Abstract
Background:
Iatrogenic instability following laminectomy occurs in patients with degenerative lumbar canal stenosis. Long segment fusions to obviate postoperative instability result in loss of motion of lumbar spine and predisposes to adjacent level degeneration. The best alternative would be an adequate decompressive laminectomy with a nonfusion technique of preserving the posterior ligament complex integrity. We report a retrospective analysis of multilevel lumbar canal stenosis that were operated for posterior decompression and underwent spinaplasty to preserve posterior ligament complex integrity for outcome of decompression and iatrogenic instability.
Materials and Methods:
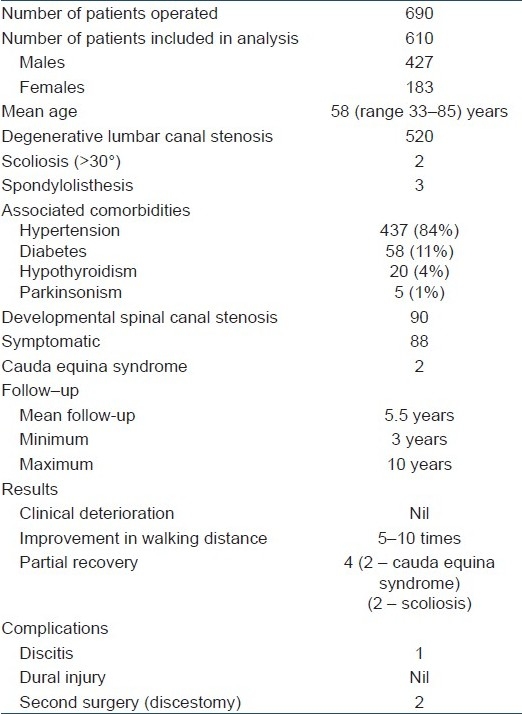
610 patients of degenerative lumbar canal stenosis (n=520) and development spinal canal stenosis (n=90), with a mean age 58 years (33–85 years), underwent multilevel laminectomies and spinaplasty procedure. At followup, changes in the posture while walking, increase in the walking distance, improvement in the dysesthesia in lower limb, the motor power, capability to negotiate stairs and sphincter function were assessed. Forward excursion of vertebrae more than 4 mm in flexion–extension lateral X-ray of the spine as compared to the preoperative movements was considered as the iatrogenic instability. Clinical assessment was done in standing posture regarding active flexion–extension movement, lateral bending and rotations
Results:
All patients were followed up from 3 to 10 years. None of the patients had neurological deterioration or pain or catch while movement. Walking distance improved by 5–10 times, with marked relief (70–90%) in neurogenic claudication and preoperative stooping posture, with improvement in sensation and motor power. There was no significant difference in the sagittal alignment as well as anterior translation. Two patients with concomitant scoliosis and one with cauda equine syndrome had incomplete recovery. Two patients who developed disc protrusion, underwent a second operation for a symptomatic disc prolapse.
Conclusion:
Spinaplasty following posterior decompression for multilevel lumbar canal stenosis is a simple operation, without any serious complications, retaining median structures, maintaining the tension band and the strength with least disturbance of kinematics, mobility, stability and lordosis of the lumbar spine.
Keywords: Decompression, laminectomies, lumbar canal stenosis, multilevel, posterior ligamentous complex, spinaplasty
INTRODUCTION
Decompressive laminectomy has been widely used as an operative treatment for lumbar canal stenosis.1–5 Laminectomy including removal of spinous process, supraspinous ligament, interspinous ligament, lamina and ligamentum flavum jeopardize the integrity of posterior complex of the spine. However, iatrogenic instability following extensive laminectomy occurs in some patients with degenerative lumbar canal stenosis or in patients who are associated with pre-existing spondylolisthesis.6–9 Long segment fusions have been done by some workers to obviate postoperative instability; however, such operations result in loss of motion of lumbar spine and predisposes the spine for adjacent level degeneration.10–13 It is important in the treatment of spinal stenoses to achieve adequate spinal decompression while maintaining the spinal stability.1 The preservation of posterior spinal elements associated with minimally invasive surgery could minimize the risk of developing de novo postoperative changes in spinal alignment and/or acceleration of facet and disc degeneration. The best alternative would be an adequate decompressive laminectomy in degenerative lumbar canal stenosis with a nonfusion technique of preserving the posterior ligament complex integrity. The integrity of posterior ligament complex is preserved by repairing the median structures, i.e. spinous process, intraspinous ligaments and supraspinous ligaments, by lifting them as one piece and repairing after the spinal decompression. The paraspinal muscles are sutured to the repaired median raphae. We describe this procedure as spinaplasty. We report a retrospective analysis of cases of multilevel lumbar canal stenosis that were operated for posterior decompression and underwent spinaplasty for outcome of decompression and iatrogenic instability.
