Abstract
Background:
Adhesion molecules play a role in leukocyte recruitment during central nervous system (CNS) inflammation.
Aim:
This study was designed to compare serum, cerebrospinal fluid (CSF) concentrations of adhesion molecules in children with meningitis and sepsis, and to evaluate their sources.
Setting:
This study was carried out at Pediatric Department, King Abdulaziz University Hospital from January 2007 to June 2008.
Design:
Serum and CSF samples were collected on admission from meningitis (n = 40), sepsis (n = 20) patients, and sera from controls (n = 20).
Materials and Methods:
Endothelial (E), leukocyte (L), platelet (P) selectins intercellular cell adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecules-1 (VCAM-1) were measured using ELISA.
Statistics:
ANOVA and Spearman's correlations were used. Adhesion molecules with albumin concentration were estimated in CSF/serum to calculate concentration quotients.
Results:
In meningitis, serum sE-, sL-, sP-selectins sICAM-1, sVCAM-1 levels were higher than controls. Compared to sepsis, serum sE-selectin, sL-selectin, sVCAM-1, CSF-sL-selectin, CSF-sVCAM-1, VCAM-1 ratio and index were higher, while serum sP-selectin was lower than meningitis. sE-selectin ratio, CSF sICAM-1 were higher in meningitis with positive than negative culture. The sE-selectin index was higher in meningitis with neurological complication than those without it. In meningitis, correlation was found between CSF protein and CSF white blood cell counts (WBCs), CSF sICAM-1, CSF sVCAM-1 and between CSF sE-selectin and CSF sICAM-1.
Conclusions:
This study supports the role of adhesion molecules especially sL-selectin, sVCAM-1 in meningitis and suggests further research to determine their use as biomarkers for meningitis and use of their antagonists as therapeutic for CNS inflammation. The presence of discrepancy of CSF/serum ratios for molecules of same molecular weight suggest intrathecal shedding in addition to diffusion through the blood-CSF barrier.
Keywords: Adhesion molecules, children, immunoglobulin family, meningitis, selectin, sepsis
Introduction
Despite progress in antibiotic and supportive therapies, meningitis remains an important problem in pediatric practice, marked by continuing significant mortality.[1,2] Furthermore, recovered patients may suffer from neurological damage, including cerebral infarction, recurrent seizures, hearing loss, learning disabilities, and/or mental retardation.[3] Pathophysiology and molecular mechanisms of meningeal inflammation are still not well understood.
An essential step in the development of meningitis is the migration of leukocytes into cerebrospinal fluid (CSF) through the blood–brain barrier which changes from a nonpermissive to a permissive phenotype for hematopoietic cell recruitment.[4] This migration of leukocytes into sites of inflammation is mediated by numerous factors such as adhesion molecules belonging to selectin (E- and P-selectin expressed on endothelial cells, and L-selectin on leukocytes), integrin (CD11a/CD18, CD11b/CD18, and CD11c/CD18), and immunoglobulin superfamily (IgSF) mainly [intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)] expressed on endothelial cells; however, ICAM-1 has also been shown to be expressed on monocytes ,[5] and CNS cells, e.g., astrocytes[6] as well as fibroblasts, leukocytes and epithelial cells.[7] These molecules work together in mediating leukocyte rolling, firm adhesion, and subsequent extravasation[8] by forming multiple receptor–ligand pairs that act either in a sequential, orchestrated fashion,[9] or in parallel pathways forming bottlenecks.[10]
Up-regulation of the expression of membrane-bound forms of adhesion molecules on activated leukocytes and their corresponding ligands on endothelial cells is induced by inflammatory mediators, such as tumor necrosis factor-alpha (TNF-α) and interleukin-1beta (IL-1β). Soluble (s) isoforms of adhesion molecules shed from the surface of activated cells can be quantified in biological fluids allowing insights into early events of leukocyte recruitment.[8] Controversial reports exist regarding the origin of soluble adhesion molecules in the CSF during cerebral inflammation either due to passive diffusion from blood via a weakened blood–CSF barrier or due to local intrathecal secretion, or both.[11,12]
Aim
The current study focused on evaluating concentrations of sE-selectin, sP-selectin, sL-selectin, sICAM-1, sVCAM in serum, and CSF of febrile pediatric patients with meningitis and sepsis without neurological infection on admission. Also, to determine whether enhancement of CSF concentrations of soluble adhesion molecules in meningitis are indicative of inflammatory disturbances of blood–CSF barrier permeability or an in situ endothelial- or epithelial-derived secretion or both. By using multivariate statistical analysis, the association between measured adhesion molecules and parameters-indicated in the disturbed blood–brain barrier was also investigated.
