Abstract
Aim:
To determine the effect of conductive keratoplasty (CK) for the treatment of induced hyperopia and astigmatism after complicated myopic laser in situ keratomileusis (LASIK) or photorefractive keratectomy (PRK).
Materials and Methods:
In this interventional case series, 11 eyes of seven subjects with a history of previous LASIK or PRK with inadequate stromal bed or flaps complications were enrolled. Inclusion criteria included residual spherical hyperopia of 1.00 to 3.00 diopters (D) and cylinder of –0.75 to –3.00 D. The modified Refractec nomogram and the LightTouch technique of CK were performed on all eyes. To treat cylinder, a pair of spots per –0.75 D of cylinder were delivered to the flat meridian. Uncorrected visual acuity at near and far (UCVAN and UCVAF respectively, logMAR), best corrected VA at near and far (BCVAN and BCVAF respectively, logMAR) were measured. Refractive outcome, contrast sensitivity, wave front aberrations were measured preoperatively and postoperatively. Statistical analysis was performed with the Wilcoxon signed rank test and the repeated measures analysis of variance with P<0.05 indicating statistically significant change from preoperatively to 1 year postoperatively.
Results:
The mean preoperative sphere (MS) was 2.57 ± 1.19 D and cylinder (MC) was –1.5 ± 0.49 D. Postoperatively, there was a significant decrease in MS to 0.36±0.98 D (P=0.003) and MC to –1.25 ± 0.76 D at 1 year (P<0.05, both cases). Spherical equivalent (SE) significantly decreased from +2.13 ± 1.09 D to –0.47 ± 1.29 D (P<0.001). The mean UCVAN significantly improved from 0.56 ± 0.32 preoperatively to 0.17 ±0.16 postoperatively (P=0.003). The mean UCVAF was 0.29 preoperatively and 0.22 postoperatively (P=0.353). Mean BCVAN was 0.18 and 0.02 after surgery, and mean BCVAF for far was 0.07 (P>0.05, both cases).
Conclusions:
CK is a predictable and reliable method to correct hyperopia after LASIK and PRK, however cylinder correction may induce irregular and unpredictable outcomes and a modified nomogram is required for further studies.
Keywords: Astigmatism, Conductive Keratoplasty, Hyperopia, Laser In Situ Keratomileusis, Photorefractive Keratectomy
INTRODUCTION
The prevalence refractive error has been reported to be up to 75% of the population, with the incidence of myopia and astigmatism far higher than hyperopia.1 Fortunately, all types of refractive error, regardless of their magnitude, can be corrected using a variety of surgical or optical techniques.2–4 Hyperopia and or astigmatism induced after laser in situ keratomileusis (LASIK) or photorefractive keratectomy (PRK) can be challenging for both patient and surgeon.5–8 Treatment of residual hyperopia after flap-related complications during LASIK or and inadequate residual stromal thickness after PRK (or LASIK) often require non-ablative procedures such as laser thermo-keratoplasty (LTK) or conductive keratoplasty (CK).
Refractive outcomes and stability of CK for low to moderate hyperopia have been reported to be equivalent or better than hyperopic LASIK or non-contact LTK.9 CK may result in an improvement in corneal optics and vision in subjects with complicated LASIK or PRK.10 The ViewPoint CK system from Refractec, Inc. [Figure 1] used in this study consists of a console that produces radiofrequency energy (RF), a disposable pen-shaped hand piece connected to the console, a foot pedal to turn the current on and off, and an electrically grounded speculum. The energy level is 0.6 W with a 0.6 s exposure time. The tip of the probe contains a disposable stainless metal needle with a diameter of 90 μm and length of 450 μm that transmits the RF electrical energy directly to the corneal stroma. The probe contains a proximal curvature of 45° and a distal angulation of 90µ to easily access the eye over the eyebrows and nose.11–13
Figure 1.

The CK system consists of a marker, a keratoplasty hand piece and its tip, a pedal, and an electrically grounded eyelid speculum. The console weighs 14 pounds
The outcomes of CK for the treatment of residual hyperopia following LASIK have been previously reported. Alio et al., found that CK was effective but predictability was poor and suggested that stability required longer follow-up.14 Asbell et al., reported CK to be effective and very safe for the treatment of low ranges of primary hyperopia and astigmatism.15 The present study was conducted to determine the efficacy of CK for the treatment of residual hyperopia and/or astigmatism after LASIK and PRK.
