | 77 ± 17 (39–143) | 479 ± 387 (53–2 173) | ||
| 2204 | Single dose, | A | 155 | 152 | 35 ± 12 (18–64) | 71 ± 12 (48–91) | 186 ± 124 (47–620) |
| 2101 | 12-week washout | A | 180 | 180 | 38 ± 13 (18–65) | 71 ± 10 (46–90) | 204 ± 114 (73–719) |
| Q0673g‡ | 47 weeks plus washout | A | 47 | 47 | 31 ± 8 (19–55) | 77 ± 13 (51–112) | 535 ± 249 (204–1 255) |
Study 10E1 was an extension to study 10 in which patients randomized initially to omalizumab or placebo received only omalizumab.
The epidemiologic study EXCELS had only a small subset of patients in which IgE measurements were taken. The numbers in the table reflect only the data set that was utilized at the time of analysis. Studies 2204 and 2101 were used only for the estimation of the absorption rate constant and volume for the omalizumab–IgE complex, as these parameters require rich sampling in the first week post dose.
Study Q0673g was not used in the model-building process.
In addition to EXCELS and study 10/10E1, the model-based analysis-included data from six other clinical trials, five of which were phase III, randomized, double-blind, placebo-controlled, parallel-group, multicentre trials. Studies 8 [7, 32] and 9 [5, 33] were both phase III studies with 7-month treatment periods and 5-month blinded extension periods that enrolled adolescents and adults with moderate to severe allergic asthma requiring daily treatment with inhaled corticosteroids. Study 11 was a phase III 32-week pilot study to assess the potential for corticosteroid reduction during omalizumab therapy in adolescents and adults with severe allergic asthma requiring daily treatment with high dose-inhaled corticosteroids, with or without oral corticosteroids [34]. Study IA05 was a phase III 1-year study in children (aged 6 to <12 years) with moderate to severe, persistent, inadequately controlled allergic asthma [35]. Study 2306 was a phase III 7-month study of patients with severe atopic (IgE mediated) allergic asthma [8]. Studies 2204 [36] and 2101 were single-dose, parallel-group investigations of omalizumab bioequivalence (150 and 300 mg s.c.) in healthy atopic volunteers with total IgE above normal concentrations (30–300 IU ml−1) at the screening visit. Q0673g was a phase I open-label study investigating the safety, tolerability, pharmacokinetics and IgE pharmacodynamics of high doses of omalizumab in 47 patients with perennial allergic rhinitis, with or without asthma. All studies were approved by Institutional Review Boards and all patients gave informed written consent. The studies were conducted in accordance with the declaration of Helsinki and Good Clinical Practice guidelines.
Analysis of omalizumab, total and free IgE
The methods used for analysis of omalizumab, total IgE and free IgE in serum samples have been reported previously [27–29]. The limits of quantitation and precision were 16 ng ml−1 and 4.9% CV (at the LOQ) for omalizumab. For free IgE the assay range was 0.78 ng ml−1 to 150 ng ml−1 with 10.1%CV at 1.51 ng ml−1 and for total IgE 2.4 ng ml−1 and 13.6% at 3.6 ng ml−1. Seventeen (0.2%) omalizumab samples were excluded from the analysis (13 being below the limit of quantitation). For free IgE, 272 (3%) of the samples were excluded from the analysis, six being below the lower limit of quantitation and 244 above the upper limit of quantitation. Ten (0.1%) total IgE samples were excluded from the analysis, eight being below the limit of quantification.
Data for model development
The data analysed were the omalizumab pharmacokinetic concentrations as well as the free and total IgE pharmacodynamic biomarker concentrations during and after treatment through the washout period. The numbers of patients included in the analysis and their baseline demographic data are summarized in Table 1. In general, patients were excluded from the analysis if there were missing data on baseline IgE, or no pharmacokinetic or IgE samples, without which the analysis could not be performed. Data were not imputed. In study 10, 28 patients were excluded from the placebo group because their free IgE concentration was at least threefold less compared with the concomitant total IgE value. In the EXCELS study, visits were scheduled about every 6 months. Subjects who reported at least two missed doses in any 6-month interval were excluded from the analysis, on the grounds that their dosing history could not be reconstructed with enough confidence. The phase I study Q0673g, which had extensive data collection over 1 year, was not used in model building, but was used to check the predictions of the model and how total IgE could be used to provide information on IgE production.
Omalizumab–IgE model
The basic non-linear model of omalizumab–IgE turnover and binding has been described previously [27, 28]. Briefly, the binding of omalizumab with IgE was written as a system of three differential equations, one for the subcutaneous administration site, one for total omalizumab (free plus complex) and another for total IgE (free plus complex). The equations, in terms of molar masses of omalizumab, IgE and the complex, with time expressed in days, were given by:
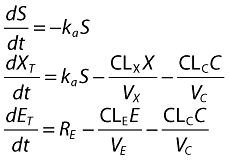 |
(1) |
where C (complex) is the solution to the quadratic for the equilibrium binding equation:
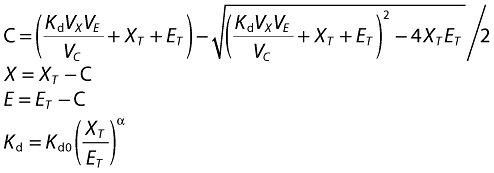 |
(2) |
and S is the amount of omalizumab in the subcutaneous site, XT and ET are molar masses of total omalizumab and IgE; X and E are free omalizumab and IgE, ka is the absorption rate constant, RE is the rate of production or expression of IgE, CLn and Vn are the clearances and volumes of free omalizumab, free IgE and the complex, Kd is the in vivo, apparent equilibrium binding constant and α is the change in the affinity of binding between omalizumab and IgE as a function of the molar ratio of total omalizumab to total IgE. Amounts in the above equations were converted to molar units using the molecular weights of omalizumab (150 kDa) and IgE (190 kDa). It is apparent from the above equation that the production of IgE was assumed to be constant over time, with a fixed rate RE.
The initial observations on the collected data from omalizumab, free and total IgE that prompted the search for time-dependent changes in IgE production was a slight misfit in a previously published model (e.g. see Figure 1 in Lowe et al. [28]) together with observations from paediatric study 10 that total IgE was decreasing after the initial increase due to the formation of complexes [25]. Further-more, following the patients from study 10E after drug washout, the total IgE values were, on average, lower than at baseline, indicating there to be less IgE in the system. A test was therefore constructed comparing two PK–IgE models, one specifying that IgE production did not change with time and the other allowing changes in turnover. The PK–IgE binding model had two parameters describing IgE turnover: production and clearance. The potential for these to change with time was explored using models where an IgE turnover parameter could change over time from a baseline value to a new state according to an exponential function:
Figure 1.