MATERIALS AND METHODS
Six hundred and ninety patients suffering from multilevel lumbar canal stenosis underwent multilevel laminectomies and spinaplasty procedure from 1995 to 2005. Out of these, patients suffering from degenerative lumbar canal stenosis (n=520) and symptomatic development spinal canal stenosis(n=90), with a mean age of 58 years (33–85 years), were followed up for 3 years or more were included in the analysis. There were 427 men and 183 women. The patients of developmental canal stenosis were younger (33–59 years); 88 of them were symptomatic essentially due to development canal stenosis while 2 of them presented with cauda equine syndrome. The elderly group of 520 patients (age 60–85 years) were symptomatic because of degenerative lumbar canal stenosis. They presented with pain in lower limbs while walking (neurogenic claudication), attaining a stooping posture to get some pain relief while walking, with radiating pains in lower limb. Over time, it led to progressive reduction in the walking distance, weakness by one grade in dorsiflexor or planter flexor or both.3 There was difficulty in walking on toes at one or both sides (n=31, 6%) difficulty in walking on heels at one or both sides (n=26, 5%) or weakness of both the groups(n=10, 2%). Each patient had symptoms for 6 months to 5 years preoperatively and had tried adequate conservative treatment for 3 months or more in the form of spinal exercises (flexion and extension), physiotherapy measures such as short wave diathermy and/or ultrasound therapy, nonsteroidal anti-inflammatory drugs (NSAIDs) with or without light lumbosacral brace. The plain X-ray pelvis including both hips (AP view) and lumbosacral X-ray (D12–S2, AP, lateral and flexion extension view) were done to rule out any structural vertebral pathology and define localized instability if present. Magnetic resonance imaging (MRI) was done in all cases.
Common comorbidities in the elderly group were hypertension (n=437, 84%), diabetes (n=58, 11%), hypothyroidism (n=20, 4%) and parkinsonism (n=5, 1%). In the elderly group, two patients had concomitant scoliosis of 30° or more and three patients had concomitant preexisting degenerative spondylolisthesis (L4–L5) prior to the index operation. Complete compatibility between the clinical pictures and MRI was considered mandatory before considering patients for operative intervention. We performed decompression and spinaplasty when the patient had one or more of the following clinical features: disturbance of sphincter functions, diminution of motor power in lower limbs, hypoesthesia in soles and/or at saddle area, reduction in walking distance to less than 200 m and disabling pain (dysesthesia) in legs. The patients included in the analysis had minimum of 2 level and maximum of 4 level decompression. Very obese patients with body mass index (BMI) more than 30 were excluded. All patients with comorbidities were treated and stabilized by physicians and anesthesiologists before inclusion for operative intervention. Lumbar canal stenosis associated with prior operation on the spine, ankylosing spondylitis, fluorosis, and achondroplasia and those who had symptoms in tandem with cervical canal stenosis were excluded [Table 1].
Table 1.
Inclusion and exclusion criteria for the study
Operative technique
This operation was performed in lateral decubitus position, with more symptomatic side pointing toward the ceiling. A midline incision was made through the skin and subcutaneous tissues. Two incisions were now made, one on each side of the median structures, keeping approximately 1.5 cm width of supraspinous ligaments intact. Median structures including supraspinous and interspinous ligaments, and spinous processes were separated from paravertebral fascia and muscles. Supraspinous and interspinous ligaments were cut at the most distal level of decompression [Figures 1 and 2]. The ligaments were cut along the proximal border of S1. Spinous processes were cut from their base in distal to proximal direction. The number of spinous processes cut depends on the number of levels to be decompressed. For a 4 level decompression, three spinous processes were cut. The required spinous processes attached to their supraspinous and interspinous ligament were lifted up as a continuous median mass still attached proximally.
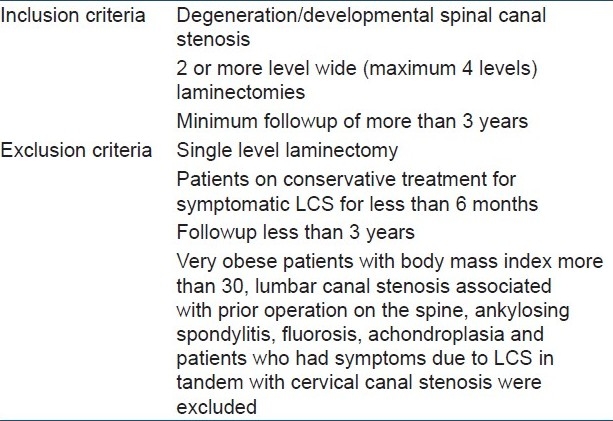
Figure 1.