MATERIALS AND METHODS
Seven subjects (11 eyes) were enrolled in this interventional 1 year study conducted at the Noor Eye Hospital and Clinic during 2006-2007. Inclusion criteria included subjects with post-LASIK or post-PRK hyperopia from 1.00 to 3.00 D and astigmatism from 0.75 to 3.00 D for a year longer, and were not candidate for further ablation due to inadequate residual stromal bed thickness or flap-related complications such a thin or incomplete flap. Other inclusion criteria were a best corrected visual acuity (BCVA) of 20/40 or better and a minimum corneal thickness of 560 μm within the central 7.0 mm optical zone. Subjects with corneal disease, glaucoma, diabetes, or retinal disease were excluded. The study proposal was reviewed and approved by the Review Board of Noor Ophthalmology Research Center and all subjects consented to participate in this study.
Surgery was performed under topical anesthesia by instilling two drops of tetracaine 0.5% separated by a 5 min interval. In astigmatic subjects, the corneal periphery was marked with an insulin needle tip at 6 and 12 o’clock at the slit lamp. Once the patient was supine under the surgical microscope, the lid speculum was inserted for maximum globe exposure. The patient was instructed to fixate on the microscope light and the optical center of the cornea was marked with the tip of a Sinskey hook. A 7 mm optical zone marker with eight marks soaked in Gentian violet was used to mark the placement of spots on the cornea.
Hyperopia was corrected using the Light Touch surgical technique and the modified nomogram provided by Refractec, Inc. (Irvine, CA, USA). For the treatment of astigmatism, a pair of CK spots for every –0.75 D was applied to the 7.0 mm optical zone on the flat meridian base to the Orbscan (Bausch and Lomb Inc., Rochester, NY, USA) axial (keratometric) topography map.
Mixed hyperopic astigmatic subjects were treated according to the nomogram for hyperopia with extra spots applied on the flat meridian.
Postoperative care included placement of bandage contact lenses and instilling topical chloramphenicol every 6 h for 3 days, artificial tears every 4 h for 2 weeks, and diclofenac (Voltaren) 0.1% every 6 h for 2 days after surgery. In the first week and first and third months follow-up visits, refraction, uncorrected visual acuity, and best corrected visual acuity for far and near (UCVAF, BCVAF, UCVAN, BCVAN, respectively) were measured. Near vision was tested monocularly at 40 cm. Safety and efficacy indices were calculated. The safety index was defined as postoperative BSCVA/preoperative BSCVA. The efficacy index was calculated as postoperative UCVA/preoperative BSCVA. At 1 year postoperatively, depth of focus which is the range of clear near vision in centimeters was measured under cycloplegia 30 min after instilling two drops of 0.5% cyclopentolate with 5 min intervals and adequate reading glasses. Contrast sensitivity was measured with the CSV-1000 HGT instrument (VectorVision, Greenville, OH, USA) with and without glare with best spectacle correction. All measurements were obtained under photopic conditions (85 cd/m2). Wave front analysis was performed on pupils dilated with phenylephrine hydrochloride and tropicamide 1% eye drops with the Zywave aberrometer (Bausch and Lomb Inc., Rochester, NY, USA). Ocular (whole eye) higher order wave front aberrations are reported to the fifth-order Zernike aberrations for spherical aberration (SAB), coma, trefoil, and mean root mean square (RMS) values for a 6 mm pupil diameter.
Statistical analyses were performed with SPSS 11.5 software (IBM Inc., Somers, NY, USA). As the variables were paired and the objective was to assess the effect of the intervention through the comparison of preoperative and postoperative data with a non-normal distribution, we used the Wilcoxon signed rank test to compare 12 month outcomes to preoperative data. To assess differences between preoperative data and data at 1 month, 3 months, and 1 year postoperatively, we used the repeated measures analysis of variance. All assumptions were tested at 95% confidence levels.
RESULTS
All 11 subjects completed the 1 year postoperative follow-up. The mean age of the cohort was 46.3 ±6.2 years. Four eyes had a history of PRK and seven eyes had undergone LASIK surgery. Hyperopia was greater than +1.00 D in all cases and 45% of the cases had astigmatism equal to or greater than 1.00 D [Table 1]. The mean sphere (MS) decreased statistically significantly from 2.57±1.19 D to 0.36±0.98 D (P=0.003). The mean cylinder was -1.5±0.49 D preoperatively which decreased statistically significantly to -1.25±0.76 D at 1 year (P=0.025). The mean spherical equivalent (SE) decreased statistically significantly from +2.13±1.09 D to -0.47±1.29 D at 1 year (P<0.001). Postoperatively, four eyes (36.4%) were within ± 0.5 D and six eyes (54.5%) were within ±1 D of emmetropia.