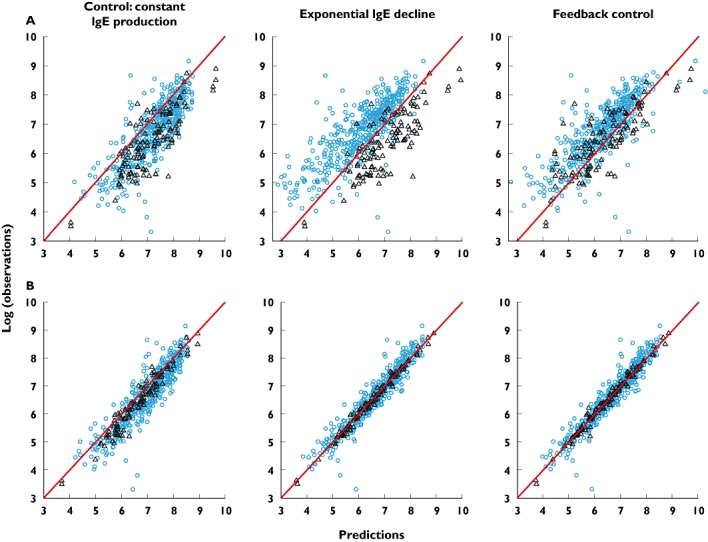
Comparison of individual predicted vs. observed for total IgE data for the competing models. The first row, A, contrasts observed total IgE and individual predictions from models based on data with up to 1 year of treatment. Row B contrasts observed total IgE and individual predictions from models based on data with up to 5 years of treatment. The comparisons are given for observations from the 3–5 year data only. The blue dots are data from paediatric study 10E1, the black triangles from the adult EXCELS study. The line of identity is red. Note that minimization for the control model in row B terminated with errors and should be regarded cautiously. 10E1 ( ); EXCELS (▵)
); EXCELS (▵)
| (3) |
where RB denotes the baseline IgE production rate, RN the IgE production rate after reaching new equilibrium and kE the rate of change over time in IgE production. All three parameters, RB, RN and kE, were allowed to vary randomly between patients, with kE, especially, able to be either positive or negative. This was important to enable the IgE production for placebo-control patients (kE,P) to change over time, either up or down. Parameter kE,P, for the placebo patients, was, effectively, a disease progression parameter, estimated separately from patients treated with omalizumab (kE,X). Initially, this model was run on an early data set consisting of phase III studies 8, 9 and 2306, plus bioequivalence data. The objective function was significantly lower, by 2147 points, for the time changing IgE production model compared with the control-fixed production model, with the residual error variance for total IgE decreasing from 25.0% to 21.1% CV. Alternative hypotheses were also investigated. If IgE clearance was allowed to increase, the objective function decreased by 2053 points and total IgE residual variance decreased to 21.6% CV. When changes in both IgE production and clearance were specified, the model became over-parameterized with a singularity in the R matrix and nonsensical values for some of the parameters, such as the projected steady-state IgE clearance (1.78 × 10−11 l day−1) and inter-individual variance in the rate of change of IgE clearance (0.14% when expressed as CV).
The second alternative was a feedback model. This assumed that IgE production is, effectively, under the control of free IgE, through the binding of IgE-allergen complexes to receptors. IgE production can therefore increase or decrease with time. This was implemented by applying a positive feedback such that free IgE controls IgE production through a modulating differential equation, which is shown below together with the updated differential equation for total IgE:
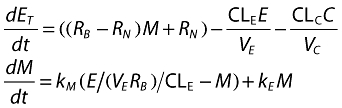 |
(4) |
where RB denotes the baseline IgE production rate prior to anti-IgE therapy, RN the IgE production rate after reaching new equilibrium, kE the background rate of change in IgE production, the disease progression parameter, to account for IgE changes in placebo treated patients. Finally, kM is the turnover rate of the modulator, accounting for physiological and biochemical delays between changes in free IgE concentrations and changes in IgE production. The three parameters RB, RN and kE were allowed to vary randomly between patients; when tested on kM, inter-individual variability was very small and not included in the model. In the final model, the value of kE (and associated inter-individual variability) was fixed following a separate fit of an exponential function to IgE data from patients receiving a matched placebo to omalizumab. Two parameters, the absorption rate constant, ka and volume of complex, VC, as well as their associated inter-individual variances, were fixed using improved first order conditional with interaction estimates obtained from a separate analysis of two richly sampled, single-dose bioequivalence studies, using the control (invariant IgE production) model.
The above models were compared using a fixed starting covariate adjustment based on previous modelling of the data. Covariates included age less than 12 years, bodyweight, body mass index (BMI), race (Caucasian, Black, Oriental and other), gender and baseline IgE concentration. The effect of covariates was multiplicative, i.e. for continuous covariates coefficients were exponents whereas for categorical covariates they were interpreted as ratios relative to the reference category. Continuous covariates were normalized relative to historical reference values: 70 kg for bodyweight, 365 ng ml−1 for baseline IgE and 20 kg m−2 for BMI. The starting inter-individual variability structure was also taken from the same previous model. Random effects characterizing inter-individual variability acted multiplicatively on selected model parameters through log-normal distributions. There were multiple criteria for model selection: (i) significant changes in the log-likelihood objective function; (ii) reduced in inter- and/or intra-individual variances; (iii) increased precision of the parameter estimates, whether they be structural or random effects (variances); (iv) diagnostic plots of predicted vs. observed, or time or predicted concentrations vs. residuals which were without overt bias; and, finally, (v) improved ability to predict the total IgE response in the 3–5 year period of time and for rich data from a phase I study q0673g. For the latter, predictions from the control (invariant production) and feedback models were created for total IgE samples from these patients, conditional upon the population parameters from the main analysis, using the post hoc procedure from NONMEM (setting MAXEVAL = 0 in the $EST statement). These were then compared with the original data as weighted residuals. Further covariate searches on the newly introduced parameters, as well as refinement of the interindividual variance structure, were undertaken after identification of the best model in the initial comparison. Random effects acted multiplicatively on the new parameters except for kE where an additive effect was assumed. The criterion for addition or removal of a covariate was a significant change in the objective function (P < 0.05, χ2-test with the appropriate degrees of freedom).
The final model from this analysis is provided as an electronic supplement available from the British Journal of Clinical Pharmacology website.
Software and settings
Estimation of population PK–PD parameters and their variances, followed by calculation of individual patient omalizumab, free and total IgE concentration–time predictions, were carried out using NONMEM (Version VI level 1.0) with the ADVAN6 subroutine. This utilized the Runge-Kutta integrator, for which a tolerance of five was specified. The first-order estimation method was used for the majority of the modelling, as the run time was long, of the order of 12 days with the final model, on a Sun Grid Engine computing environment. Prior experience with the first-order conditional estimation method suggested that this would require more than 90 days to run [28]. When random effects are not too large, as in this case, the first-order method performs reasonably well. NONMEM was run under UNIX (XL Fortran compiler 8.1.1). Data sets and descriptive tables were computed with SAS 8.2. NONMEM, output data sets were read by SAS 8.2 and graphics were created using R 2.8.1.
