Abstract
Background:
This study was designed to evaluate the effect of intensive insulin control (IIT) on outcomes for traumatically injured patients as a function of injury severity score (ISS) and age.
Patients and Methods:
A retrospective review of 2028 adult trauma patients admitted to the surgical intensive care unit (SICU) in a Level I trauma center was performed. Data were collected from a 48-month period before (Pre-IIT) (goal blood glucose 80–200 mg/dL) and after (Post-IIT) (goal blood glucose level 80–110 mg/dL), an IIT protocol was initiated. Patients were stratified by age and ISS. The primary endpoint was mortality.
Results:
There were 784 Pre-IIT and 1244 Post-IIT patients admitted. There was no significant difference between Pre-IIT vs. Post-IIT for the mechanism of injury or ISS. Values for the Pre-IIT group were significantly higher for mortality (21.5% vs. 14.7%, P<0.001) and hospital, but not ICU length of stay were decreased. A significant improvement in mortality was demonstrated between Pre-IIT vs. Post-IIT stratified within the age groups of 41–50, 51–60, and 61 but not the groups 18–30 and 31–40. Mean glucose levels (mg/dL) decreased significantly after the institution of IIT (144.7±1.4 vs. 130.9±0.9; P<0.001). In addition, the occurrence per patient of blood glucose levels <40 mg/dL increased (0.77% vs. 2.86%; P=0.001) and blood glucose levels greater than 200 mg/dL was similar (39.1% vs. 38.8%; P=0.892) in the Pre-IIT and Post-IIT groups, respectively. Glycemic variability, reflected by the standard deviation of each patient's mean glucose level during ICU stay, as well as mean glucose level were lower in survivors than in nonsurvivors. Finally, multivariable logistic regression analysis identified both mean glucose level and glycemic variability as independent contributors to the risk of mortality.
Conclusions:
The implementation of IIT has been associated with a decrease in both hospital length of stay as well as mortality. Average glucose value and glucose variability are independent predictors of survival. Trauma patients with moderate, severe, and very severe injuries benefit most from IIT. These observational data suggest that patients over 40 years of age benefited a great deal more than their younger counterparts from IIT. This study supports the need for a randomized controlled trial to investigate the role of IIT in traumatically injured patients.
Keywords: Age, glucose, glycemic control, ISS, insulin, intensive insulin control, trauma
INTRODUCTION
Hyperglycemia in the critically ill patient has been a topic of great debate in the 21st century. Critical illness and/or injury disrupts the normal homeostasis of the body through alterations in autonomic and cytokine responses.[1] Trauma-induced hyperglycemia is thought to be secondary to elevated levels of epinephrine, glucagon, and cortisol.[2,3] Views of this phenomenon as a protective response mechanism were brought into question by Van den Berghe et al. who showed that morbidity and mortality in the SICU could be reduced by intensive insulin control (IIT) (80–110 mg/dL).[4] These results prompted multiple investigations evaluating the effects of IIT in other critically ill medical and surgical populations with varied results.[3,5–10] Despite this lack of consensus, IIT has been proposed as the standard of care and endorsed as a level I recommendation by the Institute for Health Care Improvement.[11]
While trauma patients are often mentioned as a subpopulation in larger reports, studies focusing on the outcomes of trauma patients undergoing IIT have been somewhat limited.[4,6] Outcomes of trauma patients following IIT protocols were compared to historical controls, with improved mortality in patients having fewer episodes of blood glucose greater than 150 mg/dL.[12] However, two recent studies found that mortality remained unchanged or increased with the implementation of IIT.[3,7] Multiple studies have discovered independent associations between hyperglycemia and mortality, infectious co-morbidities, and length of stay in the hospital (HLOS) and intensive care unit (ILOS).[1,13] In addition, several authors have suggested a strong association between increased glycemic variability and increased mortality in critically ill patients.[14,15]
It remains unclear if IIT benefits trauma patients or if hyperglycemia is a marker for increased injury severity. Our objective was to evaluate the effect of a IIT protocol on trauma patients and to better characterize the patient populations which benefit from this intervention.