Table 1.
Subject data and treatment before and 1 year after conductive keratoplasty
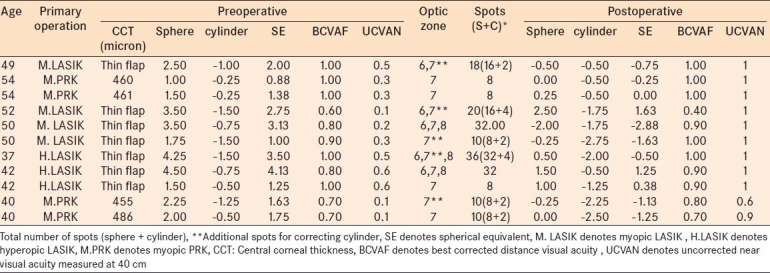
The mean preoperative logMAR UCVAF was 0.28±0.15 (0.50 decimal notation) and BCVAF was 0.07± 0.08 (0.80 decimal notation) with no statistically significant differences at 1 year postoperatively (P=0.353 and P=0.726 respectively) [Figure 2].
Figure 2.
Distance uncorrected corrected visual acuity and best corrected visual acuity. LogMAR notation
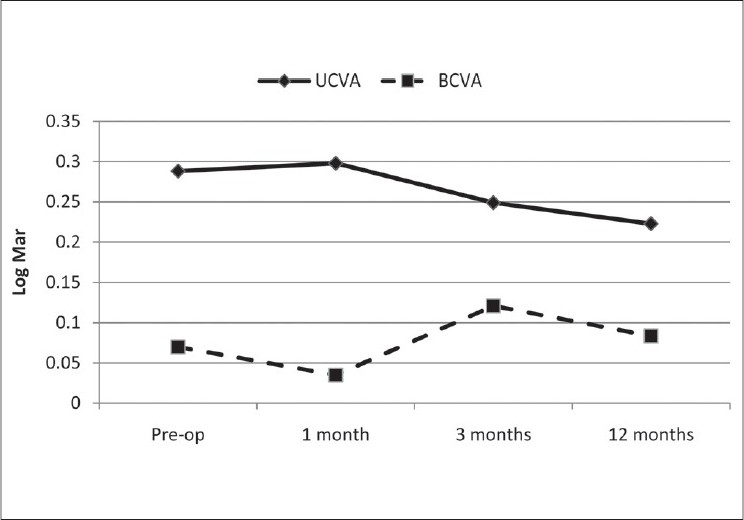
The mean UCVAN improved statistically significantly from 0.56±0.32 (0.30 or J8) to 0.17± 0.16 (0.70 or J3) at 1 year postoperatively. The mean BCVAN was 0.18±0.29 (0.67 J3) preoperatively and 0.02± 0.60 (0.9 or J2) postoperatively (P=0.104) [Figure 3]. The postoperative BCVAF [Table 1] did not show significant change and 45.4% of cases (five eyes) had no change in visual acuity and 36.4% (four eyes) gained 1 line and two eyes lost 1 line and one eye lost 2 line of visual acuity. The distance vision efficacy index was 0.80 and the safety index was 1.01. The mean depth of focus with correction was 18.5 cm which increased to 23.1 cm at 1 year postoperatively (P=0.285).
Figure 3.
Uncorrected corrected visual acuity at near and best corrected visual acuity at. LogMAR notation
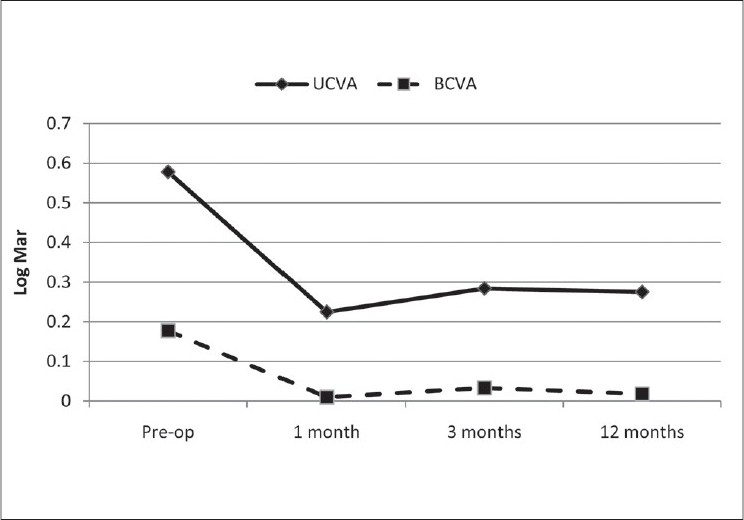
There was no statistically significant change in mean total aberration RMS spherical aberration (SAB), coma, and trefoil at 1 year postoperatively [Figure 4].