Results
Comparison of models – empirical exponential and semi-mechanistic feedback vs. control
To ascertain whether the production rate of IgE was decreasing with time, the exponential and feedback models of IgE production were compared with the control constant IgE production model. While the constant IgE production model could adequately fit the early (up to 1-year data) excluding studies 10E1 and EXCELS, it failed to converge on the full data set, demonstrating that the constant IgE production hypothesis was incapable of describing long-term data. The exponential and feedback models gave similar structural parameter values (Table 2), although some interpatient random effects, such as that on the projected IgE production at treatment equilibrium, were smaller (Table 3). There was increased precision of the estimates of several parameters with the feedback model (Tables 2, 3). Most importantly, the log-likelihood objective function values for the exponential and interim feedback models were highly significantly improved, being 4674 and 4755 points better than the control model (Table 3). The feedback model had the same number of parameters as the exponential model and was statistically significantly better, as judged by the log-likelihood objective function value (OFV) being 82 points lower. Interestingly, when the ability of the three models to predict 3–5 year observations using parameters estimated from data for treatment periods up to 1-year was evaluated, it was evident that the control model overpredicted the long-term concentrations of total IgE (Figure 1). The overprediction was countered with the exponential model, although the predictions were different for children vs. adults. The result was more balanced for the feedback model, although there may have been a tendency to overcorrection, the predicted total IgE being lower than observed (Figure 1A). When the model parameters were estimated with the full set of patient data with treatment up to 5 years, the overprediction of the control model was still present (although this should be regarded cautiously as NONMEM terminated with rounding errors) whereas the IgE down-regulation models gave good correspondence between predictions and observations (Figure 1B). Because of its mechanistic nature, potential post-treatment predictive properties and good fit to the data, the feedback model was retained for further evaluation.
Table 2.
Structural parameter estimates for the models
| Population mean, θ (%RSE) | ||||
|---|---|---|---|---|
| Omalizumab or IgE parameter* | Control, constant | Exponential | Feedback interim | Feedback final |
| Clearance omalizumab, CLX/F (l day−1) | 0.214 | 0.207 (2.4) | 0.207 (2.3) | 0.206 (2.1) |
| Clearance IgE, CLE/F (l day−1) | 3.21 | 2.83 (3.5) | 2.83 (3.6) | 2.87 (4.3) |
| Clearance complex, CLC/F (l day−1) | 0.717 | 0.538 (3.5) | 0.533 (3.4) | 0.535 (4.3) |
| Volume omalizumab and IgE, VX/F and VE/F (l) | 9.41 | 8.61 (1.5) | 8.61 (1.5) | 8.62 (1.4) |
| Volume complex, VC/F) (l) | 7.15 (1.8)** | |||
| Absorption rate, ka (day−1) | 0.446 (3.2)** | |||
| Binding dissociation constant, Kd (nm) | 1.93 | 2.11 (4.5) | 2.10 (4.4) | 2.15 (4.7) |
| Kd change with total omalizumab to total IgE ratio, α | 0.0945 | 0.0635 (19) | 0.0659 (18) | 0.0532 (24) |
| IgE production prior to omalizumab, RB (µg day−1) | 982 | 966 (3.6) | 965 (3.6) | 967 (4.4) |
| IgE production at new equilibrium, RN (µg day−1) | – | 188 (31) | 168 (17) | 253 (13) |
| Rate of change in IgE production with omalizumab, kM (% year−1) | – | 53.9 (12) | 58.5 (5.1) | 53.8 (8.4) |
| Background (placebo) rate of change in IgE production, kE (% year−1) | 0.197 ± 1.47*** | |||
These analyses used data from studies in Table 1 except study Q0673g. Note that minimization for the control (constant) model terminated with errors and should be regarded cautiously. RSE, relative standard errors; these are missing from the control model as it terminated with rounding errors and did not run the $COV procedure.
Depending on covariate adjustment for each parameter, values are given for a 70 kg, 20 kgm−2 BMI, Caucasian male aged >12 years with baseline IgE value of 365 ng ml−1.
Values for VC and ka were fixed from a separate first order conditional with interaction estimation using data from single-dose bioequivalence studies.
The background rate of change in IgE production was estimated independently from the placebo data and was fixed in the estimation process; the standard error is shown on the original scale.
Table 3.
Random effect (variance) parameter estimates for the models
| Interindividual random effects, ω, as %CV (%RSE) [Shrinkage*] | ||||
|---|---|---|---|---|
| Omalizumab or IgE parameter | Control, constant | Exponential | Feedback interim | Feedback final |
| Clearance omalizumab, CLX/F | 33% | 32% (11%) | 32% (11%) | 37% (11%) [17%] |
| Clearance IgE, CLE/F | 16% | 13% (45%) | 13% (46%) | 45% (31%) [58%] |
| Clearance complex, CLC/F | 23% | 19% (24%) | 19% (25%) | 36% (38%) [55%] |
| Volume omalizumab and IgE, VX/F and VE/F | 24% | 24% (22%) | 24% (22%) | 26% (23%) [28%] |
| Volume complex, VC/F | 21%† (13%) [76%] | |||
| Absorption rate, ka | 57%† (7.9%) [51%] | |||
| Binding dissociation constant, Kd | 12% | 17% (21%) | 17% (21%) | 23% (18%) [38%] |
| IgE production prior to omalizumab, RB | 32% | 29% (15%) | 30% (15%) | 56% (20%) [38%] |
| IgE production at new equilibrium, RN | – | 180% (54%) | 93% (27%) | 73% (21%) [63%] |
| Rate of change in IgE production with omalizumab‡ | – | 835 (31%) | No random effect | |
| Background rate of change in IgE production, kE‡ | – | 1050§ (12%) [44%] | ||
| Residual random effects, σ, as %CV (%RSE) [Shrinkage¶] | ||||
|---|---|---|---|---|
| Omalizumab | 36% | 37% (17%) | 37% (17%) | 36% (18%) [13%] |
| Total IgE | 34% | 27% (6.8%) | 28% (6.7%) | 27% (6.7%) [16%] |
| Free IgE | 37% | 34% (5.8%) | 35% (5.8%) | 35% (5.9%) [13%] |
| Objective function value | −18 178 | −22 847 | −22 927 | −23 446 |
| Terminated with rounding errors | −4 669 from control P < 0.001 for 4 d.f. | −4 748 from control P < 0.001 for 4 d.f. | −512 from feedback interim P < 0.001 for 14 d.f. | |
Note that minimization for the control model terminated with rounding errors and should be regarded cautiously. RSE, relative standard errors; these are missing from the control model as it did not run the $COV procedure. Note that for the final model a covariate search was conducted for patient factors that may affect the change in IgE production with time and that covariance was allowed between the parameters CLX, CLE, CLC, VX, RB and Kd.
Shrinkage in the post hoc ETA estimates (ηph) was calculated as 1 − SD(ηph)/√ω.
Values for VC and ka were fixed from a separate first order conditional with interaction estimation using data from single-dose bioequivalence studies.
Variance reported in original units rather than as CV =√(exp(ω) − 1) as an additive error model was specified.
Estimated independently from placebo data and fixed in the models.
Shrinkage in the residual error ε was calculated as 1 − SD (residual)/√σ. Subjects receiving placebo were discarded for PK-related parameters.