Diagrammatic representation of spinaplasty operation. (a) Line diagram of spine in sagittal view shows normal attachment of supraspinous and interspinous ligaments. (b) Supraspinous and interspinous ligaments are cut at most distal level of the planned decompression. Spinous processes are cut from the base and lifted up as a continuous median mass still attached to the suraspinous and interspinous ligament and their undisturbed proximal attachment. (c) After adequate decompression and suturing of the deepest layer of muscles over the dura, the median structures are sutured to the distal bed and paraspinal muscles (d) line diagram of axial view and (e) axial view of CT scan shows laminectomy and remains of spinous process after spinaplasty
Figure 2.
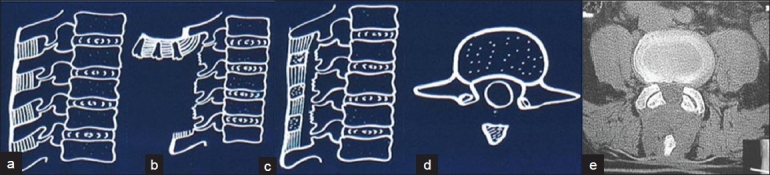
Intraoperative photographs of the operative steps: (a) median mass of supraspinous, interspinous ligaments and spinous processes exposed; (b) median structures lifted up en masse still attached proximally (arrow); (c) median structures sutured to the distal bed and to paraspinal muscles and aponeurosis; (d) paraspinal muscles are stitched to median structures
A standard laminectomy was performed, ligament flavum was excised, and any offending osseous or discogenic compressing material (n=11) was removed to achieve adequate decompression of the entire stenotic width and length of the dural tube and its contents. Deroofing of the nerve root (n=9) canals was performed when required. The epidural space was closed by stitching the deepest layer of muscles. Under (deeper) surface of the spinous processes was trimmed by 3–4 mm. The central mass of spinous processes with its supraspinous and interspinous ligaments was sutured to its distal bed. The paravertebral muscles and fascia were sutured to the median structures. Thus posterior caudal ligament was repaired meticulously, as paraspinal muscles were stitched back.
The wound was closed in layers over the suction drain. No blood transfusion was used for decompression up to 3 levels. Seventeen patients with decompression of 4 levels required blood replacement of 1 unit. The drain was removed after 48–72 hours and the patients were encouraged to walk with a light brace on 3rd or 4th postoperative day. Exercises of the spine taught preoperatively were encouraged as soon as the postoperative pain subsided by 7th day of surgery. Most of the patients were performing exercises of the spine which they were trained to do before surgery in the recumbant position, such as active spinal extension, lifting of lower limb against gravity to strengthen the abdominal wall muscles and muscles of the hip joint. The spinal brace was gradually discarded about 3 months after the operation except in patients who had scoliosis of 30° or more. No restrictions were imposed on the physical activities of the patients after 3 months of the operation.
On followup, the patient and his/her close relations were enquired about any changes in the posture while walking, increase in the walking distance, improvement in dysesthesia in lower limb, the motor power, capability to negotiate stairs and sphincter function. The patients were called 2–3 weeks after discharge from the hospital for clinical assessment and stitch removal. They were evaluated at 3 months after the procedure, and thereafter at 6–12 months interval. Patients were followed up by periodic clinical examination and X-rays anteroposterior and lateral view of lumbosacral spine in maximum flexion and extension at 1 year and last followup. Any forward excursion of vertebrae more than 4 mm on flexion–extension lateral X-ray of the spine as compared to the preoperative was considered as the instability created or added by the index operation. All patients with minimum 3 years followup were included in the analysis.
RESULTS
Six hundred and ten patients which could be followed up for 3 years or more were included in the study. All patients were followed up from 3 to 10 years after the operation. They had no difficulty in doing active flexion-extension movement, active lateral bending and rotations smoothly, without “catch” or apprehension [Figures 3a,b,4d,e]. They were steady and had no hesitation or pain or “catch” on movement. No patient deteriorated neurologically in the immediate postoperative period. At their last followup, (3–10 years after the index operation), walking distance improved by 5–10 times from that of preoperative status. Patients experienced marked relief (70–90%) from neurogenic claudication and they observed marked improvement in their preoperative stooping posture. Clinical assessment of neurological signs at 3 months after operation revealed no objective deterioration of preoperative neural signs in any patient. When assessed at 3 years after the operation, every patient showed improvement in sensation and motor power. Two young male patients who had presented with cauda equina syndrome due to severe developmental canal stenosis showed partial recovery of sphincters, sexual functions and neurology. Least neural recovery was seen in two patients of degenerative lumbar canal stenosis with concomitant scoliosis of 30° or more [Table 2]. One young female patient developed postoperative discitis at L4–L5 level despite the fact that no intervention was done at the disc while performing decompression for developmental canal stenosis. Two patients aged 62 and 76 years, respectively, underwent a second operation for a symptomatic disc prolapsed at L5–S1 level, 6 and 8 years after the index surgery. Six ambulatory patients suffering from parkinsonism maintained their ambulatory function with some improvement in their walking distance and neurogenic claudication. Three months after the operation, the lumbosacral spinal support was gradually discarded. All the patients were permitted and could do their normal activities without restriction except for crude/strenuous activities (boxing or wrestling or jumping). LS belt was still advised to be used for long travels in public transport.