Materials and Methods
All consecutive patients aged 1 month to 15 years old admitted from January 2007 to June 2008 to the Pediatric department, King Abdulaziz University Hospital, Jeddah, Saudi Arabia with suspected meningitis at the time of admission were enrolled in this study. Inclusion was dependent on the decision to perform a lumbar tap, at the discretion of the attending physicians. This study was conducted according to the principles established in Helsinki and approved by King Abdulaziz University Hospital ethics committee. Informed consent was obtained from the parents/guardian of the subjects.
Data collection
Clinical and laboratory data were collected prospectively as follows: (a) demographics [age, gender, body mass index (BMI), hospital admission date]; (b) coexisting medical conditions, antibiotic pretreatment, vaccination status, temperature and duration of fever at the time of presentation, and occurrence and numbers of seizures; (c) physical examination findings (presence of rash, meningeal signs, and papilledema) Children were scored using Glasgow meningococcal septicemia prognostic score (GMSPS);[13](d) routine tests for biochemistry and hematology on the date of the lumbar tap including CSF finding (white cell count and differential, protein, glucose, Gram-stained CSF smear examination and culture); (e) Upon hospital discharge (survivors) or death (non-survivors) summary data, including total hospital stay and complications, were collected for all subjects.
Group definition
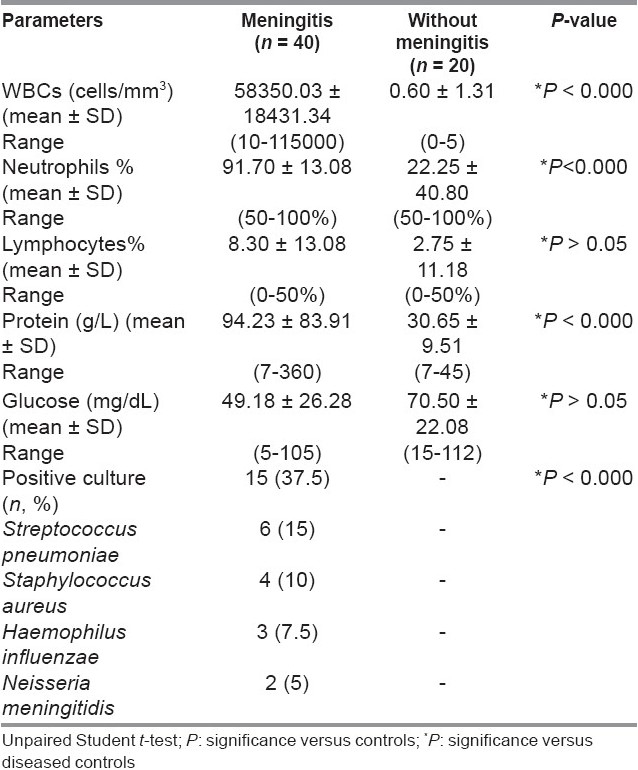
According to the clinical presentation and CSF findings, patients were classified into three groups: Patients suffering from meningitis (group I, n = 40) and septicemia, patients without neurological infection (group II, n = 20) served as diseased controls, and healthy control subjects (group III, n = 20). The diagnosis of meningitis was based on an acute febrile illness, with headaches, nuchal rigidity, and/or confusion, stupor, seizures, neurological deficit; in a laboratory test CSF grows bacteria or virus on culture. The causative organisms (n = 15, 37.5%) were Streptococcus pneumoniae (n = 6, 15%), Staphylococcus aureus (n = 4, 10%), Haemophilus influenzae (n = 3, 7.5%), and Neisseria meningitidis (n = 2, 5%) in CSF culture. Patients with negative culture (n = 25, 62.5%) were diagnosed to have bacterial meningitis if they had the presence of CSF polymorphonuclear cell count >5 mm3. CSF: Blood glucose ratio < 60% or CSF: Glucose < 40 mg/dL and protein >40 mg/dL in the absence of meningeal hemorrhage with either CSF gram-stain positive or blood culture positive or in the absence of both a normal chest x-ray and a negative montoux test. The relative low culture positive results obtained in this study may be due to multiple factors, including the use of antibiotics before admission, a common problem in many other developing countries. In addition, the yield of CSF culture may have been significantly impaired by some of the logistical difficulties in rapidly processing CSF cultures. Septicemic patients without neurological infection (group II) were those with culture-proved sepsis associated with confusion or agitation, white cell count of < 5/mm3 in the CSF, normal glucose and protein levels, and negative results of bacterial and viral CSF cultures. The criteria used for the definition of sepsis were those proposed by the American College of Chest Physicians and the Society of Critical Care Medicine Consensus Conference.[14] Group II included pulmonary infection (n = 9, 45%), urinary tract infection (n = 8, 40%), and bacterial colitis (n = 3, 15%).
