Abstract
Whole slide imaging (WSI), or “virtual” microscopy, involves the scanning (digitization) of glass slides to produce “digital slides”. WSI has been advocated for diagnostic, educational and research purposes. When used for remote frozen section diagnosis, WSI requires a thorough implementation period coupled with trained support personnel. Adoption of WSI for rendering pathologic diagnoses on a routine basis has been shown to be successful in only a few “niche” applications. Wider adoption will most likely require full integration with the laboratory information system, continuous automated scanning, high-bandwidth connectivity, massive storage capacity, and more intuitive user interfaces. Nevertheless, WSI has been reported to enhance specific pathology practices, such as scanning slides received in consultation or of legal cases, of slides to be used for patient care conferences, for quality assurance purposes, to retain records of slides to be sent out or destroyed by ancillary testing, and for performing digital image analysis. In addition to technical issues, regulatory and validation requirements related to WSI have yet to be adequately addressed. Although limited validation studies have been published using WSI there are currently no standard guidelines for validating WSI for diagnostic use in the clinical laboratory. This review addresses the current status of WSI in pathology related to regulation and validation, the provision of remote and routine pathologic diagnoses, educational uses, implementation issues, and the cost-benefit analysis of adopting WSI in routine clinical practice.
Keywords: Consultation, diagnosis, digital, education, frozen section, imaging, informatics, telepathology, validation, virtual microscopy, whole slide imaging
INTRODUCTION
Whole slide imaging (WSI), also commonly referred to as “virtual” microscopy, involves the digitization or scanning of glass slides to produce “digital slides” for viewing by humans or subjecting them to automated image analysis. The creation of digital slides is intended to simulate light microscopy. Since the introduction of whole slide scanners almost a decade ago (around 1999), WSI technology has evolved to the point where digital slide scanners are currently capable of producing high-resolution digital images within a relatively short time. Scanning of slides at multiple magnifications and focal planes (so-called z axis) is also possible. Compared to static digital images, WSI have been shown to be more beneficial for educational and some diagnostic purposes.[1] However, there appear to be several technical and logistical barriers to be overcome before WSI becomes a widely accepted modality in the practice of Pathology. For example, current scanning technology does not satisfactorily accommodate thick smears and three-dimensional cell groups in cytopathology.[2,3] With tissue sections, scanners are currently unforgiving when encountering tissue folds, bubbles and poor staining of material to be scanned.[4] Unless significant modifications to workflow are made centered around digital pathology (e.g. automation, continuous flow processes, quality of the histology presented to the WSI devices), placing WSI systems in the clinical pathology laboratory has been shown to stress the system in terms of reliability and throughput.[5]
In the United States, regulatory issues regarding digital pathology are also in flux. The Food and Drug Administration (FDA) convened a panel hearing in October 2009 that focused on how best to regulate whole slide digital imaging systems used for primary pathologic diagnosis. At present, there are unclear regulatory standards related to image capture and display, validation, and clinical use of WSI. This review addresses the current status of WSI regulation and validation and the use of WSI for remote and routine pathologic diagnosis and education. We also discuss implementation issues and cost-benefit considerations.
REGULATION AND VALIDATION
In the United States, federal regulations set forth in the Food, Drug and Cosmetic Act of 1938 and the Medical Device Amendments of 1976 provide the FDA with limited authority over medical devices. Some of these devices are subject to premarket review through 510(k) premarket notification process or premarket approval application (PMA). These US federal regulations pertain primarily to manufacturers of whole slide digital imaging systems, and potentially also to laboratories that incorporate WSI in diagnostic services. The FDA convened a panel hearing in October 2009 that focused on how best to regulate WSI systems that are to be used for primary diagnosis in surgical pathology.[6] Details of the events and debates of this FDA advisory panel meeting are available on The Daily Scan blog.[7] While WSI systems are clearly medical devices subject to FDA regulation, there are a number of open issues the FDA will need to address before the regulatory environment is clarified:
Will the FDA choose to regulate these devices, or exercise discretion on the grounds that they are similar to conventional microscopes, which the FDA has chosen not to regulate?
If regulation is contemplated, will it be applied to entire WSI systems or will WSI components be regulated separately (i.e., image capture, image storage and manipulation, display screens, other aspects of the user interface, specialized software functions)? [Figure 1]
Figure 1.
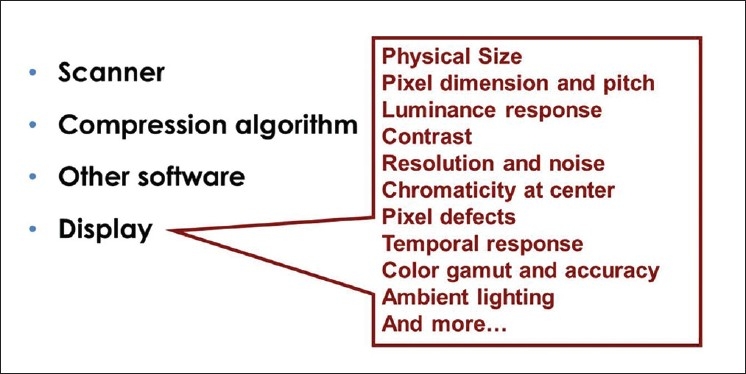
Qualities of a digital display device. WSI systems can be regulated as a whole, or individual components such as displays can be regulated separately
How will regulation be applied to care models in which components of WSI are purchased and operated by different entities (e.g., image capture in one facility, image hosting and manipulation in a second, and interpretation in a third facility)?