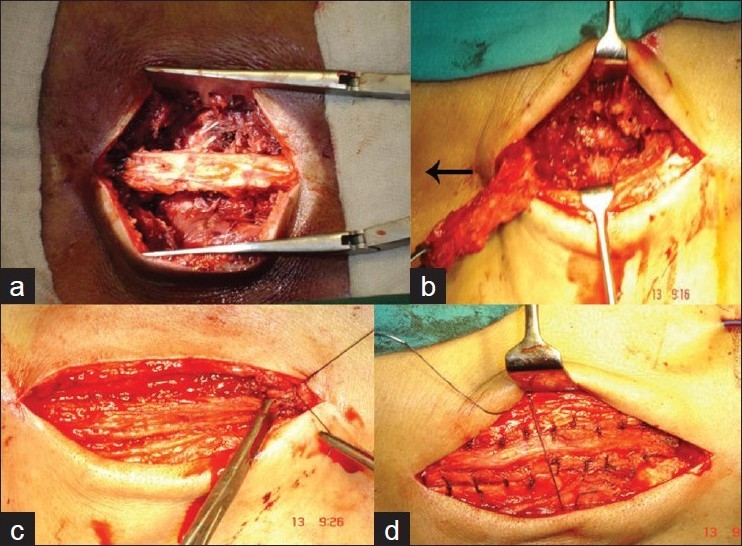
Figure 3.
Clinical photographs of a 76 years old patient of degenerative canal stenosis at 6 years followup shows good forward flexion (a) and extension (b). X-ray (lateral view) in flexion (c) and extension (d) shows stable spine
Figure 4.
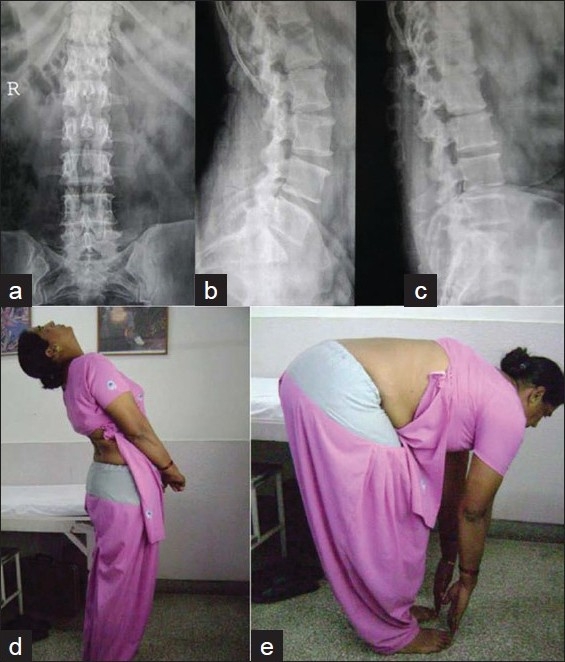
Plain X-ray lumbar spine antero-posterior view of a young patient of developmental spinal stenosis at 8 years follow-up shows adequate decompression following 3-level laminectomies. The arrow shows a shadow of retained tip of spinous process. Lateral X-ray in extension (b) and flexion (c) shows a stable spine. Clinical photograph in extension (d) and flexion (e) shows clinical stability
Table 2.
Demographics, results and complications of the spinaplasty
Radiological evaluation
All patients had radiographic assessment between 3 and 9 years postoperatively. There was no significant difference in the sagittal alignment as seen on flexion–extension X-rays. The preoperative spondylolisthesis did not show any deterioration or improvement. Postoperative scoliotic angle remained unchanged in those who had scoliosis. Two patients who developed discogenic pain 6–8 years postoperatively had MRI evaluation which showed newly developed evidence of disc protrusion at L5–S1 level.
Flexion–extension lateral X-rays did not show any significant anterior translation [Figures 3–5]. Even in the patient(n=3) who had preexisting spondylolisthesis, any change in the excursion was by less than 4 mm. The slip angle did not show any significant variation in flexion–extension X-rays though we found this assessment inconsistent and undependable because of irregularity of bone surfaces in the analyzed clinical material.
Figure 5.