PATIENTS AND METHODS
With approval of our institutional review board, we performed a retrospective cohort review of all trauma patients ≥18 years of age seen at our level I trauma center. Patient records were excluded for incomplete data. Patient records were separated into those admitted in the 48 months prior to (Pre-IIT) the implementation and the 48 months following (Post-IIT) the implementation of a IIT protocol in our unit. Changes in outcome measures were compared between the Pre-IIT and Post-IIT groups. Prior to initiating our IIT protocol, there was no specific blood glucose control protocol, and hyperglycemia was treated primarily with subcutaneous insulin, with overall target blood glucose of 80-200 mg/dL. The individual patient goal blood glucose range was determined by the trauma surgery attending and usually not initiated until blood sugars were noted to be consistently greater than 200 mg/dL. The IIT protocol calls for maintenance of blood glucose values between 80 and 110 mg/dL utilizing an insulin infusion standardized order set which is part of the ICU admission packet. Blood glucose levels were checked every 2 h by bedside blood glucose analyzer (Accucheck) and infusion titrated based on a nomogram to increase the rate of infusion and IV push insulin for values over 241 mg/dL. Blood samples were obtained from either capillary finger stick or arterial catheter samples. This protocol was utilized for all patients admitted to the intensive care unit (ICU). Patients were transitioned to sliding scale insulin with basal insulin administration upon discharge from the ICU. Demographic and outcome data gathered from these patients’ records included: age, injury severity score (ISS), ILOS, HLOS, method of injury (blunt vs. penetrating), closed head injury (CHI), past medical history, admission glucose level (ADMglucose), average blood glucose levels (AVEglucose), single episode of hypoglycemia (Hypo 40) (glucose <40 mg/dL), single episode of hyperglycemia (Hyper 200) (glucose >200 mg/dL), and the primary outcome variable, in-hospital mortality. Glucose variability was also evaluated as the standard deviation of glucose values for the admission. A univariate and multivariate risk factor analysis was performed for mortality.
Potential confounders for mortality included age and severity of injury as depicted by ISS. These variables were included in a subgroup analysis. Stratification by age was then analyzed with groups consisting of patients aged 18–30, 31–40, 41–50, 51–60, and 60+. Patients were also stratified by ISS: mild (1–8), moderate (9–15), severe (16–24), and very severe (25+). Mortality was assessed and compared between the Pre-IIT and Post-IIT cohorts. A statistical analysis was applied to all data comparisons looking for significant changes in outcomes following IIT protocol implementation using the number cruncher statistical system (NCSS) software package version 2004 (NCSS, Kaysville, UT). Unpaired t-tests were performed on continuous data (summarized using means and standard error of the mean or median and range) and either χ2 or Fisher's exact tests were used when analyzing nominal qualitative data (summarized using counts and percentages). Differences were considered statistically significant at P<0.05. A logistic regression model was used to evaluate IIT as a predictor of death independent of other potential factors based on univariate analysis with inclusion of factors with P<0.1.
RESULTS
Description of the study population
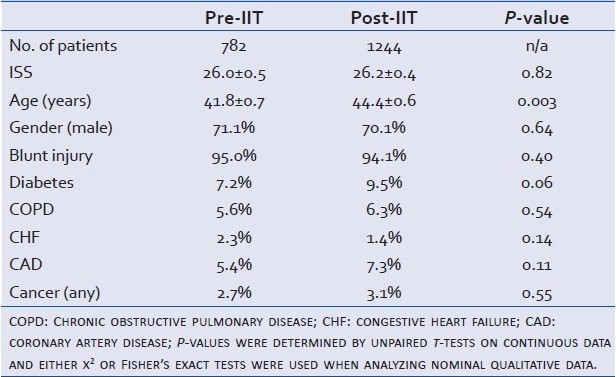
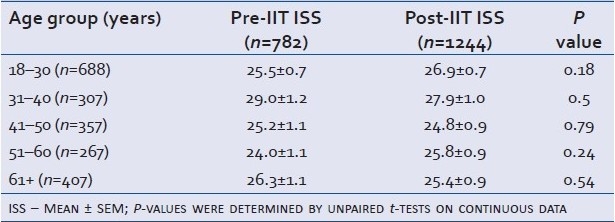
A total of 2028 trauma patients ≥ 18 years of age were admitted to the trauma intensive care unit over the 96-month period. There were 784 patients in the Pre-IIT group and 1244 in the Post-IIT group. Two patients in the Pre-IIT group had incomplete records without an ISS and were excluded leaving a total of 782. Demographic data are shown in Table 1.
Table 1.