Exclusion criteria
Excluded from the study were children having other systemic or chronic diseases necessitating inpatient antibiotic therapy (e.g., urinary tract infections in infants < 3 months, periorbital cellulites, deep abscess, bone or joint infections, or known bacteremia), immunodeficiency, ventriculoperitoneal shunt, recent neurosurgery, purpura, developmental delay, or impairment of hearing prior to the diagnosis of acute bacterial meningitis.
Sample collection
Serum and CSF samples were collected on admission in sterile tubes. All samples were tested for white blood cell count with differential, bacterial, and viral cultures. Levels of CSF glucose, and protein were determined by routine laboratory procedures. The remaining serum and CSF samples were spun down, and supernatant was frozen at -80°C until assayed.
Mediator assays
sE-selectin, sL-selectin, SP-selectin, sICAM-1, and sVCAM-1 were measured in the serum and CSF samples using specific sandwich immunoassays (ELISA kits from Bender MedSystems Europe, Vienna, Austrin) based on recombinant soluble adhesion molecules supplied by the manufacturer's standards. All serum samples were diluted in diluent buffer provided with the kits, while the CSF assay was performed on non-diluted samples according to the manufacturer's instructions. The samples and standards were run in duplicate. For sE-selectin, sL-selectin, SP-selectin, sICAM-1, and sVCAM-1, the sensitivity of the kits were 0.3, 0.198, 1.06, 2.2, and 0.6 ng/mL and intraassay coefficients of variations were 5.4%, 3.7%, 2.4%, 7.7%, and 3.1%, respectively). The albumin was measured by the Eliza kit obtained from Egyptian Company for Biotechnology; Cairo, Egypt; the detection limit of the assay was 1.0 g/dL.
Statistical analysis
Data were expressed as mean ± SD or as a percentage when appropriate. One-way analysis of variance (ANOVA) was used for comparison among all different groups using the SPSS version 10 software packages for windows. When the analysis of variance was significant, post hoc testing of differences between groups was performed using the least significant difference multiple comparison test. The CSF × 103/serum albumin index values were also calculated to determine the integrity of the blood–brain barrier. The adhesion molecule ratio was calculated as follows: Adhesion molecules (CSF) × 103/adhesion molecules (serum). Since considerable amounts of these soluble adhesion molecules are already present in normal serum, an “adhesion molecule index” in analogy to the IgG index was used to correct blood–brain barrier disruption.[15] The Spearman's or Pearson correlation coefficient test was used to evaluate associations of adhesion molecules. For all tests, P < 0.05 was considered significant.
Results
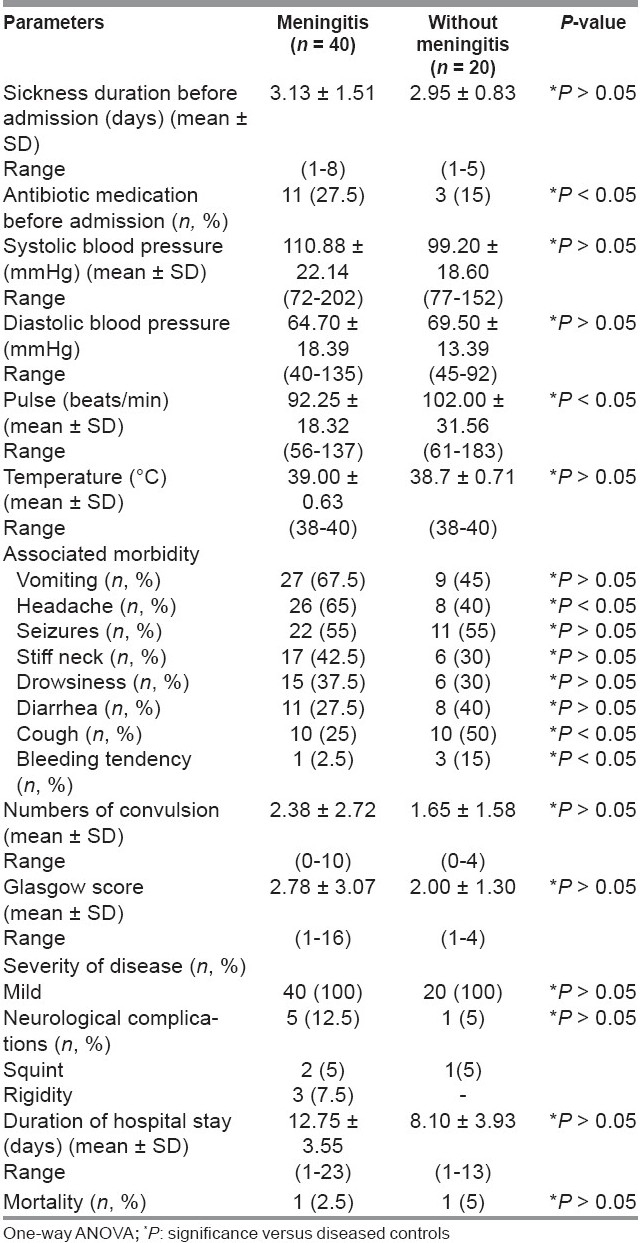
Table 1 showed the demographic characteristics of all the studied groups. There were no significant differences regarding age or body mass index (BMI) between the studied groups.