Will regulatory approval of WSI cover all types of diagnostic work, or will some tissue types, disciplines, analyses, or diagnostic entities be excluded? Current WSI approval, for example, is limited to HER2/neu, estrogen receptor (ER) and progesterone receptor (PR) analysis.[8]
In addition to FDA requirements, the Clinical Laboratory Improvement Amendments (CLIA) impacts clinical laboratories using WSI systems. If used in a clinical laboratory for an application not explicitly cleared or approved by the FDA, an argument can be made that the laboratory is employing a laboratory-developed test (LDT) and is subject to CLIA validation requirements pertaining to LDTs. Finally, professional and scientific standards require pathologists to assume responsibility for the methods they employ in the care of patients, including WSI.
How should WSI be validated? Validation is traditionally defined as confirmation, through the provision of objective evidence, that the requirements for a specific intended application have been fulfilled. In the case of a clinical laboratory test the validation process must take into account the purpose for which a test is intended, performance claims that the test must meet to be suitable for the intended application, and an assessment of the risks that may prevent the test from serving its intended purpose. Tests themselves are said to be validated after all of the individual performance claims appropriate for the clinical application are found to be valid. Performance claims can be of a number of types, including claims about analytic bias, reproducibility, suitability of certain specimen types, and turnaround time. Claims can concern the accuracy of diagnosis or the accuracy of an individual measurement (e.g., tumor size).
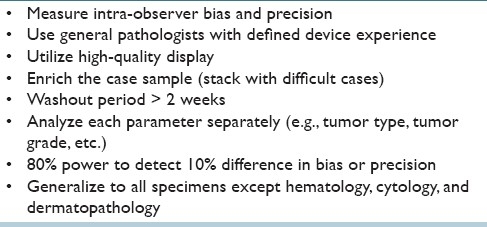
It is impossible to use the scientific method to affirmatively prove that a claim is valid. “Validity” is a matter of informed judgment. Reasonable people may differ over the degree of assurance required or the types of procedures that should be performed to assess a claim, and may have different views about the types of claims that should be tested to consider a test fit for a particular use. Table 1 lists some of the specific validation issues raised by WSI. Although limited validation studies have been published using WSI,[9–11] no generally-accepted standard guidelines are available to validate WSI for diagnostic use in the clinical laboratory. Evaluators must consider a range of issues that include sample size and statistical power, separating pathologist performance issues from device performance issues, the scope of cases to include in a challenge set, whether the set should be “enriched” with difficult cases, washout (time interval before asking a pathologist to review the same diagnostic material), the time it takes pathologists to become facile with WSI instruments, and the setting in which validation is assessed. Table 2 lists one of the authors’ (PNV) personal preferences for WSI validation.
Table 1.
Issues to consider in the validation of WSI for routine diagnostic application

Table 2.
Preferences for WSI validation for routine diagnostic application
PRIMARY FROZEN SECTION DIAGNOSIS AND TELEPATHOLOGY
WSI in recent years has been effectively utilized by several groups for telepathology, including primary frozen section diagnosis and secondary/tertiary teleconsultation.[12–20] The advantages of using WSI for this purpose include access to an entire digitized slide or even an entire case (set of slides), automated scanning, the high resolution of images available for review, rapid interpretation time, and the ability to exploit simultaneous viewing (teleconferencing). The University Health Network (UHN) in Ontario, Canada has extensive experience using WSI for telepathology.[21,22] UHN is a multi-site academic institution in downtown Toronto, comprising the Princess Margaret Hospital (PMH), Toronto Western Hospital (TWH) and Toronto General Hospital (TGH) which houses UHN's consolidated pathology department. TWH has no on-site pathologist and is located approximately one mile to the west of TGH. It is also the only UHN site where neurosurgery is performed, generating up to 10 frozen sections in a typical week. Sending a single pathologist to TWH to cover this small volume of frozen sections, most of which come from neurosurgery, created several challenges including delays in regular case sign-out at TGH, delays in carrying out academic responsibilities at TGH and no possibility of consulting with colleagues on difficult frozen sections. The latter issue created the risk of compromised diagnostic accuracy and/or unnecessarily deferred frozen section diagnoses. Telepathology was identified as a viable solution to these challenges and has been in use at UHN for over seven years.
At UHN, a team that consisted of a pathologist, a senior histotechnologist and an information technology (IT) support person was formed in 2003 to select a digital pathology vendor, validate the system to be used for frozen section diagnosis, train new users and carry out due diligence that included consultation with the medical malpractice insurance provider, development of a protocol for approval by UHN's Medical Advisory Committee and engagement of the surgeons at TWH. After an 18-month development period, the system went live in November of 2004 initially using a robotic microscope (Leica TPS2, Leica Microsystems) for making frozen section diagnoses at TWH in the absence of an on-site pathologist. The robotic microscope was used until October 2006 to report 350 frozen sections. While the robotic system was found to provide diagnostic accuracy that was equivalent to a light microscope, it typically took 10 min to review a single frozen section slide and produced total turnaround times (TAT) of > 20 min. This created challenges with respect to meeting CAP accreditation benchmarks for TAT.