Cohort demographics: age and ISS presented as mean ± SEM
Outcome measures
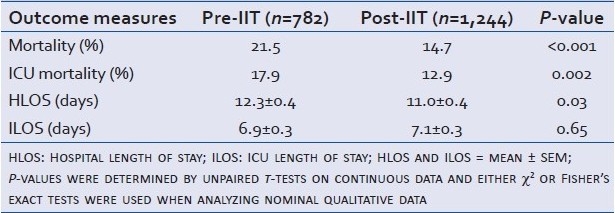
The outcome measures used to judge the significance of the IIT protocol are shown in Table 2. Unadjusted mortality decreased significantly following IIT implementation, with a relative risk of mortality in the Post-IIT group of 0.68 (95% CI: 0.57–0.83).
Table 2.
Comparison of treatment outcomes: Pre-IIT vs. Post-IIT
Effect of ISS
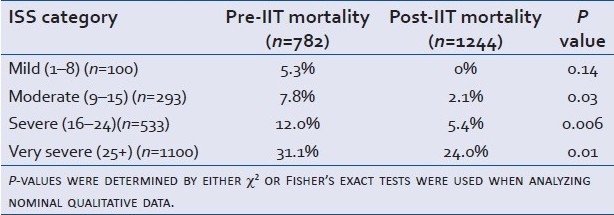
Table 3 illustrates the differences in mortality seen Pre-IIT and Post-IIT, stratified by ISS. Mortality did not change significantly among the mildly injured. It did, however, decrease in those with moderate, severe, and very severe injuries.
Table 3.
Mortality comparison stratified by ISS
Effect of age
Stratification of our data by age is shown in Table 4. The two youngest populations—those aged 18–30 and 31–40—failed to show statistically significant drops in mortality following IIT. However, our data provide strong evidence that the older populations aged 41–50, 51–60, and 61+ benefited from IIT implementation with regard to mortality. These differences were independent of ISS as seen in Table 5.
Table 4.
Mortality comparison stratified by decade of life
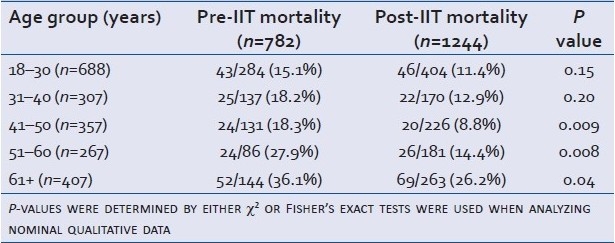
Table 5.
ISS comparison stratified by decade of life
Combined effect of age and ISS
After determining that patients benefited differently, based on both age and ISS, survival differences were determined based both on their severity of injury and by their age. Of the 782 Pre-IIT, 421 were between the ages of 18 and 40. The remaining 361 were aged 41 years and older. In the Post-IIT group (n=1,244), 574 were 18–40 years old and 670 were 41 or older. The results presented below [Table 6] show the percent decreases in mortality for these categories. This aggregate data demonstrate a significant improvement in mortality in the older cohort for moderate-to-very severely injured patients. In addition, a trend toward improved mortality can be observed in the combined <40-year-old cohort overall.
Table 6.
Mortality comparison stratified by ISS designation and patient age being 18–40 years and 41+ years

Effect on glycemic control
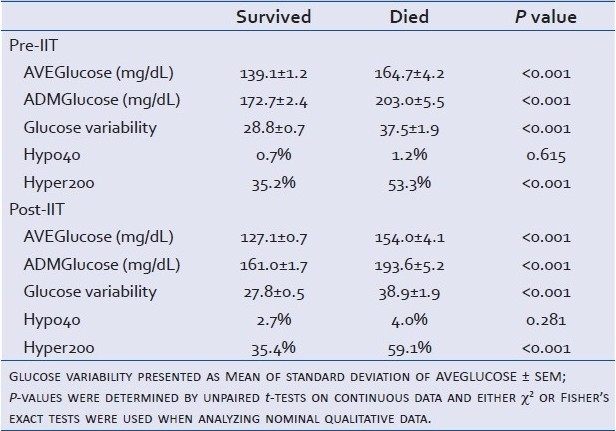
Mean glucose levels (mg/dL±SEM) decreased significantly after the institution of IIT (144.7±1.4 vs. 130.9±0.9; P<0.001); however, glucose variability (mg/dL ± SEM) based on standard deviation of glucose values remained similar (30.74±0.7 vs. 29.33±0.5; P=0.061). In addition, the occurrence per patient of blood glucose levels less than 40 mg/dL increased (0.77% vs. 2.86%; P=0.001) and blood glucose levels greater than 200 mg/dL were similar (39.1% vs. 38.8%; P=0.892) in the Pre-IIT and Post-IIT groups, respectively. However, the AVEglucose, ADMglucose, glucose variability, and HYPER 200 values were significantly decreased in survivors in both the Pre-IIT and Post-IIT groups [Table 7].