Covariates – patient demographics which affect the omalizumab–IgE turnover system
Given the extensive data set available, a search for patient demographic covariates confirmed that, as expected from prior work, bodyweight was a significant covariate for omalizumab clearance, volume and IgE production (Table 4). For clearance, body mass index (BMI) and race, although statistically significant, were minor in terms of the extent of their effects; the largest, a power of 0.25 for BMI, would only give a 19% increase in clearance for a doubling of this factor. Bodyweight and baseline IgE were the most important predictors of the rate at which IgE was produced with exponents of 0.91 and 0.73, respectively. Few others were noteworthy in terms of the extent of the effects. The omalizumab–IgE binding constant was largely unaffected by patient characteristics, save for the affinity of binding being slightly lower in children less than 12 years of age with a Kd higher by 20%. Gender and race did not appear to have any notable effects on the rate at which IgE production changed, although the Oriental and other races appeared to down-regulate IgE production to lower levels than Caucasian and black populations. Intriguing was that the rate at which IgE production was modulated, parameter kM, was 71% faster in children less than 12 years of age compared with adults, with bodyweight and baseline IgE having lesser effects (32% and 13% increases for doubling of these two factors, respectively). A model with a smooth transition with age would, however, be both more useful and physiologically more plausible. Two further models were tested to explore the age effect on kM. In one, a continuous (exponential) relationship starting from age 5 years then gradually transitioning to the adult value gave a worse fit than the dichotomous test (age discrete: OFV –23445.52; age exponential: OFV –23429.81, a difference of 15.71). In the second, a sigmoid hyperbolic function from a value for children transitioning smoothly to the adult value of kM was used. This gave an objective function value not significantly different from the dichotomous model (age discrete: OFV –23445.52; age hyperbolic: OFV –23446.98, a difference of 1.46). The parameters for the smooth transition model were such that kM would be the same for ages 5–11 years then rapidly transition (sigmoidicity, i.e. gamma or Hill coefficient, of 49.6) to the adult value at age 11.8 years; effectively, the hyperbolic function approximated a step function and supported discretization of age.
Table 4.
Covariate parameter estimates from the final feedback model
| Covariate | Estimate (± SEM) | Covariate | Estimate (± SEM) |
|---|---|---|---|
| Clearances | Binding constant, Kd | ||
| Bodyweight on CLX/F, CLE/F, CLC/F, RB | 0.914 ± 0.037 | Baseline IgE on Kd | 0.047 ± 0.010 |
| Body mass index on CLX/F | 0.246 ± 0.060 | Age (<12 vs.≥12 years) on Kd | 1.20 ± 0.023 |
| Race (Black vs. Caucasian) on CLX/F | 1.06 ± 0.025 | Race (Black vs. Caucasian) on Kd | 0.95 ± 0.026 |
| Race (Oriental vs. Caucasian) on CLX/F | 1.10 ± 0.064 | Race (Oriental vs. Caucasian) on Kd | 0.80 ± 0.100 |
| Race (Other vs. Caucasian) on CLX/F | 1.10 ± 0.027 | Race (Other vs. Caucasian) on Kd | 0.96 ± 0.031 |
| Baseline IgE (inverted) on CLE/F | 0.233 ± 0.014 | ||
| Volumes | Rate of production of IgE at new equilibrium on treatment, RN | ||
| Bodyweight on VX/F, VE/F, VC/F | 1.05 ± 0.030 | Bodyweight on RN | 1.09 ± 0.184 |
| Age (<12 vs.≥12 years) on VX/F, VE/F | 0.97 ± 0.021 | Baseline IgE on RN | 0.723 ± 0.085 |
| Rate of production of IgE at start of treatment, RB | Gender (Female vs. male) on RN | 0.740 ± 0.098 | |
| Baseline IgE on RB | 0.733 ± 0.015 | Age (<12 vs.≥12 years) on RN | 1.27 ± 0.257 |
| Age (<12 vs. ≥12 years) on RB | 0.973 ± 0.018 | Race (Black vs. Caucasian) on RN | 1.17 ± 0.280 |
| Gender (Female vs. Male) on RB | 0.975 ± 0.014 | Race (Oriental vs. Caucasian) on RN | 0.712 ± 0.315 |
| Race (Black vs. Caucasian) on RB | 1.02 ± 0.028 | Race (Other vs. Caucasian) on RN | 0.520 ± 0.114 |
| Race (Oriental vs. Caucasian) on RB | 1.17 ± 0.086 | ||
| Race (Other vs. Caucasian) on RB | 0.95 ± 0.027 | ||
| Rate of change in IgE production with omalizumab, kM | |||
| Age (<12 vs. ≥12 years) on kM | 1.71 ± 0.231 | Gender (Female vs. male) on kM | 1.01 ± 0.082 |
| Bodyweight on kM | 0.400 ± 0.123 | Race (Black vs. Caucasian) on kM | 1.11 ± 0.151 |
| Baseline IgE on kM | 0.170 ± 0.057 | Race (Oriental vs. Caucasian) on kM | 1.29 ± 0.267 |
| Race (Other vs. Caucasian) on kM | 1.01 ± 0.104 | ||
The covariates were exponents for the continuous covariates bodyweight, body mass index and and baseline IgE, or ratios for categorical covariates age <12 years, race and gender. SEM, standard error of the mean.
For the clearance of omalizumab, there was little shrinkage (17%) indicating good sampling design for this parameter (Table 3). The volume, baseline IgE production and binding parameters were somewhat shrunk towards the mean (28%, 38% and 38%, respectively) due to lack of samples around Cmax. Other parameters showed a fair degree of shrinkage indicating that individual patient values should be used with caution. However, there was little shrinkage in the residual variances indicating that there was good discriminatory ability to select an appropriate model structure. Furthermore, given that the first-order estimation method had been used (due to the immense amount of time it would take to use first-order conditional estimation, the first-order method taking about 12 days to run) the model described the data well. There were no notable deviations of the final model curves from the observed data, as illustrated in Figure 2 by the diagnostic plots of residuals vs. both concentration and time for the long-term studies 10E1 and EXCELS. The majority of measured samples were within 0.5 loge units, i.e. within +65% or −39%, of the individual patients' predicted curves. Therefore, it was judged that the model fitted well the long-term data from patients with severe persistent allergic asthma. Examples of individual predicted curves from the final omalizumab–IgE model applied to the two studies contributing long-term data to the analysis are presented in Figure 3.
Figure 2.
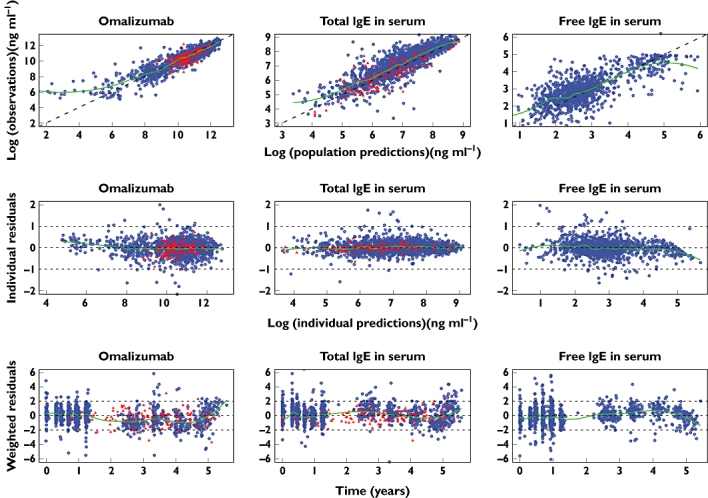
Diagnostic plots for assessment of the final feedback model fit to data. Blue dots: study 10E1; red triangles: EXCELS. First row: observations vs. population prediction; second row: individual residuals vs. individual predictions; third row: weighted population residuals vs. time. The line through the centre is a local regression with a span of 0.25 generated using the LOESS function in R. Given that an additive error model was used on log-transformed data, the units for the individual residuals are loge of the concentrations in ng ml−1; 0.5 loge unit is 1.6-fold, or, on the linear scale, +65%, −39%
Figure 3.