In September 2006, UHN began parallel testing between the robotic microscope and a WSI platform (Aperio ScanScope CS). After only 30 cases, it was apparent that WSI was going to provide superior image quality, a user experience that more closely replicated light microscopy than the robotic device and a four to fivefold reduction in the amount of time required to review a frozen section slide. The TAT (time from receiving tissue to calling the surgeon with a diagnosis) was approximately 15 min for WSI versus 20 min per single block frozen section using the robotic microscope. Since October 2006, UHN pathologists have used WSI to make over 1800 primary frozen section diagnoses in the absence of an on-site pathologist. WSI has provided diagnostic accuracy that is equivalent to that experienced with light microscopy and facilitates the reporting of single block frozen sections with total TATs in the range of 14 to 16 min. They have experienced a 5% deferral rate with at least two pathologists reviewing the case before a deferred diagnosis is given, a quality measure that is not possible with a lone on-site pathologist reporting frozen sections by light microscopy.
Several factors have contributed to the success of the UHN program including a well-defined clinical application in the form of a small volume of neuropathology frozen sections, an uncomplicated frozen section workflow where most cases involve single pieces of tissue < 10 mm in size, an implementation period of approximately 18 months that allowed all team members to build confidence in the system and a team approach involving pathologists, histotechnologists, IT support staff, vendors and surgeons committed to making the program work. It has been the UHN experience that consistently high-quality frozen section slides produced by a skilled histotechnologist is an absolute requirement in order to have image quality that is sufficient to allow reliable frozen section diagnoses to be made via WSI [Figure 2]. System failure, requiring a pathologist to travel from TGH to TWH to report a frozen section, has occurred on six occasions (0.3% of cases) with a 15-min delay in TAT for the affected cases. The WSI failures included an unexpected hospital network shutdown (one case), moving the scanner to another network drop in the frozen section room associated with a loss of connectivity due to an IP address problem (one case), scanner failing to scan small (~2 mm) pale pieces of edematous brain tissue (two cases; the problem was resolved by adjusting the scanner gains to create a “faint slide” scanning protocol), excess mounting media on a frozen section slide that fouled the scanner objective requiring a thorough cleaning of the scanner objective and stage (one case), and a burned out light bulb in scanner light source (one case). The UHN has found WSI technology to be safe, accurate and reliable for making frozen section diagnoses in settings where there is no on-site pathologist. Successful implementation requires: effective planning and communication, a willingness to adjust old routines without compromising quality, and histotechnologists who are able to provide consistently high-quality frozen section slides.
Figure 2.
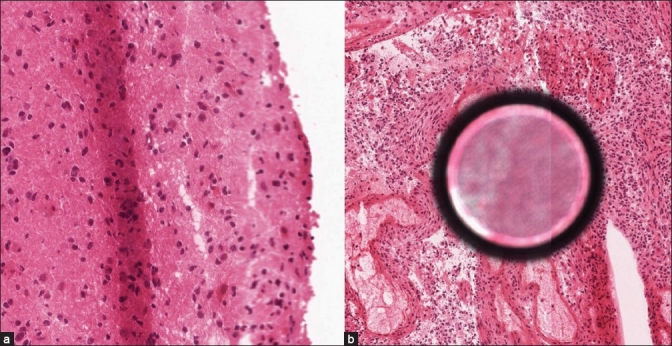
This figure shows two examples to illustrate the impact of suboptimal frozen section slides on image quality generated by WSI devices. (a) A diffuse astrocytoma with a tissue fold in the center of the field is shown that has caused the edge of the section (right edge) to be out of focus, (b) A high-grade astrocytoma with a large air bubble under the coverslip
ROUTINE PATHOLOGICAL DIAGNOSIS
WSI is increasingly being used in the day-to-day practice of surgical pathology, particularly for teleconsultation. Digitized slides have been used for certain quality assurance practices, such as obtaining second opinions. However, the question on most pathologists’ minds is whether WSI will be utilized for making routine pathologic diagnoses, ushering in the era of the “slideless” laboratory. The adoption of digital pathology has been slower than the adoption of digital images in radiology. This is partly related to the fact that pathology digital data is acquired in a slightly different manner from that in radiology. Although both disciplines require an imaging modality to collect primary data,[23] in radiology, images begin as digital data whereas pathology images have to be converted from an analog substrate into a digital format. Other differences between radiology and pathology digital imaging are the picture archiving systems (i.e., Picture Archiving and Communication System or PACS) and associated standards (e.g., Digital Imaging and Communications in Medicine or DICOM) available for radiology, larger file size and associated metadata of pathology digital image files, and workflow efficiencies in radiology.[23,24] Some of the barriers to the adoption of digital pathology images are related to the performance, workflow efficiency, infrastructure, integration with other software, and exposure to digital images.[25] Despite significant increases in technology, current adoption of WSI in the clinical space has been restricted and limited largely to niche practices.
The general pathology laboratory at Kalmar County Hospital in Kalmar, Sweden, is unique in that for around two years they have been digitizing all of their glass slides.[26] They scan around 60,000 histopathology slides per year, and over 75% of their histopathology diagnostic work is performed using digital pathology. Their impetus to go “slideless” was related to ergonomics as well as the need to network with colleagues in a country where there was a shortage of pathologists. Essential requirements for their success included: full integration with the digital pathology system and laboratory information system (LIS), reliable scanning, running the slide scanner continually with limited use of lab personnel, and good image quality. Obtaining consultations on their difficult cases in a timely manner was greatly facilitated through digital slide sharing and conferencing. More institutions are following suit; for example, a clinical trial at the University of Pittsburgh Medical Center (UPMC) evaluating the feasibility of signing out a high volume of surgical pathology cases using only digitized slides is currently underway.