Table 7.
Glucose variables stratified by Pre-IIT and Post-IIT: AVEGlucose and ADMGlucose presented as mean ± SEM
Univariate and multivariate analysis
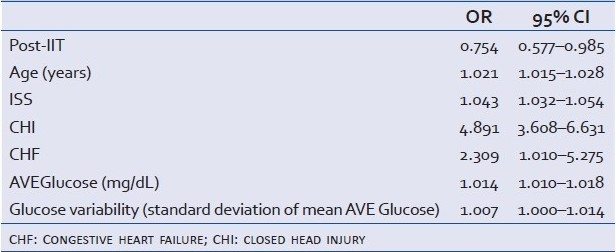
Univariate logistic regression analysis of the relationship of age, ISS, Pre-IIT, CHI, diabetes, COPD, CHF, CAD, cancer, ADMglucose, AVEglucose, hypo 40, hyper 200, and glucose variability to mortality showed a significant relationships (P<0.1) between all variables and mortality except COPD and hypoglycemia [Table 8]. Multivariate logistic regression analysis, incorporating those variables with P<0.1, showed that the odds ratio of mortality in the Post-IIT group was 0.754 (95% CI: 0.577–0.985) [Table 9].
Table 8.
Cohort demographics and glucose variables: Age, ISS, AVEGlucose, and ADMGlucose presented as mean ± SEM
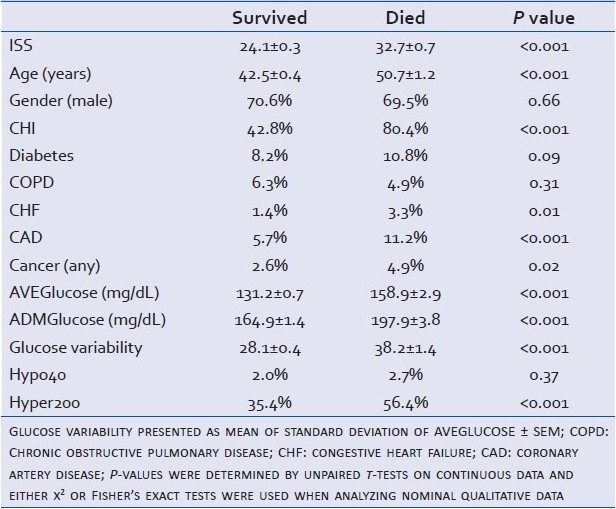
Table 9.
Multivariate analysis
DISCUSSION
Trauma-induced hyperglycemia has been hypothesized to be a protective mechanism of the body, providing increased substrate for metabolism in glucose-dependent tissues such as white blood cells, brain tissue, and smooth muscle. In 2001, this concept was brought into question by a landmark article from Van den Berghe et al.[4] The origin of hyperglycemia in the acutely stressed patient is multifactorial and represents the influence of both endogenous and exogenous forces. In spite of often normal insulin levels, catecholamine, glucocorticoid, glucogon, and cytokine levels are all increased in the stressed patient.[16–19] The optimal treatment for this resultant hyperglycemia in trauma patients has been the subject of several investigations and continues to be a topic of great debate since the publication of the NICE-SUGAR trail results noting that trauma patients were a population which may benefit from IIT.[5] Clearly in both our Pre-IIT and Post-ITT groups decreased AVEglucose, ADMglucose, glucose variability, and HYPER 200 are associated with survival.
IIT has become a standard practice in the ICU, although the patients who would benefit most from this therapy remain to be determined. The identification of these populations has become of greater importance after the Joint Commission and Accreditation of Health Organizations identified glucose control as a “core measure” in certain populations to decrease hospital-acquired infections.[3]
Multiple investigators have noted that hyperglycemia at admission and during the hospital stay is associated with increased mortality.[13,20] Mean glucose concentration decreased from 145 to 130 mg/dL after the initiation of an IIT protocol in our study. These results are similar to the effects noted by Krinsley who noted that the average glucose levels in their subjects decreased from 152 to 131 mg/dL after instituting IIT.[21]
The risk of hypoglycemia resulting from insulin therapy is the most common concern in the literature.[4,6,8,9,22] In our study, the occurrence of hypoglycemia per patient, glucose <40 mg/dL, increased from 0.77% to 2.86% with the institution of IIT. This indicates that IIT may be implemented with a limited, but real increase in the incidence of hypoglycemia. However, short- and long-term sequellae of hypoglycemia observed, such as seizure, headache, dysarthria, or impaired judgment, are very difficult to quantify.[22] We did not observe an association between hypoglycemia and mortality which is consistent with a recent report by Mowery et al.[23]
Debate exists over the need for IIT in patients outside of the ICU. Van den Berghe et al. noted in their mixed medical/surgical ICU evaluation of IIT that patients with ILOS <3 days did not show a mortality benefit from IIT.[21] In our study, stratification by ISS revealed that the mildly injured patients (ISS <9) failed to confirm any mortality benefit from IIT, while the reverse was true for patients with ISS ≥ 9. With only 100 patients in the ISS <9 group, our study is underpowered to reveal any significant effect in this grouping due to low expected mortality rate. Given this, IIT may provide a survival benefit for all traumatically injured patients.