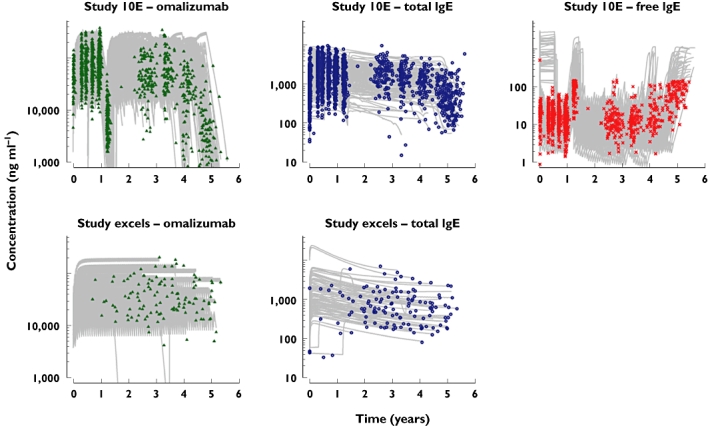
Fit of the final feedback omalizumab–IgE model to data from patients in the long-term studies 10E1 and EXCELS. The symbols are the measured data, the grey lines the individual patient predicted curves. For free IgE the upper limit of quantitation was 150 ng ml−1; hence, the measured data cannot span the same ranges as the predictions from the model. However, given that the model is simultaneously fitting omalizumab free and total IgE and there is support from free IgE samples within the range of the assay, an accurate prediction for free IgE when data are not directly available can be made [37]. Please note that the ordinate is logarithmic
Prediction checking and specific cases
To further illustrate how measured total IgE and, by implication, IgE production, can change with time and how the feedback model went some way to explaining these changes, a phase I study (Q0673g) not used in parameter estimation was used for prediction checking. The results of a comparison of predictions vs. observations, expressed as weighted residuals, for all the subjects in this study are shown in Figure 4. Although, overall, the residuals were reasonably centralized (i.e. unbiased prediction), the control model did not predict the time course of total IgE accurately. There was a clear trend to underprediction by, on average, 0.5 standard deviation units in the first 6–12 weeks, counterbalanced at the end of the treatment period by an overprediction. The feedback model corrected much of this, although there remain interesting trends that may warrant further investigation.
Figure 4.
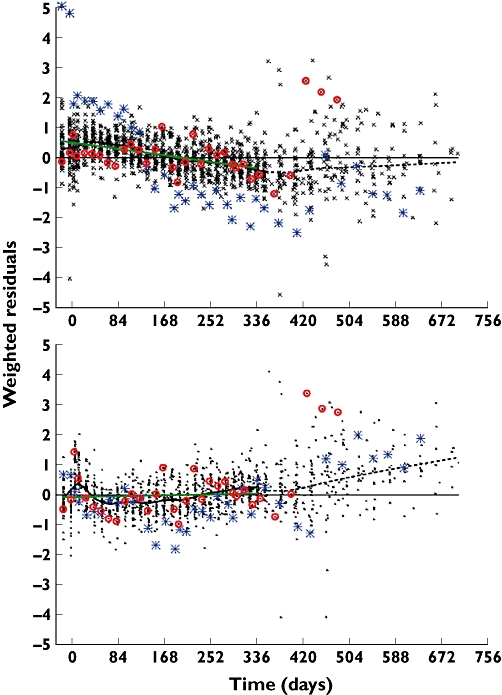
Comparison of predicted vs. observed total IgE for study Q0673g. The symbols are the weighted residuals for the comparison of observed vs. model predicted total IgE. The upper panel shows the result for the control model where parameters were estimated from 1-year data, the lower panel the final feedback model fitted to the 5-year data. The blue stars and red circles highlight the residuals for the two patients shown in Figure 5. The green solid lines are least squares fits for the drug treatment period to 336 days. The dotted black line is a Gaussian LOESS with span of 0.2 (S-Plus)
For the best responder in study Q0673g (Figure 5, left panel) after the initial increase due to the formation of omalizumab–IgE complexes, total IgE decreased over the subsequent weeks, even though the overall concentrations of omalizumab were at steady state. During this time, free IgE concentrations also tended to decrease. However, being a less precise assay, it was more difficult to detect the trend in this marker. When the control and IgE feedback models were used to predict these data, it was apparent that a constant IgE production and clearance could not explain it, although the model gave a good fit to the omalizumab PK data. The feedback model provided a better fit, although some minor features in the data were still not fully explained, such as decreasing total IgE from 120 to 210 days. The feedback model estimated that the production of IgE had, most likely, decreased from 1523 to 216 µg day−1, about 86%, in just over 500 days. At the other extreme (Figure 5, right panel) another patient had little or no decrease in total IgE or IgE production. Both the constant and feedback models fitted quite well.
Figure 5.
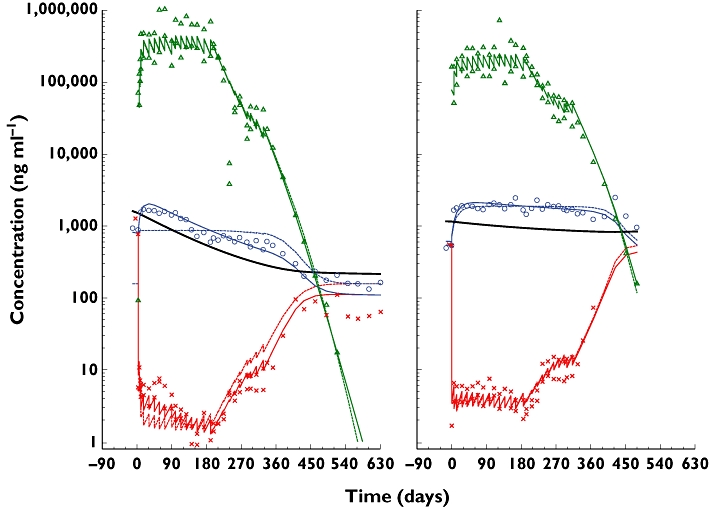
Example individual fits for the control (no change in IgE production) and feedback models for a phase I study (Q0673g) in patients with allergic rhinitis. The solid lines are the feedback model, the dashed the control and the symbols are the measured data. On the left is a 49-year-old, 58.5-kg, 152-cm Asian woman with 388 IU ml−1 (931 ng ml−1) baseline IgE given 0.030 mg kg−1 bodyweight per IU ml−1 baseline IgE every 2 weeks for 26 weeks then 0.0015 mg kg−1 per IU ml−1 for 18 weeks. IgE production decreased from 1523 to 216 µg day−1 over 517 days (thick solid line). The decrease in IgE production paralleled the decrease in total IgE. On the right is a 22-year-old, 81.9-kg, 152-cm Asian man with 205 IU ml−1 (492 ng ml−1) baseline IgE, given 0.030 then 0.005 mg kg−1 bodyweight per IU ml−1 baseline IgE every 2 weeks. The model estimated IgE production decreased from 1188 to 859 µg day−1 over 397 days. Omalizumab ( ); total IgE (
); total IgE ( ); IgE production (—); free IgE (
); IgE production (—); free IgE ( )
)
Implications of changes in IgE production
To evaluate the long-term implications of treatment with omalizumab, the final model was used to predict total IgE concentrations after 1-, 3- or 5-year treatment periods for a 35-year-old, 65 kg, Caucasian male subject with a screening IgE concentration of 1320 ng ml−1 (550 IU ml−1). The resulting simulation, displayed in the top panel of Figure 6, suggested that treated patients would be expected to approach new equilibria in IgE concentrations approximately 5 years after initiating treatment. The bottom panel in Figure 6 shows the corresponding predictions for the IgE production rate. These curves parallel the total IgE curves in the top panel, thereby suggesting that total IgE could be utilized as a biomarker to monitor individual patient changes in IgE production during and after treatment with omalizumab. Figure 6 insets show that although the modulation rate constant was 71% faster in children, combining this with the effects of age on the binding constant (20% worse) and the rate of IgE production at its new equilibrium (27% higher) were such that the down- and predicted up-regulation would be no different between children and adults.