Rendering routine pathologic diagnoses using WSI is feasible if the images truly represent an accurate digital reproduction of the scanned glass slide which can be saved, archived, reviewed and later retrieved without degradation of the image. Moreover, apart from integration with the LIS, the routine use of WSI in pathology laboratories will require seamless connectivity over broadband networks, efficient workstations, cost-effective storage solutions, and standards-based informatics transactions for integrating information with WSI.[27,28] It is difficult to think of WSI for diagnostic purposes without considering the rest of the electronic medical record. It seems unlikely that pathologists will render diagnoses without access to additional medical information. One of the reasons for reported discrepancies between digital and glass slide diagnoses is attributed to inadequate clinical data, apart from other factors such as image quality, missed tissue on the digital slide and the pathologists’ lack of experience using a WSI system.[29] It was demonstrated in one telepathology study using a virtual slide system that the correct diagnosis was made in 66% of cases without clinical data provided compared to a correct diagnosis of 76% with clinical data provided.[17] Therefore, in order for WSI to become an accepted diagnostic modality the provision of adequate medical information (e.g. gross pathology description, prior pathology reports, clinical history, etc.) will need to be weaved into the imaging system. Additional concerns that have yet to be satisfactorily addressed relate to malpractice and liability issues, as well as reimbursement for technical services related to producing the WSI.
Digital slides offer several advantages over glass slide review in terms of fidelity of the diagnostic material, portability, ease of sharing and retrieval of archival images, and ability to make use of computer-aided diagnostic tools (e.g. image algorithms).[30] Image analysis tools can automate or quantify with greater consistency and accuracy than light microscopy.[31] WSI has also permitted new business models of care in pathology. One such example is the virtual immunohistochemistry service provided by large national laboratories. After the remote reference laboratory performs technical staining and slide scanning services, the referring pathologist is provided with full access to these immunostained slides for their interpretation or referral to a teleconsultant. This has allowed some pathology practices to re-capture a portion of the reimbursement for professional interpretation services that has previously been diminished by these business practices. In the near future, the adoption of standards, validation guidelines, automation of workflow, creation of new revenue streams, and nuances of clinical digital practice will likely dictate a new standard of care for primary pathologic interpretations.
EDUCATION, TUMOR BOARDS AND PRESENTATIONS
WSI has gained tremendous acceptance for education, at tumor boards, and for presentations. WSI are much more interactive than glass slides, easy to share anywhere at any time, and can help standardize training material. The successful use of WSI in undergraduate medical education and pathology resident training has been highlighted by several authors,[32–38] including the creation of digital slide teaching sets.[39–41] Unlike glass slide teaching sets, digital slides will not fade, break or disappear. Digital slides also offer the ability to standardize images, permit annotation, and can provide a wide case range for trainees, including rare cases. Digital teaching sets that can be accessed on a server over a network are available to multiple users, and can be developed to contain test modules for trainees. Not surprisingly, many medical schools are abandoning the light microscope. Collaboration among students is easier with WSI, and this technology supports the creation of a virtual-slide laboratory in medical schools. WSI also allows one to track how users view, pan and zoom around a WSI.[42,43] This function has been shown to be particularly helpful with respect to tutoring and assessing trainees [Figure 3], as well as for the development of image processing tools.
Figure 3.
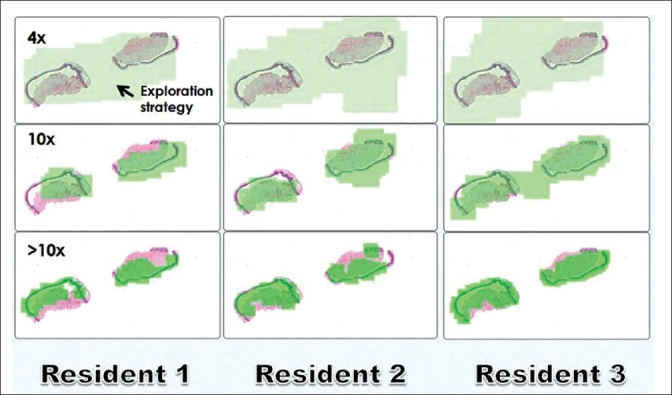
Search maps of WSI of inflammatory skin biopsies. Using a “light” version of SlideTutor a user's interaction with the digital image is recorded. The green highlighted areas represent the areas of the image that were viewed (search map). The search maps of three different residents are shown at different magnifications. Images courtesy of Dr. Claudia Mello-Thoms, Department of Biomedical Informatics and Department of Radiology, University of Pittsburgh, USA
WSI have also had a positive impact on pathologists presenting cases at tumor boards in several institutions.[44,45] This is because WSI offers higher quality images with annotation, greater educational value for clinicians, involves less preparation time than photographing cases, and permits real-time flexibility (e.g. easy to add on cases, perform side-by-side viewing, and gives access to the entire slide which allows one to answer “on-the-spot” questions). WSI has also permeated into other areas such as E-education, virtual workshops, digital images in pathology journals and for proficiency testing.[46–48]
APPLICATIONS AND CAVEATS FOR SERVICE IMPLEMENTATION
In order to integrate WSI into routine practice, an infrastructure needs to be developed in the pathology department. This infrastructure consists of: (i) hardware for scanning slides, storing the scanned images, transmission of the images to pathologists, and the interfaces necessary to display the images and report interpretations; and (ii) the software to facilitate the workflow of the image movement, display, and reporting of the results. Following development of the internal infrastructure, the addition of remote teleconsultation requires that other features be considered in the system. These include security of protected patient information, process validation, as well as regulatory, medico-legal, and billing issues all to be added to the software overlay. And finally, when telconsultations are coming from outside the institution's firewall, engagement of IT resources in order for systems to “talk” to one another successfully.