One of the greatest criticisms of the 2001, Van den Berghe et al. article is that subsequent randomized controlled trials (RCTs) have failed to reproduce this mortality improvement.[24] A recent meta-analysis of RCTs evaluating IIT noted a wide range in the mean age (46–75 years).[24] Our data indicate a mortality benefit for patients over 40 years of age. Several of the studies that failed to show a mortality improvement had study populations with lower mean ages than did the original 2001 article, which could offer some explanation to the mixed results seen by many researchers trying to confirm the findings of the Van den Berghe et al. study.[4,20,24] Two possible hypothesis for this difference would be an increased insulin resistance or decreased insulin production in critically injured patients at different ages.
As with all studies of a retrospective nature, our data have several limitations. One must consider if other changes in care protocols and guidelines over the time period studied may have influenced the outcome. Management of the critically ill patient is rapidly evolving with regular changes in treatment thresholds for acute respiratory distress syndrome, blood transfusions, infections, and shock. In a similar study design, Scalea et al. noted that hyperglycemia may be a result of less aggressive management by the attending trauma surgeon or patients who, in spite of aggressive glycemic management, are challenging to control.[16] In addition, with any subgroup analysis, the power of the study must be considered based on the number of patients in each group. This may have limited our ability to demonstrate a difference in mortality in the younger age groups. Lastly, our IIT protocol used bedside glucose monitors to determine levels from both arterial and capillary blood samples. The accuracy of these glucose detectors compared to laboratory values was not calibrated. This could introduce a measurement bias into the study that is impossible to control for retrospectively. Although there are many potential confounding issues with this study design, the subgroup analysis demonstrates populations which IIT may lead to a lower mortality in trauma patients.
In conclusion, we found that IIT, resulting in glucose values with a mean of 130.9 mg/dL, was associated with decreased blood glucose values, mortality, and HLOS in trauma patients at our Level I trauma center. When controlling for age and ISS, we found IIT, lower average glucose value, and lower glucose variability are independent predictors of decreased mortality. Trauma patients over age 40 and those moderately, severely, or very severely injured showed the greatest reduction in mortality through the use of the IIT protocol. These findings, in addition to the NICE-SUGAR results, support the need for a randomized controlled trial to investigate the role of IIT in traumatically injured patients.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Scalea TM, Bochicchio GV, Bochicchio KM, Johnson SB, Joshi M, Pyle A. Tight glycemic control in critically injured trauma patients. Ann Surg. 2007;246:605–10. doi: 10.1097/SLA.0b013e318155a789. [DOI] [PubMed] [Google Scholar]
- 2.Collier B, Dossett LA, May AK, Diaz JJ. Glucose control and the inflammatory response. Nutr Clin Pract. 2008;23:3–15. doi: 10.1177/011542650802300103. [DOI] [PubMed] [Google Scholar]
- 3.Wahl WL, Taddonio M, Maggio PM, Arbabi S, Hemmila MR. Mean glucose values predict trauma patient mortality. J Trauma. 2008;65:42–7. doi: 10.1097/TA.0b013e318176c54e. [DOI] [PubMed] [Google Scholar]
- 4.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 5.Finfer S, Chittock DR, Su SY, Blair D, Foster D, Dhingra V, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–97. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 6.Krinsley JS. Glycemic control, diabetic status, and mortality in a heterogeneous population of critically ill patients before and during the era of intensive glycemic management: Six and one-half years experience at a university-affiliated community hospital. Semin Thorac Cardiovasc Surg. 2006;18:317–25. doi: 10.1053/j.semtcvs.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Toschlog EA, Newton C, Allen N, Newell MA, Goettler CE, Schenarts PJ, et al. Morbidity reduction in critically ill trauma patients through use of a computerized insulin infusion protocol: A preliminary study. J Trauma. 2007;62:1370–5. doi: 10.1097/TA.0b013e318047b7dc. [DOI] [PubMed] [Google Scholar]