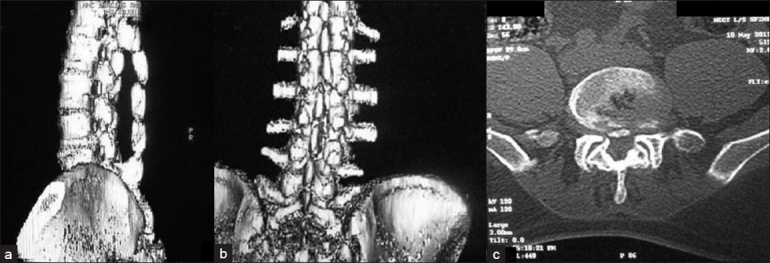
3D reconstructed sagittal (a) coronal (b) and axial CT (c) of 10 years follow-up in a 55-year-old patient who underwent spinaplasty following laminectomy, showing adequate decompression and healing and continuity of midline structure
DISCUSSION
Verbiest (1954)2 deserves the credit for making the medical world aware about the condition, which is now called lumbar canal stenosis. It is a common spinal problem in people over the age of 65 years. Surgery is required with increasing frequency in such patients.15–21 The incidence of postoperative (5–10 years) instability after extensive decompressive laminectomy is reported as 3–20%.7–10 Many workers have combined extensive laminectomy with fusion with or without instrumented stabilization.21–24 However, there is no convincing evidence to suggest that concomitant fusion has increased patients’ satisfaction.25–29 Fusion of mobile segments of the spine entails hypermobility of the adjacent unfused joints which may lead to instability (more than 3 mm of anteroposterior translation) or premature disc degeneration or stenosis of the canal. The incidence of adjacent segment degeneration in patients followed up for 5 years or more after fusion has been reported as 10–30%.11–13 Such changes may reflect the natural history of lumbar spondylosis over the years;25,26 however, these are of significance if associated with clinical symptoms of radiculopathy, discogenic pain and stenosis referable to that level.25,26 Juxta fusion level changes account for substantial percentage of revision spine surgery. Most of such subsequent operations have involved neural decompression rather than further extension of spinal fusion. Adequate decompression of the stenotic area therefore should be mandatory in the first operation to minimize the necessity of a second decompression. In the absence of preexisting spinal instability, arthrodesis is unnecessary after decompression of lumbar spine for canal stenosis. No difference was found in the improvement of walking distance and relief of pain (followup more than 2 years) in patients who had decompression alone or decompression with fusion.11,21–25,27–29
The integrity of the spinous process, supraspinous ligament and interspinous ligament complex is important to prevent instability following decompressive laminectomies. Daily activities (stress) on the lumbar spine and lumbosacral transition make great static and dynamic demands. Nature has provided this part of the spine with large massive vertebrae and strong muscle ligament structures. Therefore, we must consider sparing each component of posterior complex unit. The paraspinal muscles also lose their levers and their function and becomes weaker with separation of the muscles with dissection of their tendinous part free from their body attachments and with excision of osseous part. The superspinous and interspinous ligament provide resistance at the limit of flexion.
A biomechanical evaluation of graded posterior element removal between total laminectomy, interlaminar approach and minimally invasive approach was conducted in a cadaveric model. It demonstrated increased flexion–extension and axial rotation at the surgical site, particularly after total laminectomy. The author concluded that minimization of bone and ligament removal associated with minimally invasive surgery could minimize the risk of developing de novo postoperative changes in spinal alignment.30
In an experimental study, biomechanical comparison of lumbar spine instability between laminectomy and laminotomy was analyzed. It was noted that intervertebral displacement during flexion load on laminectomy specimen was greater than in a laminotomy specimen, suggesting that the posterior complex integrity is less likely to develop segmental instability than a lumbar spine with a destroyed anchoring point for supraspinous ligament.31
Similarly, Chan et al.32 had tested the effect of interspinous ligament integrity on adjacent segment instability after lumbar instrumentation and laminectomy on a fresh porcine lumbar spine. It was observed that under flexion, the intervertebral displacement on adjacent disc with complete laminectomy was statistically larger than those of integrity and partial laminectomies. This study implies than an instrumented spine with integrity of posterior complex is less likely to develop adjacent segment instability than a spine with destruction of anchoring point for supraspinous ligament. The importance of muscle forces in flexible and rigid instrumented stabilization was studied on a cadaveric lumbar spine to assess the functional impairment of the lumbar spine after laminectomy. The co-activation of agonist and antagonist muscle forces resulted in increased stability under the load condition of bending and rotation. It was observed that functional impairment following laminectomy was corrected by ligamentoplasty and by means of muscle forces. Hence, authors concluded that ligamentoplasty appears as an alternative to decompression with spondylodesis.33
Similarly, the osteoplastic laminectomy and total facetectomy was evaluated for biochemical instability on fresh frozen human cadaver lumbar spine specimen. It was observed that flexion–extension ROM increased significantly after total facetectomy, but not after osteoplastic laminectomy. The axial rotation increased remarkably after total facetectomy, but only moderately after osteoplastic laminectomy. Thus, it was concluded that osteoplastic laminectomy preserves the spinous process as well as facet joints, which maintain greater stability.34
We have added a procedure to multilevel laminectomies by keeping the midline raphe of supraspinous, interspinous ligament and spinous process intact. This will act as an anchoring point for paraspinal muscle and suggested a term for this procedure as “spinaplasty”.