There are a number of methods for receipt of WSI teleconsultation cases. For institutions communicating cases regularly, a secure permanent connection such as a virtual private network (VPN) is an optimal solution in terms of security. For ad hoc consultation cases, coming from a variety of remote sites, internet transmission and security may be enabled via a variety of commonly used encryption modalities. It is implicit that devices and image formats must be compatible across institutions. In order to facilitate consultations from pathologists at outside institutions to this subspecialty-based pathology practice at the Massachusetts General Hospital (MGH) in Boston, devices and software were “agnostized” and thereby able to process raw images in any format. Remote sites scan slides and enter clinical and demographic information using the MGH department's website. Images are queried via their software and directed first to a “hot seat” review station where they are then triaged to the appropriate subspecialist consultant, who also has the ability to share the images with other intranet users. Finalized cases are reported in the same system.
As already alluded to above, the advent of rapid whole slide scanning has several applications. In fact, the use of WSI for primary diagnosis and rapid teleconsultation is now not only possible, but may be preferred over routine microscope-based tasks. However, barriers to widespread adoption of WSI for teleconsultation that still need to be overcome include the high cost of scanning devices, validation of the process of interpretation of WSI for primary diagnosis (all specimen types may not perform similarly), the potential for FDA regulation, and legal issues related to teleconsultation across states and internationally.
EFFICIENCIES AND COSTS
While the advantages of WSI for digital pathology are well established,[27,49] formal evaluation of the parameters that impact the costs and benefits of various digital pathology activities based on WSI have not been rigorously evaluated. Analyses based on cost have traditionally focused on direct costs (for both hardware and software) and indirect costs (support personnel), while evaluations of the opportunities provided by WSI have usually focused on operational factors such as ease of use, scalability, etc. However, analyses of this nature largely ignore a fundamental workflow issue in diagnostic surgical pathology that is part of routine practice, namely that the histological sections on glass slides that are a necessary and intrinsic component of diagnostic surgical pathology must be produced as part of any WSI process.
The department of pathology at the Washington University School of Medicine in St. Louis, MO in the USA developed a rigorous “value-added” approach that focuses on specific operational measures (cost, time, and accuracy), and the various clinical settings in which they can provide enhancement, to determine the settings in which WSI is able to improve surgical pathology practice.[50] The perspective for their value-added analysis is a tertiary care medical center surgical pathology practice characterized by a large volume of high-complexity cases; a subspecialty emphasis sign-out model; multiple sign-out areas; numerous training programs; and an academic pathology department. The results of their value-added approach depend upon this practice setting.
Value-added is defined by purely operational measures, specifically cost savings, time savings, or improvements in accuracy. Value-added can be assessed on a number of different scales. While the value-added approach described below focuses largely on the analysis-related patient care activities, WSI also adds value to educational activities and research. Some aspects of digital pathology based on WSI are specifically not value-added in the Washington University practice setting. For example, the mere capability of being able to produce a digital image that can be used for primary diagnosis (digital sign-out) in and of itself is not value-added, since the diagnosis based on the routine histological section is already possible from conventional light microscopy. However, aspects of digital sign-out that are not value-added in this tertiary care model may well provide a benefit in other practice settings, such as support of subspecialty consultation or the opportunity to view special stains produced by outside laboratories.
Overall, WSI as a tool for complete diagnostic sign-out was not yet economically viable. However, there were five specific areas in which WSI provided capabilities that were found to enhance the pathology practice at Washington University, which were either superior to currently existing workflow processes, or were unavailable at the time [Table 3]. Using these five specific capabilities, the pathologists identified several areas in which WSI did not necessarily improve diagnostic accuracy, but nonetheless improved patient care. First, the use of WSI of selected slides from cases sent in consultation provided them with the opportunity to enhance patient care by allowing an immediately-available permanent record of the slides to guide frozen section diagnosis at the time of subsequent definitive excision; for comparison at sign-out of subsequent excision or post-therapy specimen; for presentation at patient care conferences; in QA activities; and so on. Second, WSI of selected slides sent to other institutions as requested or required by their policies for patient care, or slides encumbered by medico-legal proceedings, provided a permanent record for use in patient care activities even though their department lost control of the original glass slides. Third, WSI of original H and E slides that would be destroyed as part of ancillary testing made it possible to retain the diagnostic content of the slides; given the demonstration that molecular tests can be performed on nucleic acids collected from glass slides, the electronic record of slides produced by WSI will likely become more important. Fourth was the use of WSI for digital image analysis (e.g., HER-2/neu analysis) to support emerging slide-based diagnostic paradigms.
Table 3.