Figure 4.
Ocular wave front aberrations preoperatively and 1 year after surgery. All eyes were measured with a pharmacologically dilated pupil. Wave front data are reported for a 6 mm pupil plotted to the fifth Zernike order
There was no statistically significant change in photopic contrast sensitivity with and without glare at 3, 6, 12, and 18 cycles/degrees at1 year postoperatively (P>0. 05 in all cases).
During this study there was no case of infection, and four eyes (36.4 %) had a transient anterior chamber reaction which disappeared in within a week, and half of the subjects complained of moderate ocular pain during the early postoperative period (3--5 days).
DISCUSSION
The present paper documents our experience with CK for the treatment of residual hyperopia and or astigmatism after complicated LASIK and PRK. One important limitation was the small number of subjects, which could have influenced the significance of the correlations, and second was the lack of access to the subjects for longer follow up. However, increasing the sample size would be time consuming as the cohort consisted of subjects who had undergone previous surgery and had a complication that result in a hyperopia which is rare given the success of myopic LASIK. The sample size in similar studies was not significantly different.14,16,17 Initially, we had 13 eyes of eight subjects, but 2 were excluded due to failure to present for follow-up visits.
In this study the goal was to determine the efficacy of CK for the correction of hyperopia and/or astigmatism after complicated LASIK or PRK. The decrease in the mean SE and sphere values was significant. Corneal topographic changes verify this change as a central corneal steepening due to CK [Figure 4], which induces a myopic shift of the eye toward emmetropia to correct the induced hyperopia. In the present study, the SE in 36.4% (4 eyes) was within ±0.50 D and in 54.5% (6 eyes) SE was within ±1.00 D of emmetropia. These data are similar to those reported by Alio et al., who found 49% ±0.50 D and 71% ±1.00 D.14 Despite the small sample size in our study, it seems that subjects with previous myopic LASIK or PRK had a better response and less regression after CK compared to those with previous hyperopic LASIK or PRK.
The mean MC change only +0.25 D postoperatively (P>0.05), indicating that astigmatism correction with CK was not efficacious or predictable in our study. However further studies with a larger sample size of astigmatism and vector analyses are needed to assess the correction of astigmatism after previous LASIK or PRK.
Postoperative UCAF was 0.3 log Mar (0.5) in 81.9% (9 eyes) which is somewhat higher than 69% reported by Alio et al.14 The mean postoperative UCVAN of 0.17 logMAR was statistically significant better than preoperatively (P=0.003). The mean UCVAN in the present study is similar to the 0.18 (0.67 or J3) reported by Stahl et al., on 10 near plano presbyopes.16,17 The improvement in UCVAN in the present study can be attributed to the induced multifocality and the increase in the asphericity (more prolate) of the cornea after CK, resulting in better near vision.
The loss of BCVAF in the present study was similar to that reported by Alio et al.12 The loss in BCVAF can be due to decentration, irregular astigmatism, failure to apply spots evenly, and corneal hydration. Complications included anterior chamber reaction in two subjects during a few days after surgery. This reaction disappeared without sequelae. Moshirfar, et al., also reported a similar finding.18 The observed improvement in mean BCVAN from 0.18 ±0.29 (0.5 or J5) to 0.02 ±0.06 (0.95±0.18 or J1) after surgery indicated the safety of the procedure in terms of near vision [Figure 3]. Wave front analysis and contrast sensitivity measurement showed no statistically significant changes after CK surgery despite the induced multifocality. Currently there are no similar studies in the literature for comparison.
The outcomes of the present study indicate that CK can correct mild to moderate hyperopia after complicated LASIK and PRK in which further ablation is impossible due to flap-related complications or inadequate residual stromal thickness. Pending further studies on the safety of the procedure, the induction of irregular astigmatism or decreased vision remains a risk.14
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.