Spinous process plasty following laminectomy as an effect on lumbar spine stability was evaluated by Verankonic et al. in a series of patients. Forty-one patients were operated for laminectomy, followed by spinous process plasty for spinal stenosis and dorsomedial herniated disc and recurrent disc herniation. The group where spinous process plasty (n=41) was done was compared with the one without spinous process plasty (n=11) for radiological instability at minimum 2-year followup. Only 3.8%developed (radiological) instability in comparison to 25% patients without spinous process plasty. In this study, 2 level decompression was done in 53.6% cases.35
The difference in the technique of spinous process plasty was that distal spinous process was divided into half and the fixation of spinous process with wire to achieve bony healing to median raphe. 2 wire breakage were reported, but we relied on soft tissue healing and interspinous ligament was cut below the distal level and then repaired.
The functional impairment following laminectomy was corrected by ligamentoplasty and by mean of muscle forces. Ligamentoplasty appears to be an alternative to decompression with spondylodesis. To obviate the post-fusion adjacent level degenerative changes in the spine and perceived spinal instability as a result of extensive laminectomy, nonfusion technologies are being explored and analyzed.6,36–41 Graf artificial ligament stabilization is one such technique. In our technique of spinaplasty, the strongest ligament–osseous complex offers a natural biological option. Niggemeyer et al., after a meta-analysis of the literature, stated that the best results are obtained by the least invasive surgical procedure.19 Ours is a simple and safe procedure with satisfactory long term results although the retrospective nature of the study is our limitation.
CONCLUSION
Spinaplasty is a simple procedure without any serious complications. Multilevel lumbar canal stenosis of any variety (developmental, degenerative or combined variety), with or without concomitant intraspinal pathology, can be decompressed satisfactorily. The retained median structures maintain the tension band and the strength and possibly the proprioceptive sensations of the lumbar spine, which ensure least disturbance of kinematics, mobility, stability and lordosis of the operated lumbar spine and concomitant discectomy, root-canal deroofing and intertransverse fusion can be done comfortably when indicated.
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Arnoldi CC, Brodsky AE, Cauchoix J, Crock HV, Dommisse GF, Edgar MA, et al. Lumbar spinal stenosis and nerve root entrapment syndromes: Definition and classification. ClinOrthopRelat Res. 1976;115:4–5. [PubMed] [Google Scholar]
- 2.Verbiest H. Results of surgical treatment of idiopathic developmental stenosis of the lumbar vertebral canal; Areview of twenty-seven years experience. J Bone joint SurgBr. 1977;59:181–8. doi: 10.1302/0301-620X.59B2.141452. [DOI] [PubMed] [Google Scholar]
- 3.Turner JA, Ersek M, Herron L, Deyo R. Surgery for lumbar spinal stenosis: attempted meta-analysis of the literature. Spine (Phila Pa 1976) 1992;17:1–8. doi: 10.1097/00007632-199201000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Atlas JA, Deyo RA, Keller RB, Chapin AM, Patrick DL, Long JM, et al. The Maine lumbar spine study Part III: 1 year outcomes of surgical and nonsurgical management of lumbar spinal stenosis. Spine (Phila Pa 1976) 1996;21:1787–95. doi: 10.1097/00007632-199608010-00012. [DOI] [PubMed] [Google Scholar]
- 5.Thomas NW, Rea GL, Pikul BK, Mervis LJ, Irsik R, McGregor JM. Quantitative outcome and radiographic comparisons between laminectomy and laminotomy in the treatment of acquired lumbar stenosis. Neurosurgery. 1997;41:567–75. doi: 10.1097/00006123-199709000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Postacchini F, Cinotti, Perugia D, Gumina S. The surgical management of central lumbar stenosis: multiple laminotomy compared with total laminectomy. J Bone Joint SurgBr. 1993;75:386–92. doi: 10.1302/0301-620X.75B3.8496205. [DOI] [PubMed] [Google Scholar]