Specific added benefits of WSI

In their evaluation of WSI at Washington University, it became clear that the faculty and trainees at their institution varied in their comfort level and experience with the various software packages for image analysis, and also showed marked variation in their willingness to incorporate digital image analysis into their routine practice. The faculty members were unanimous in their unwillingness to incorporate a digital imaging process requiring that they move back and forth between different software packages; many staff were unwilling to have two computer monitors so that both software packages could be open at the same time; and the faculty demanded that any WSI process was operational both locally and remotely. In collaboration with several vendors, they therefore pursued a model of one-stop-shopping in which a seamless interface was created between the imaging software (Aperio Spectrum) and their LIS (Cerner Copath). Development of this new functionality required both system architecture design and new software code, and was associated with a significant additional investment in time and money. Implementation of this interface had an overall cost of approximately $70,000 ($27,000 for software development for the Aperio interface and the CoPath HL7 interface; $45,000 for purchase of the underlying CoPath Advanced Bar Coding and Tracking (AB and T) module. The need for development of this custom interface emphasizes additional hidden costs that are often overlooked in the evaluation of the utility of WSI in routine pathology workflow. Off-the-shelf hardware and software packages, regardless of the vendor, have generic functionality and integration into specific practice environments may likely require custom software changes.
The aforementioned value-added approach appears to have been successful in identifying settings at Washington University in which WSI added utility to the surgical pathology practice, based on several metrics:
Number of scans. The number of cases scanned per year has shown consistent growth (at least 33% per year over the last three years).
Acceptance. Although faculty and trainee acceptance is difficult to measure directly and objectively, faculty and trainee demands for IT support for use of WSI via remote access by laptop computers, iPads (and similar tablets), and iPhones (and other smart phones) are interpreted as evidence that their faculty and trainees are integrating WSI into their routine workflows.
Expanded utilization. The initial value-added approach identified WSI of slides seen in consultation as an enhancement to patient care; interest from faculty to extend WSI to include select in-house cases is interpreted as evidence of the increasing recognition of a role for WSI in patient care activities.
CONCLUSION
Digital pathology systems offer pathologists an alternate, emerging mechanism to manage and interpret information. They offer increasingly fast and scalable hardware platforms for slide scanning with software that facilitates remote viewing, slide conferencing, archiving and image analysis. Initially deployed and validated largely within the research and biopharmaceutical industries, WSI is increasingly being implemented for direct patient care. Improvements in image quality, scan times and image-viewing browsers will hopefully allow pathologists to more seamlessly convert to digital pathology, much like our radiology colleagues have done before us. However, WSI creates both opportunities and challenges. While there are clearly successful niche applications of WSI technology for clinical, educational and research purposes, it is evident that several areas still require attention and/or careful consideration before more widespread clinical adoption of WSI takes place. These include regulatory issues, development of standards of practice and validation guidelines, workflow modifications, as well as defining situations where WSI technology will really improve practice in a cost-effective way. Current progress concerning these and other issues, along with improving technology, will no doubt pave the way for increased adoption over the next decade, allowing the pathology community as a whole to harness the true potential of WSI for patient care. The digital decade will likely redefine how pathology is practiced and the role of the pathologist.
COMPETING INTERESTS
All authors do not have financial conflicts of interest. DCW is on the scientific advisory board for Corista, LLC.
AUTHORS’ CONTRIBUTIONS
All authors contributed to the writing, editing and review of this manuscript.
ACKNOWLEDGMENTS
The contents of this article were presented orally at the College of American Pathologists’ companion meeting held at the USCAP meeting in San Antonio, Texas, USA on Saturday, February 26, 2011.
Footnotes
Available FREE in open access from: http://www.jpathinformatics.org/text.asp?2011/2/1/36/83746
REFERENCES
- 1.Pantanowitz L. Digital images and the future of digital pathology. J Pathol Inform. 2010:15. doi: 10.4103/2153-3539.68332. 1pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pantanowitz L, Hornish M, Goulart RA. The impact of digital imaging in the field of cytopathology. Cytojournal. 2009;6:6. doi: 10.4103/1742-6413.48606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilbur DC. Digital cytology: Current state of the art and prospects for the future. Acta Cytol. 2011;55:227–38. doi: 10.1159/000324734. [DOI] [PubMed] [Google Scholar]
- 4.Bautista PA, Yagi Y. Improving the visualization and detection of tissue folds in whole slide images through color enhancement. J Pathol Inform. 2010;1:25. doi: 10.4103/2153-3539.73320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilbertson J, Yagi Y. Histology, imaging and new diagnostic work-flows in pathology. Diagn Pathol. 2008;(Suppl 1):S14. doi: 10.1186/1746-1596-3-S1-S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lusky K. Where will FDA land on whole-slide digital? CAP Today. 2009;23(12):1. [Google Scholar]
- 7.FDA advisory panel meets, debates digital pathology. The Daily Scan. Blog posted by Ole Eichhorn Oct 26 2009. [Last accessed on 2011 Jun 20]. Available from: http://blog.aperio.com/2009/10/fda-advisory-panel-meets-debates-digital-pathology.html .