REFERENCES
- 1.Hyman L. Myopic and hyperopic refractive error in adults: An overview. Ophthalmic Epidemiol. 2007;14:192–7. doi: 10.1080/09286580701535517. [DOI] [PubMed] [Google Scholar]
- 2.Keeney AH, Fintlemann EW, Estlow BR. Refractive correction and associated factors in spectacle glass injuries. Trans Am Ophthalmol Soc. 1971;69:321–35. [PMC free article] [PubMed] [Google Scholar]
- 3.Lee YC, Lim CW, Saw SM, Koh D. The prevalence and pattern of contact lens use in a Singapore community. CLAO J. 2000;26:21–5. [PubMed] [Google Scholar]
- 4.Fong CS. Refractive surgery: The future of perfect vision. Singapore Med J. 2007;48:709–18. [PubMed] [Google Scholar]
- 5.Lui MM, Silas MA, Fugishima H. Complications of photorefractive keratectomy and laser in situ keratomileusis. J Refract Surg. 2003;19:S247–9. doi: 10.3928/1081-597X-20030302-16. [DOI] [PubMed] [Google Scholar]
- 6.Ambrosio R, Jr, Wilson SE. Complications of laser in situ keratomileusis: Etiology, prevention, and treatment. J Refract Surg. 2001;17:350–79. doi: 10.3928/1081-597X-20010501-09. [DOI] [PubMed] [Google Scholar]
- 7.Gimbel HV, Penno EE, van Westenbrugge JA, Ferensowicz M, Furlong MT. Incidence and management of intraoperative and early postoperative complications in 1000 consecutive laser in situ keratomileusis cases. Ophthalmology. 1998;105:1839–47. doi: 10.1016/s0161-6420(98)91026-0. [DOI] [PubMed] [Google Scholar]
- 8.Wilson SE. LASIK: Management of common complications. Laser in situ keratomileusis. Cornea. 1998;17:459–67. doi: 10.1097/00003226-199809000-00001. [DOI] [PubMed] [Google Scholar]
- 9.McDonald MB, Davidorf J, Maloney RK, Manche EE, Hersh P. Conductive keratoplasty for the correction of low to moderate hyperopia: 1-year results on the first 54 eyes. Ophthalmology. 2002;109:637–49. doi: 10.1016/s0161-6420(01)01022-3. [DOI] [PubMed] [Google Scholar]
- 10.Hersh PS, Fry KL, Chandrashekhar R, Fikaris DS. Conductive Keratoplasty to Treat Complications of LASIK and Photorefractive Keratectomy. Ophthalmology. 2005;112:1941–7. doi: 10.1016/j.ophtha.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Du TT, Fan VC, Asbell PA. Conductive keratoplasty. Curr Opin Ophthalmol. 2007;18:334–7. doi: 10.1097/ICU.0b013e3281df2cf0. [DOI] [PubMed] [Google Scholar]
- 12.McDonald MB. Conductive keratoplasty: A radiofrequency-based technique for the correction of hyperopia. Trans Am Ophthalmol Soc. 2005;103:512–36. [PMC free article] [PubMed] [Google Scholar]
- 13.Hashemi H, Habibollahi AR. Conductive keratoplasty to correct Residual Hyperopia and Astigmatism after cataract surgery. Iran J Ophthalmol. 2008;20:3–9. [Google Scholar]
- 14.Alio JL, Ramzy MI, Galal A, Claramonte PJ. Conductive keratoplasty for the correction of residual hyperopia after LASIK. J Refract Surg. 2005;21:698–704. doi: 10.3928/1081-597X-20051101-07. [DOI] [PubMed] [Google Scholar]
- 15.Asbell PA, Maloney RK, Davidorf J, Hersh P, McDonald M, Manche E. Conductive keratoplasty for the correction of hyperopia. Trans Am Ophthalmol Soc. 2001;99:79–84. [PMC free article] [PubMed] [Google Scholar]
- 16.Stahl JE. Conductive keratoplasty for presbyopia: 3-year results. J Refract Surg. 2007;23:905–10. doi: 10.3928/1081-597X-20071101-07. [DOI] [PubMed] [Google Scholar]
- 17.Stahl JE. Conductive keratoplasty for presbyopia: 1-year results. J Refract Surg. 2006;22:137–44. doi: 10.3928/1081-597X-20060201-10. [DOI] [PubMed] [Google Scholar]
- 18.Moshirfar M, Feilmeier M, Kumar R. Anterior chamber inflammation induced by conductive keratoplasty. J Cataract Refract Surg. 2005;31:1676–7. doi: 10.1016/j.jcrs.2005.07.029. [DOI] [PubMed] [Google Scholar]