Figure 6.
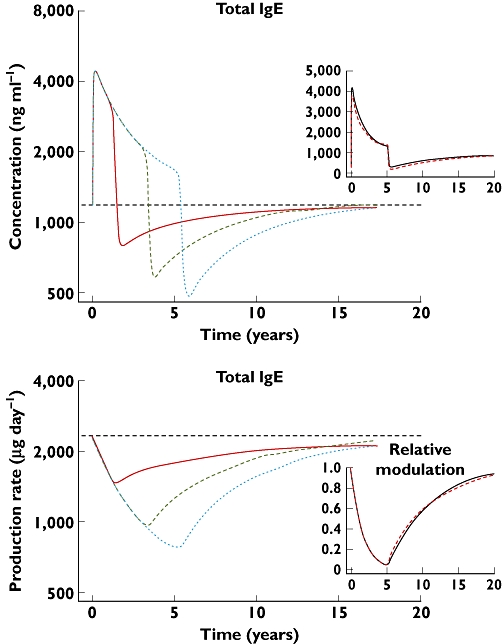
Long-term predictions of total IgE and IgE production rate, based on the final feedback model, following 1, 3 or 5 years of treatment with omalizumab 375 mg for a typical 35 year old, 65 kg, Caucasian male subject with screening IgE concentration of 550 IU ml−1 or 1320 ng ml−1. The insets show the effect of age on total IgE and the relative change in the modulator of IgE production. The simulations are for 5 years treatment of patients initially aged 6 and 35 years. Note that the scales for the main figures are logarithmic to show the parallelism of total IgE and IgE production, the insets linear to show better the extent of the changes. For the IgE modulation scale, 1 is the up-regulated state before treatment, 0 the background production. For the main figures, the lines are: 1 year ( ); 3 years (
); 3 years ( ); 5 years (
); 5 years ( ). For the insets: 25-year-old (
). For the insets: 25-year-old ( ); 6-year-old (—)
); 6-year-old (—)
Discussion
The analyses presented herein demonstrate that the production of IgE can decrease when a patient is treated with omalizumab. By adding a simple exponential decline in IgE production over time, from an initial value at the commencement of treatment to a new steady-state value, a highly significant improvement in model fit was achieved. Indeed, it has been shown that the PK–IgE model previously published [20, 27–29], in which IgE production was assumed to be constant, was incapable of converging on and describing total IgE data once long-term 3–5 year observations were included in the data set. When comparing whether the explanation for the decreasing total IgE was due to decreasing production or increasing clearance, two factors were taken into account. First, baseline IgE has been shown to have a greater dependence on IgE production than IgE clearance [27, 28]. Second, the projected steady-state IgE clearance for a time changing IgE clearance model was 15.1 l day−1, which, when taking the volume into account (10.3 l), gave an apparent half-life of 0.47 days. However, Waldmann et al. demonstrated a range of IgE half-lives of 1.8–3.1 days using a direct injection of radiolabelled IgE [38]. It was therefore considered physiologically unlikely that IgE clearance should be increased over threefold in atopic asthmatics. IgE production, on the other hand, can often be up-regulated to levels many fold higher. Furthermore, the estimates for the down-regulated steady-state production of IgE from the time changing IgE production models were in the same range as those reported by Waldmann et al. [38]. Taken together, the varying IgE production model appeared physiologically more reasonable, although it cannot be ruled out that there may be some changes in IgE clearance in allergic atopic patients, compared with the healthy state.
Two different modelling concepts were applied to describe, understand and, it is hoped, predict what should happen to IgE concentrations after treatment cessation. The empirical exponential model was only suitable for investigating changes in IgE production during treatment with omalizumab. With this model, IgE production would remain at the new steady-state concentration upon withdrawal of treatment. Although useful to describe the treatment effect, this model, unlike the feedback version, would not correctly represent the ability of the body to continue to respond to allergen exposure, with the potential to up-regulate IgE production based upon the interaction of allergen-loaded IgE with IgE receptor bearing cells. Further improvements in the quality of fit, precision of the parameter estimates and ability of the model to predict total IgE after 3–5 years of treatment were made by using a semi-mechanistic feedback scheme from free IgE. Overall, the improvements in model compared with that previously published provides substantial evidence that a constant IgE production model is an incorrect description of physiological reality and that IgE production must be declining with treatment in a substantial proportion of patients in the allergic atopic population.
Although various studies have shown that there is down-regulation of B-cell FcεRI, occurring within the first 2 weeks or thereabouts of treatment [12], the IgE production system does not down-regulate so quickly. The estimated rate at which IgE production changes, about 54% per year, is such that it would take 3–5 years, for those who respond, to reach a new steady state. The trajectory of the change suggests that, with anti-IgE treatment, patients head towards normal rates of IgE production, as compared with estimates based on radiolabelled IgE [38]. A possible mechanism for this change may involve the divalent binding of omalizumab to membrane embedded IgE on B lymphocytes, analogous to that demonstrated for a probe monoclonal in an in vitro system [16] and in mice [17]. The slow decrease in IgE production would then be due to the gradual loss and non-replacement of IgE secreting plasma cells [39]. The feedback model mimics this mechanism by using free IgE (whether it be on cells or in solution) as the causative agent which controls a putative modulator system responsible for IgE production.
A useful feature of the model is that decreases in IgE production parallel the decreases in total IgE (Figures 5 and 6). The feedback model predicts that IgE production decreases with duration of treatment and will level out after about 5 years (Figure 6). Although paediatric patients had a faster modulation rate, the relative rate and extent of IgE down-regulation would be the same as in adults due to the interplay between modulation, binding and the projected down modulated rate of IgE production. On treatment withdrawal IgE production would begin to increase (albeit more slowly) taking approximately 15 years to return to baseline concentrations. At this point in time this is simply a hypothesis resulting from the model; it is, however, testable. If patients who have been treated with omalizumab for extended periods were to have their treatment withdrawn, they could be tracked for recurrence of symptoms and total IgE increases. This hypothesis is being tested in a 1-year treatment withdrawal study, XPORT [40]. That said, full verification would require long-term follow-up over many years. If symptoms were to remain stable and low, as has been reported [18], and IgE were to increase only slowly, then omalizumab would have, effectively, enabled the attainment of a less atopic state. Even if patients should need to restart treatment, with IgE production diminished, their new baseline IgE would be lower than before, such that a lower dose would be indicated.