- 8.Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–61. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 9.Van den Berghe G, Wilmer A, Milants I, Wouters PJ, Bouckaert B, Bruyninckx F, et al. Intensive insulin therapy in mixed medical/surgical intensive care units: Benefit versus harm. Diabetes. 2006;55:3151–9. doi: 10.2337/db06-0855. [DOI] [PubMed] [Google Scholar]
- 10.Vogelzang M, Nijboer JM, van der Horst IC, Zijlstra F, ten Duis HJ, Nijsten MW. Hyperglycemia has a stronger relation with outcome in trauma patients than in other critically ill patients. J Trauma. 2006;60:873–7. doi: 10.1097/01.ta.0000195715.63978.80. [DOI] [PubMed] [Google Scholar]
- 11.Marik PE, Varon J. Intensive insulin therapy in the ICU: Is it now time to jump off the bandwagon? Resuscitation. 2007;74:191–3. doi: 10.1016/j.resuscitation.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Collier B, Diaz J, Jr, Forbes R, Morris J, Jr, May A, Guy J, et al. The impact of a normoglycemic management protocol on clinical outcomes in the trauma intensive care unit. JPEN J Parenter Enteral Nutr. 2005;29:353–358. doi: 10.1177/0148607105029005353. discussion 359. [DOI] [PubMed] [Google Scholar]
- 13.Yendamuri S, Fulda GJ, Tinkoff GH. Admission hyperglycemia as a prognostic indicator in trauma. J Trauma. 2003;55:33–8. doi: 10.1097/01.TA.0000074434.39928.72. [DOI] [PubMed] [Google Scholar]
- 14.Egi M, Bellomo R, Stachowski E, French CJ, Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105:244–52. doi: 10.1097/00000542-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Krinsley JS. Glycemic variability: A strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008–13. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 16.Cherrington AD: Banting Lecture 1997: Control of glucose uptake and release by the liver in vivo. Diabetes. 1999;48:1198–214. doi: 10.2337/diabetes.48.5.1198. [DOI] [PubMed] [Google Scholar]
- 17.Gale SC, Sicoutris C, Reilly PM, Schwab CW, Gracias VH. Poor glycemic control is associated with increased mortality in critically ill trauma patients. Am Surg. 2007;73:454–60. doi: 10.1177/000313480707300507. [DOI] [PubMed] [Google Scholar]
- 18.Marik PE, Raghavan M. Stress-hyperglycemia, insulin and immunomodulation in sepsis. Intensive Care Med. 2004;30:748–56. doi: 10.1007/s00134-004-2167-y. [DOI] [PubMed] [Google Scholar]
- 19.Montori VM, Bistrian BR, McMahon MM. Hyperglycemia in acutely ill patients. JAMA. 2002;288:2167–9. doi: 10.1001/jama.288.17.2167. [DOI] [PubMed] [Google Scholar]
- 20.Bochicchio GV, Sung J, Joshi M, Bochicchio K, Johnson SB, Meyer W, et al. Persistent hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;58:921–4. doi: 10.1097/01.ta.0000162141.26392.07. [DOI] [PubMed] [Google Scholar]
- 21.Krinsley JS. Effect of an intensive glucose management protocol on the mortality of critically ill adult patients. Mayo Clin Proc. 2004;79:992–1000. doi: 10.4065/79.8.992. [DOI] [PubMed] [Google Scholar]
- 22.Krinsley JS, Grover A. Severe hypoglycemia in critically ill patients: Risk factors and outcomes. Crit Care Med. 2007;35:2262–7. doi: 10.1097/01.CCM.0000282073.98414.4B. [DOI] [PubMed] [Google Scholar]
- 23.Mowery NT, Guillamondegui OD, Gunter OL, Diaz JJ, Jr, Collier BR, Dossett LA, et al. Severe hypoglycemia while on intensive insulin therapy is not an independent predictor of death after trauma. J Trauma. 2010;68:342–7. doi: 10.1097/TA.0b013e3181c825f2. [DOI] [PubMed] [Google Scholar]
- 24.Wiener RS, Wiener DC, Larson RJ. Benefits and risks of tight glucose control in critically ill adults: A meta-analysis. JAMA. 2008;300:933–44. doi: 10.1001/jama.300.8.933. [DOI] [PubMed] [Google Scholar]