- 8.Pantanowitz L. Automated HER2 digital image analysis. Adv Lab. 2007;16:80. [Google Scholar]
- 9.Gilbertson JR, Ho J, Anthony L, Jukic DM, Yagi Y, Parwani AV. Primary histologic diagnosis using automated whole slide imaging: A validation study. BMC Clin Pathol. 2006;6:4. doi: 10.1186/1472-6890-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nielsen PS, Lindebjerg J, Rasmussen J, Starklint H, Waldstrøm M, Nielsen B. Virtual microscopy: an evaluation of its validity and diagnostic performance in routine histologic diagnosis of skin tumors. Hum Pathol. 2010;41:1770–6. doi: 10.1016/j.humpath.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 11.Jukić DM, Drogowski LM, Martina J, Parwani AV. Clinical examination and validation of primary diagnosis in anatomic pathology using whole slide digital images. Arch Pathol Lab Med. 2011;135:372–8. doi: 10.5858/2009-0678-OA.1. [DOI] [PubMed] [Google Scholar]
- 12.Costello SS, Johnston DJ, Dervan PA, O’Shea DG. Development and evaluation of the virtual pathology slide: A new tool in telepathology. J Med Internet Res. 2003;5:e11. doi: 10.2196/jmir.5.2.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furness P. A randomized controlled trial of the diagnostic accuracy of internet-based telepathology compared with conventional microscopy. Histopathology. 2007;50:266–73. doi: 10.1111/j.1365-2559.2006.02581.x. [DOI] [PubMed] [Google Scholar]
- 14.Słodkowska J, Pankowski J, Siemiatkowska K, Chyczewski L. Use of the virtual slide and the dynamic real-time telepathology systems for a consultation and the frozen section intra-operative diagnosis in thoracic/pulmonary pathology. Folia Histochem Cytobiol. 2009;47:679–84. doi: 10.2478/v10042-010-0009-z. [DOI] [PubMed] [Google Scholar]
- 15.Fallon MA, Wilbur DC, Prasad M. Ovarian frozen section diagnosis: Use of whole-slide imaging shows excellent correlation between virtual slide and original interpretations in a large series of cases. Arch Pathol Lab Med. 2010;134:1020–3. doi: 10.5858/2009-0320-OA.1. [DOI] [PubMed] [Google Scholar]
- 16.Williams S, Henricks WH, Becich MJ, Toscano M, Carter AB. Telepathology for patient care: What am I getting myself into? Adv Anat Pathol. 2010;17:130–49. doi: 10.1097/PAP.0b013e3181cfb788. [DOI] [PubMed] [Google Scholar]
- 17.Massone C, Peter Soyer H, Lozzi GP, Di Stefani A, Leinweber B, Gabler G, et al. Feasibility and diagnostic agreement in teledermatopathology using a virtual slide system. Hum Pathol. 2007;38:546–54. doi: 10.1016/j.humpath.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Słodkowska J, Chyczewski L, Wojciechowski M. Virtual slides: Application in pulmonary pathology consultations. Folia Histochem Cytobiol. 2008;46:121–4. doi: 10.2478/v10042-008-0018-3. [DOI] [PubMed] [Google Scholar]
- 19.Wilbur DC, Madi K, Colvin RB, Duncan LM, Faquin WC, Ferry JA, et al. Whole-slide imaging digital pathology as a platform for teleconsultation: A pilot study using paired subspecialist correlations. Arch Pathol Lab Med. 2009;133:1949–53. doi: 10.1043/1543-2165-133.12.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jakate S. Application of virtual microscopy in consultation practice of gastrointestinal and liver pathology. J Pathol Inform. 2010;1:16. doi: 10.4103/2153-3539.68333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans AJ, Chetty R, Clarke BA, Croul S, Ghazarian DM, Kiehl TR, et al. Primary frozen section diagnosis by robotic microscopy and virtual slide telepathology: The University Health Network Experience. Hum Pathol. 2009;40:1070–81. doi: 10.1016/j.humpath.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Evans AJ, Kiehl TR, Croul S. Frequently asked questions concerning the use of whole-slide imaging telepathology for neuropathology frozen sections. Sem Diag Pathol. 2010;27:160–6. doi: 10.1053/j.semdp.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Montalto MC. Pathology RE-imaged. The history of digital radiology and the future of anatomic pathology. Arch Path Lab Med. 2008;132:764–5. doi: 10.5858/2008-132-764-PRTHOD. [DOI] [PubMed] [Google Scholar]
- 24.Hipp JD, Fernandez A, Compton CC, Balis UJ. Why a pathology image should not be considered as a radiology image. J Pathol Inform. 2011;2:26. doi: 10.4103/2153-3539.82051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson ES, Rayo M, Gill C, Gurcan MN. Barriers and facilitators to adoption of soft copy interpretation from the user perspective: Lessons learned from filmless radiology for slideless pathology. J Pathol Inform. 2011;2:1. doi: 10.4103/2153-3539.74940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorstenson S. Digital pathology system. Case study. Advance Lab. 2010;19:69. [Google Scholar]
- 27.Weinstein RS, Graham AR, Richter LC, Barker GP, Krupinski EA, Lopez AM, Erps KA, Bhattacharyya AK, Yagi Y, Gilbertson JR. Overview of telepathology, virtual microscopy, and whole slide imaging: prospects for the future. Hum Pathol. 2009;40:1057–69. doi: 10.1016/j.humpath.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Daniel C, Rojo MG, Klossa J, Mea VD, Booker D, Beckwith BA, et al. Standardizing the use of whole slide images in digital pathology. Comput Med Imaging Graph. 2011 doi: 10.1016/j.compmedimag.2010.12.004. [In press] [DOI] [PubMed] [Google Scholar]