- 7.Johnson B, Annertz M, Sjoberg C, Stromqvist B. A progressive and consecutive study of surgically treated lumbar spinal stenosis.Part II; Five year follow-up by an independent observer. Spine (Phila Pa 1976) 1997;22:2938–44. doi: 10.1097/00007632-199712150-00017. [DOI] [PubMed] [Google Scholar]
- 8.Johnson B, Annertz M, Sjoberg C, Stromqvist B. A progressive and consecutive study of surgically treated lumbar spinal stenosis.Part I: Clinical features related to radiographic findings. Spine (Phila Pa 1976) 1997;22:2932–7. doi: 10.1097/00007632-199712150-00016. [DOI] [PubMed] [Google Scholar]
- 9.Shenkin HA, Hash CJ. Spondylolisthesis after multiple bilateral laminectomies and facetectomies for lumbar spondylosis.Follow-up review. J Neurosurg. 1979;50:45–7. doi: 10.3171/jns.1979.50.1.0045. [DOI] [PubMed] [Google Scholar]
- 10.Lee CK. Lumbar spine instability (olisthesis) after extensive posterior spinal decompression. Spine (Phila Pa 1976) 1983;8:429–3. doi: 10.1097/00007632-198305000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Frymoyer JW, Henley EN, Jr, Howe J, Kuhlmann D, Matteri RE. A comparison of radiographic findings in fusion and nonfusion patients ten or more years following lumbar disc surgery. Spine (Phila Pa 1976) 1979;4:435–40. doi: 10.1097/00007632-197909000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann TR, Spratt KF, Tozzi JE, Weintein JN, Reinarz SJ, el-Khoury GY, et al. Long-term follow-up of lower lumbar fusion patients. Spine (Phila Pa 1976) 1987;12:97–104. doi: 10.1097/00007632-198703000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Lee CK. Accelerated degenration of the segment adjacent to lumbar fusion. Spine(Phila Pa 1976) 1988;13:375–7. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Bresnahan L, Ogden AT, Natarajan RN, Fessler RG. A biochemical evaluation of graded posterior element removal for treatment of lumbar stenosis: Compresonof a minimally invasive approach with two standard laminectomy techniques. Spine (Phila Pa 1976) 2009;34:17–23. doi: 10.1097/BRS.0b013e318191438b. [DOI] [PubMed] [Google Scholar]
- 15.Postacchini F, Cinotti G, Gumina S. Long term to mid term results of surgery in lumbar spinal stenosis. J Bone Joint SurgBr. 1993;75(Suppl -II):173–4. [Google Scholar]
- 16.Herno A, Airaksinen O, Saari T. Long term to mid term result of surgical treatment of lumbar spinal stenosis. Spine (Phila Pa 1976) 1993;18:1471–4. [PubMed] [Google Scholar]
- 17.Mcullen GM, Bernini PM, Bernstein SH, Tosteson TD. Clinical and Roetgenographic results of decompression for lumbar spinal stenosis. J Spinal Disord. 1994;7:380–7. [PubMed] [Google Scholar]
- 18.Iraksinen O, Herno A, Turnen V, Saari T, Suomlainen O. Surgical outcome of 438 patients treated surgically for lumbar spinal stenosis. Spine (Phila Pa 1976) 1997;22:2278–82. doi: 10.1097/00007632-199710010-00016. [DOI] [PubMed] [Google Scholar]
- 19.Niggemeyer O, Strauss JM, Schulitz KP. Comparison of surgical procedures for degenerative lumbar spinal stenosis: A meta-analysis of the literature from 1975 to 1995. Eur Spine J. 1997;6:423–9. doi: 10.1007/BF01834073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lguchi T, Kurihara A, Nakayama J, Sato K, Kurosaka M, Yamasaki K. Minimum 10 years outcome of decompressive laminectomy for degenerative lumbar spinal stenosis. Spine (Phila Pa 1976) 2000;25:1754–9. doi: 10.1097/00007632-200007150-00003. [DOI] [PubMed] [Google Scholar]
- 21.Herkowitz HN, Kurz LT. Degenerative lumabrspondylolisthesis with spinal stenosis: A prosepective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint SurgAm. 1991;73:802–8. [PubMed] [Google Scholar]
- 22.Bridwell KH, Sedgewick TA, O’Brien MF, Lenke LG, Baldus C. The role of fusion and instrumentation in the treatment of degenerative sopdylolisthesis with spinal stenosis. J Spinal Disord. 1993;6:461–72. doi: 10.1097/00002517-199306060-00001. [DOI] [PubMed] [Google Scholar]
- 23.diPierro CG, Helm GA, Shaffrey CI, Chadduck JB, Henson SL, Malik JM, et al. Treatment of lumbar spinal stenosis by extensive unilateral decompression and contralateral autologous bone fusion: operative technique and results. J Neurosurg. 1996;84:166–73. doi: 10.3171/jns.1996.84.2.0166. [DOI] [PubMed] [Google Scholar]