For individual patients and their attending physicians, total IgE could be a valuable biomarker, the only proviso being repetitive sampling to avoid confounding signals, such as temporary increases in IgE due to other factors such as treatment with pholcodine [37, 41]. After the initial increase due to the formation of omalizumab–IgE complexes (which should have equilibrated by the time the patients have their clinical responsiveness tested at 12–16 weeks [42]) total IgE could be monitored periodically to track IgE production. This monitoring of total IgE should enable physicians to determine whether the patient is down-regulating and, if so, when they should attain a lower, hopefully non-atopic state.
In conclusion, it has been demonstrated using omalizumab pharmacokinetic, free and especially total IgE data, analysed with a mechanism-based PK/PD model incorporating omalizumab–IgE binding and feedback for control of IgE production, that a prediction that omalizumab is able to decrease the production rate of IgE over ‘several weeks or months (some say more than 1 year)’[26], is true. With this down-regulation, it may well be that treatment with omalizumab does not have to be maintained for long periods of time, and there is now a means by which IgE synthesis can be monitored such that individual treatment decisions can be made.
Acknowledgments
We gratefully acknowledge the help and support of Aurélie Gautier, Gregory Pinault and Georges Etté of the programming group at Novartis for the extraction of data from the production server, for creating NONMEM data sets for analysis and for some of the figures and tables in the manuscript. The authors were assisted in the preparation of the manuscript by professional medical writer Paul Hutchin. Writing support was funded by the study sponsor.
Competing Interests
All authors work for and own shares in Novartis Pharma AG, manufacturers of omalizumab (Xolair®).
Supporting Information
Additional Supporting Information may be found in the online version of this article:
NONMEM code for the final model.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
REFERENCES
- 1.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 2.Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005;115:459–65. doi: 10.1016/j.jaci.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 3.Presta L, Shields R, O'Connell L, Lahr S, Porter J, Gorman C, Jardieu P. The binding site on human immunoglobulin E for its high affinity receptor. J Biol Chem. 1994;269:26368–73. [PubMed] [Google Scholar]
- 4.Presta LG, Lahr SJ, Shields RL, Porter JP, Gorman CM, Fendly BM, Jardieu PM. Humanization of an antibody directed against IgE. J Immunol. 1993;151:2623–32. [PubMed] [Google Scholar]
- 5.Soler M, Matz J, Townley R, Buhl R, O'Brien J, Fox H, Thirlwell J, Gupta N, Della CG. The anti-IgE antibody omalizumab reduces exacerbations and steroid requirement in allergic asthmatics. Eur Respir J. 2001;18:254–61. doi: 10.1183/09031936.01.00092101. [DOI] [PubMed] [Google Scholar]
- 6.Bousquet J, Cabrera P, Berkman N, Buhl R, Holgate S, Wenzel S, Fox H, Hedgecock S, Blogg M, Cioppa GD. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy. 2005;60:302–8. doi: 10.1111/j.1398-9995.2004.00770.x. [DOI] [PubMed] [Google Scholar]
- 7.Busse W, Corren J, Lanier BQ, McAlary M, Fowler-Taylor A, Cioppa GD, Gupta AA, Omalizumab an. anti-IgE recombinant humanized monoclonal antibody, for the treatment of severe allergic asthma. J Allergy Clin Immunol. 2001;108:184–90. doi: 10.1067/mai.2001.117880. [DOI] [PubMed] [Google Scholar]
- 8.Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, Beeh KM, Ramos S, Canonica GW, Hedgecock S, Fox H, Blogg M, Surrey K. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60:309–16. doi: 10.1111/j.1398-9995.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 9.Chanez P, Humbert M, Verkindre C, Tunon de Lara M, Molimard M. Diminution de l'expression des récepteurs FceRI dans l'asthme allergique persistant sévère traité par omalizumab. Rev Mal Respir. 2009;26(Suppl. 1):S144. [Google Scholar]
- 10.Beck LA, Marcotte GV, MacGlashan D, Togias A, Saini S. Omalizumab-induced reductions in mast cell Fcepsilon RI expression and function. J Allergy Clin Immunol. 2004;114:527–30. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Lin H, Boesel KM, Griffith DT, Prussin C, Foster B, Romero FA, Townley R, Casale TB. Omalizumab rapidly decreases nasal allergic response and FcepsilonRI on basophils. J Allergy Clin Immunol. 2004;113:297–302. doi: 10.1016/j.jaci.2003.11.044. [DOI] [PubMed] [Google Scholar]
- 12.MacGlashan DW, Jr, Bochner BS, Adelman DC, Jardieu PM, Togias A, Kenzie-White J, Sterbinsky SA, Hamilton RG, Lichtenstein LM. Down-regulation of Fc(epsilon)RI expression on human basophils during in vivo treatment of atopic patients with anti-IgE antibody. J Immunol. 1997;158:1438–45. [PubMed] [Google Scholar]
- 13.Streck M, Tole J, Gandhi R, Loun T, Stewart C, Lew D. Omalizumab downregulates the expression of CD23 (FceRII) on human bronchial smooth muscle cells (HBSMC) in vitro. J Allergy Clin Immunol. 2006;117(2) Suppl.:S9. [Google Scholar]
- 14.Hibbert RG, Teriete P, Grundy GJ, Beavil RL, Reljic R, Holers VM, Hannan JP, Sutton BJ, Gould HJ, McDonnell JM. The structure of human CD23 and its interactions with IgE and CD21. J Exp Med. 2005;202:751–60. doi: 10.1084/jem.20050811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jung CM, Prinz JC, Rieber EP, Ring J. A reduction in allergen-induced Fc epsilon R2/CD23 expression on peripheral B cells correlates with successful hyposensitization in grass pollinosis. J Allergy Clin Immunol. 1995;95(1 Pt 1):77–87. doi: 10.1016/s0091-6749(95)70155-9. [DOI] [PubMed] [Google Scholar]
- 16.Stämpfli MR, Miescher S, Aebischer I, Zurcher AW, Stadler BM. Inhibition of human IgE synthesis by anti-IgE antibodies requires divalent recognition. Eur J Immunol. 1994;24:2161–7. doi: 10.1002/eji.1830240934. [DOI] [PubMed] [Google Scholar]
- 17.Davis FM, Gossett LA, Pinkston KL, Liou RS, Sun LK, Kim YW, Chang NT, Chang TW, Wagner K, Bews J. Can anti-IgE be used to treat allergy? Springer Semin Immunopathol. 1993;15:51–73. doi: 10.1007/BF00204626. [DOI] [PubMed] [Google Scholar]