- 29.Jara-Lazaro AR, Thamboo TP, Teh M, Tan PH. Digital pathology: Exploring its applications in diagnostic surgical pathology practice. Pathology. 2010;42:512–8. doi: 10.3109/00313025.2010.508787. [DOI] [PubMed] [Google Scholar]
- 30.Feldman MD. Beyond morphology: Whole slide imaging, computer-aided detection, and other techniques. Arch Pathol Lab Med. 2008;132:758–63. doi: 10.5858/2008-132-758-BMWSIC. [DOI] [PubMed] [Google Scholar]
- 31.Friedberg RC, Pantanowitz L. Practice evolution: Decentralized computer-assisted immunohistochemical image analysis. Arch Pathol Lab Med. 2009;133:597–600. doi: 10.5858/133.4.597. [DOI] [PubMed] [Google Scholar]
- 32.Boutonnat J, Paulin C, Faure C, Colle PE, Ronot X, Seigneurin D. A pilot study in two French medical schools for teaching histology using virtual microscopy. Morphologie. 2006;90:21–5. doi: 10.1016/s1286-0115(06)74314-4. [DOI] [PubMed] [Google Scholar]
- 33.Bruch LA, De Young BR, Kreiter CD, Haugen TH, Leaven TC, Dee FR. Competency assessment of residents in surgical pathology using virtual microscopy. Hum Pathol. 2009;40:1122–8. doi: 10.1016/j.humpath.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Chen YK, Hsue SS, Lin DC, Wang WC, Chen JY, Lin CC, et al. An application of virtual microscopy in the teaching of an oral and maxillofacial pathology laboratory course. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:342–7. doi: 10.1016/j.tripleo.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Foster K. Medical education in the digital age: Digital whole slide imaging as an e-learning tool. J Pathol Inform. 2010;1:14. doi: 10.4103/2153-3539.68331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris T, Leaven T, Heidger P, Kreiter C, Duncan J, Dick F. Comparison of a virtual microscope laboratory to a regular microscope laboratory for teaching histology. Anat Rec. 2001;265:10–4. doi: 10.1002/ar.1036. [DOI] [PubMed] [Google Scholar]
- 37.Weaker FJ, Herbert DC. Transition of a dental histology course from light to virtual microscopy. J Dent Educ. 2009;73:1213–21. [PubMed] [Google Scholar]
- 38.Helin H, Lundin M, Lundin J, Martikainen P, Tammela T, Helin H, et al. Web-based virtual microscopy in teaching and standardizing Gleason grading. Hum Pathol. 2005;36:381–6. doi: 10.1016/j.humpath.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 39.Conran R, Fontelo P, Liu F, Fontelo M, White E. Slide2Go: A virtual slide collection for pathology education. AMIA Annu SympProc. 2007:918. [PubMed] [Google Scholar]
- 40.Li L, Dangott BJ, Parwani AV. Development and use of a genitourinary pathology digital teaching set for trainee education. J Pathol Inform. 2010;1 doi: 10.4103/2153-3539.63822. pii:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharma G, Chenevert J, Dangott BJ, Muse A, Duboy JD, Pantanowitz L, et al. Development of a head and neck pathology slide teaching set for trainee education. J Pathol Inform. 2010;1:38. doi: 10.4103/2153-3539.63822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Treanor D, Lim CH, Magee D, Bulpitt A, Quirke P. Tracking with virtual slides: A tool to study diagnostic error in histopathology. Histopathology. 2009;55:37–45. doi: 10.1111/j.1365-2559.2009.03325.x. [DOI] [PubMed] [Google Scholar]
- 43.Mello-Thoms C, Pantanowitz L, Parwani A, Ho J, Sharma G, Crowley R. Analysis of digital slide exploration characteristics of expert pathologists. J Pathol Inform. 2010;1:22. [Google Scholar]
- 44.Heffner S. Streamlining tumor board reviews. AdvLab. 2008:20. [Google Scholar]
- 45.Tecotzky R. Digital pathology enhances hospital's tumor board meetings. MLO. 2009 [Google Scholar]
- 46.Dee FR, Lehman JM, Consoer D, Leaven T, Cohen MB. Implementation of virtual microscope slides in the annual pathobiology of cancer workshop laboratory. Hum Pathol. 2003;34:430–6. doi: 10.1016/s0046-8177(03)00185-0. [DOI] [PubMed] [Google Scholar]
- 47.Rosai J. Digital images of case reports and other articles. Int J Surg Pathol. 2007;15:5. doi: 10.1177/1084713806296004. [DOI] [PubMed] [Google Scholar]
- 48.Marchevsky AM, Wan Y, Thomas P, Krishnan L, Evans-Simon H, Haber H. Virtual microscopy as a tool for proficiency testing in cytopathology: A model using multiple digital images of Papanicolaou tests. Arch Pathol Lab Med. 2003;127:1320–4. doi: 10.5858/2003-127-1320-VMAATF. [DOI] [PubMed] [Google Scholar]
- 49.Hedvat CV. Digital microscopy: past, present, and future. Arch Pathol Lab Med. 2010;134:1666–70. doi: 10.5858/2009-0579-RAR1.1. [DOI] [PubMed] [Google Scholar]
- 50.Isaacs M, Yates S, Clermont W, Rossi J, Pfeifer JD. Implementation of whole slide imaging in surgical pathology: A value added approach. J Pathol Inform [In press] 2011 doi: 10.4103/2153-3539.84232. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]