- 24.Katz JN, Lipson SJ, Lew RA, Grobler LJ, Weinstein JN, Brick GW, et al. Lumbar laminectomy alone or with instrumented or noninstrmentedarthrosesis in degenerative lumbar. Spine (Phila Pa 1976) 1997;22:1123–31. doi: 10.1097/00007632-199705150-00012. [DOI] [PubMed] [Google Scholar]
- 25.Johnson KE, Rosen I, Uden A. The natural course of lumbar spinal stenosis. ClinOrthop. 1992;279:82–6. [PubMed] [Google Scholar]
- 26.Spivak J. Degenrativelumbar spinal stenosis. J bone Joint SurgAm. 1998;80:1053–66. doi: 10.2106/00004623-199807000-00015. [DOI] [PubMed] [Google Scholar]
- 27.White A, Punjabi M. The problem of clinical instability in the human spine.Philadelphia, etc. J B Lippincott Company. 1990:277–378. [Google Scholar]
- 28.Caputy AJ, Luessehop A. Long-term evaluation of decompressive surgery for degenerative lumbar stenosis. J Neurosurg. 1992;77:669–76. doi: 10.3171/jns.1992.77.5.0669. [DOI] [PubMed] [Google Scholar]
- 29.Katz JN, Lipson SJ, Chang LC, Levine SA, Fossel AH, Liang MH. 7 to 10 years outcome of decompressive surgery of degenerative lumbar spinal stenosis. Spine (Phila Pa 1976) 1996;21:92–8. doi: 10.1097/00007632-199601010-00022. [DOI] [PubMed] [Google Scholar]
- 30.Bresnahan L, Ogden ATY, Natarajan RN, Fessler RG. A biomechanical evaluation of graded posterior element removal for treatment of lumbar stenosis: comparison of a minimally invasive approach with two standard laminectomy techniques. Spine (Phila Pa 1976) 2009;34:17–23. doi: 10.1097/BRS.0b013e318191438b. [DOI] [PubMed] [Google Scholar]
- 31.Tai CL, Hsiesh PH, Chen WP, Chen LH, Chen WJ, Lai PL. Biochemical comperison of lumbar spine instability between laminectomy and bilateral laminotomy for spinal stenosis syndrome – an experimental study in porcine model. BMC MusculoskeletDisord. 2008;11:84. doi: 10.1186/1471-2474-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen LH, Lai PL, Tai CL, Niu C, Fu TS, Chen WJ. The effect of interspinous ligament integrity on adjacent segment instability after lumbar instrumentation and laminectomy – an experimental study in porcine model. Biomed Mater Eng. 2006;16:261–7. [PubMed] [Google Scholar]
- 33.Quint U, Wilke HJ, Laqer F, Claes L. laminectomy and functional impairment of the lumbar spine: the importance of muscle forces in flexible and rigid instrumented stablization – a biochemical study in vitro. Eur Spine J. 1998;7:229–38. doi: 10.1007/s005860050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kato Y, Punjabi M, Nibu K. Biochemical study of lumbar spinal stability after osteoplastic laminectomy. J Spinal Disord. 1998;11:146–50. [PubMed] [Google Scholar]
- 35.Vrannkovic D, Splavski B, Hecimovic I, Glavina K. Spinous process-plasty following laminectomy as a contributing factor to spine stability. Arch Orthop Trauma Surg. 1996;115:211–5. doi: 10.1007/BF00434556. [DOI] [PubMed] [Google Scholar]
- 36.Gardner A, Pande KC. Graf Ligamentoplasty: A 7 year follow-up. Eur Spine J. 2002;11(Suppl 2):157–63. doi: 10.1007/s00586-002-0436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sengupta DK. Dynamic stabilization devices in the treatment of low back pain. Orthop Clin North Am. 2004;35:43–56. doi: 10.1016/S0030-5898(03)00087-7. [DOI] [PubMed] [Google Scholar]
- 38.Kanayama M, Hashimoto T, Shigenobu K. Rationale biomechanics and surgical indications for Graf ligamentoplasty. Orthop Clin North Am. 2005;36:373–7. doi: 10.1016/j.ocl.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 39.Tsuji H, Itoh T, Sekido H, Yamada H, Katoh Y, Makiyama N, et al. Expansive laminoplasty for lumbar spinal stenosis. IntOrthop. 1990;14:309–14. doi: 10.1007/BF00178765. [DOI] [PubMed] [Google Scholar]
- 40.Matsui H, Kanamori M, Ishihara H, Hirano N, Tsuji H. Expansive lumbar laminoplasty for degenerative spinal stenosis in patients below 70 years of age. Eur Spine J. 1997;6:191–6. doi: 10.1007/BF01301435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawaguchi Y, Knamori M, Ishihara H, Kikkawa T, Matsui H, Tsuji H, et al. Clinical and radiograph results of expansive lumbar laminoplasty in patients with spinal stenosis. J Bone Joint SurgAm. 2004;86-A(8):1698–703. doi: 10.2106/00004623-200408000-00013. [DOI] [PubMed] [Google Scholar]