- 18.Nopp A, Johansson SG, Ankerst J, Palmqvist M, Oman H. CD-sens and clinical changes during withdrawal of Xolair after 6 years of treatment. Allergy. 2007;62:1175–81. doi: 10.1111/j.1398-9995.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- 19.Nopp A, Johansson SG, Adedoyin J, Ankerst J, Palmqvist M, Oman H. After 6 years with Xolair; a 3-year withdrawal follow-up. Allergy. 2010;65:56–60. doi: 10.1111/j.1398-9995.2009.02144.x. [DOI] [PubMed] [Google Scholar]
- 20.Slavin RG, Ferioli C, Tannenbaum SJ, Martin C, Blogg M, Lowe PJ. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123:107–13. doi: 10.1016/j.jaci.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 21.Slavin RG, Jimenez P. Reduction of the total IgE level by omalizumab in children and adolescents. J Asthma. 2009;46:102–3. doi: 10.1080/02770900802513015. [DOI] [PubMed] [Google Scholar]
- 22.Katz RM, Rafi AW, Do L, Lin M, Mangat R, Azad N, Sender S. Efficacy of omalizumab using extended dose intervals. J Allergy Clin Immunol. 2007;119:s212. [Google Scholar]
- 23.Hanf G, Brachmann I, Kleine-Tebbe J, Seybold J, Kunkel G, Suttorp N, Noga O. Omalizumab decreased IgE-release and induced changes in cellular immunity in patients with allergic asthma. Allergy. 2006;61:1141–4. doi: 10.1111/j.1398-9995.2006.01180.x. [DOI] [PubMed] [Google Scholar]
- 24.Steiss JO, Strohner P, Zimmer KP, Lindemann H. Reduction of the total IgE level by omalizumab in children and adolescents. J Asthma. 2008;45:233–6. doi: 10.1080/02770900701883782. [DOI] [PubMed] [Google Scholar]
- 25.Berger W, Gupta N, McAlary M, Fowler-Taylor A. Evaluation of long-term safety of the anti-IgE antibody, omalizumab, in children with allergic asthma. Ann Allergy Asthma Immunol. 2003;91:182–8. doi: 10.1016/S1081-1206(10)62175-8. [DOI] [PubMed] [Google Scholar]
- 26.Lanier BQ. Unanswered questions and warnings involving anti-immunoglobulin E therapy based on 2-year observation of clinical experience. Allergy Asthma Proc. 2005;26:435–9. [PubMed] [Google Scholar]
- 27.Hayashi N, Tsukamoto Y, Sallas WM, Lowe PJ. A mechanism-based binding model for the population pharmacokinetics and pharmacodynamics of omalizumab. Br J Clin Pharmacol. 2007;63:548–61. doi: 10.1111/j.1365-2125.2006.02803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lowe PJ, Tannenbaum S, Gautier A, Jimenez P. Relationship between omalizumab pharmacokinetics, IgE pharmacodynamics and symptoms in patients with severe persistent allergic (IgE-mediated) asthma. Br J Clin Pharmacol. 2009;68:61–76. doi: 10.1111/j.1365-2125.2009.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meno-Tetang GM, Lowe PJ. On the prediction of the human response: a recycled mechanistic pharmacokinetic/pharmacodynamic approach. Basic Clin Pharmacol Toxicol. 2005;96:182–92. doi: 10.1111/j.1742-7843.2005.pto960307.x. [DOI] [PubMed] [Google Scholar]
- 30.Long AA, Fish JE, Rahmaoui A, Miller MK, Bradley MS, Taki HN, Demeo AN, Tilles SA, Szefler SJ. Baseline characteristics of patients enrolled in EXCELS: a cohort study. Ann Allergy Asthma Immunol. 2009;103:212–9. doi: 10.1016/S1081-1206(10)60184-6. [DOI] [PubMed] [Google Scholar]
- 31.Milgrom H, Berger W, Nayak A, Gupta N, Pollard S, McAlary M, Taylor AF, Rohane P. Treatment of childhood asthma with anti-immunoglobulin E antibody (omalizumab) Pediatrics. 2001;108:E36. doi: 10.1542/peds.108.2.e36. [DOI] [PubMed] [Google Scholar]
- 32.Lanier BQ, Corren J, Lumry W, Liu J, Fowler-Taylor A, Gupta N. Omalizumab is effective in the long-term control of severe allergic asthma. Ann Allergy Asthma Immunol. 2003;91:154–9. doi: 10.1016/S1081-1206(10)62170-9. [DOI] [PubMed] [Google Scholar]
- 33.Buhl R, Soler M, Matz J, Townley R, O'Brien J, Noga O, Champain K, Fox H, Thirlwell J, Della CG. Omalizumab provides long-term control in patients with moderate-to-severe allergic asthma. Eur Respir J. 2002;20:73–8. doi: 10.1183/09031936.02.00278102. [DOI] [PubMed] [Google Scholar]
- 34.Holgate ST, Chuchalin AG, Hebert J, Lotvall J, Persson GB, Chung KF, Bousquet J, Kerstjens HA, Fox H, Thirlwell J, Cioppa GD. Efficacy and safety of a recombinant anti-immunoglobulin E antibody (omalizumab) in severe allergic asthma. Clin Exp Allergy. 2004;34:632–8. doi: 10.1111/j.1365-2222.2004.1916.x. [DOI] [PubMed] [Google Scholar]
- 35.Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124:1210–6. doi: 10.1016/j.jaci.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Riviere G-J, Keubler P, Jaffer J, Yeh C-M, Reynolds C, Brookman L. Novel omalizumab liquid formulation bioequivalent to lyophilized formulation. Eur Respir J. 2008;32(Suppl. 52):345s. [Google Scholar]
- 37.Florvaag E, Johansson SGO, Öman H, Harboe T, Nopp A. Pholcodine stimuates a dramatic increase of IgE in IgE-sensitized individuals. A pilot study. Allergy. 2006;61:49–55. doi: 10.1111/j.1398-9995.2005.00933.x. [DOI] [PubMed] [Google Scholar]
- 38.Waldmann TA, Iio A, Ogawa M, McIntyre OR, Strober W. The metabolism of IgE. Studies in normal individuals and in a patient with IgE myeloma. J Immunol. 1976;117:1139–44. [PubMed] [Google Scholar]
- 39.Chang TW, Wu PC, Hsu CL, Hung AF. Anti-IgE antibodies for the treatment of IgE-mediated allergic diseases. Adv Immunol. 2007;93:63–119. doi: 10.1016/S0065-2776(06)93002-8. [DOI] [PubMed] [Google Scholar]
- 40.A Study Evaluating the Persistency of Response With or Without Xolair after Long-Term Therapy (XPORT) Study Start Date: May 2010. Available at http://clinicaltrials.gov/ct2/show/NCT01125748 (last accessed)
- 41.Harboe T, Johansson SGO, Florvaag E, Öman H. Pholcodine exposure raises serum IgE in patients with previous anaphylaxis to neuromuscular blocking agents. Allergy. 2007;62:1445–50. doi: 10.1111/j.1398-9995.2007.01554.x. [DOI] [PubMed] [Google Scholar]
- 42.Bousquet J, Rabe K, Humbert M, Chung KF, Berger W, Fox H, Ayre G, Chen H, Thomas K, Blogg M, Holgate S. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med. 2007;101:1483–92. doi: 10.1016/j.rmed.2007.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