Table 1.
Demographic characteristics of the studied groups
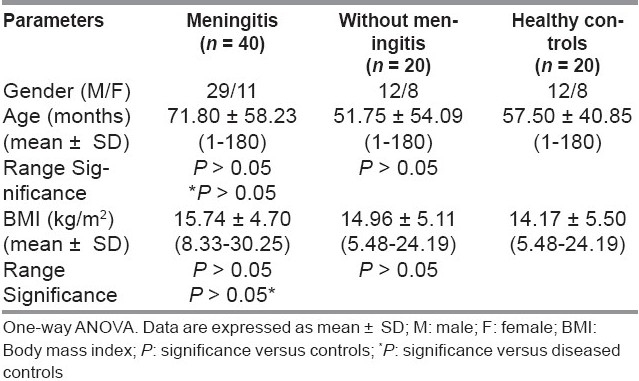
Table 2 showed vital parameters and characteristics of meningitis and non-meningitis patients. The number of meningitis patients received antibiotics before admission to hospital was more compared to the non-meningitis group (P < 0.05). The frequent presenting symptoms and signs in meningitis patients were vomiting (67.5%) followed by headache (65%) then seizures (55%), stiff neck (42.5%), drowsiness (37.5%), diarrhea (27.5%), cough (25%), and bleeding tendency (2.5%), while in non-meningitis patients were seizures (55%), cough (50%), vomiting (45%), diarrhea (40%), headache (40%), stiff neck (30%), drowsiness (30%), and bleeding tendency (15%). Headache was more, while cough and bleeding tendency were less in meningitis than in non-meningitis patients (P < 0.05). According to Glasgow score, all involved patients’ disease severity was mild. The incidence of neurological complications in meningitis patients was 12.5% (5% squint and 7.5% rigidity of upper and lower limbs), while in non-meningitis patients (5% suffer from squint). Among meningitis patients, three cases of rigidity were due to S. pneumoniae and the two cases of squint were due to H. influenzae and N. meningitidis (one case each). Mortality rates were 2.5% and 5% in both meningitis and non-meningitis patients.
Table 2.
Vital parameters and characteristics of meningitis and diseased controls
CSF white blood cell counts (WBCs), percent of neutrophils, and CSF–proteins were higher in meningitis than in non-meningitis patients (P < 0.000). In meningitis patients, CSF culture showed positively in 37.5% of cases while 62.5% showed no organism (aseptic meningitis) [Table 3].
Table 3.
Cerebrospinal fluid (CSF) parameters of meningitis and diseased controls
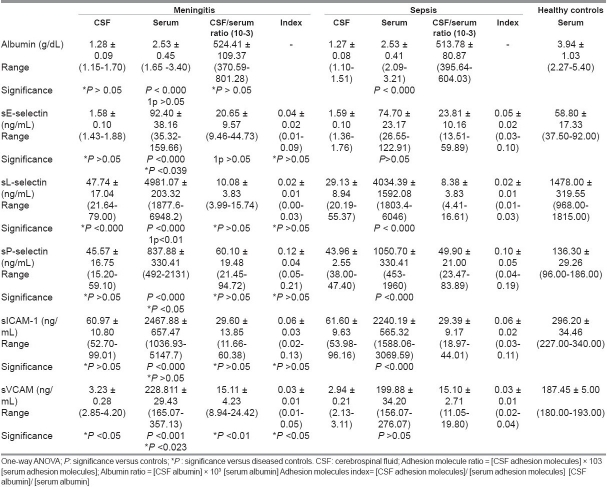
Compared to healthy controls, serum albumin was lower (P < 0.000 for both), while serum sL-selectin, sP-selectin, and sICAM-1 were higher in both meningitis and non-meningitis groups (P < 0.000 for all). Meanwhile, in the meningitis group only elevated serum levels of sE-selectin and sVCAM-1 (P < 0.000, P < 0.001) were found compared to healthy controls. In the meningitis group, serum sE-selectin (P < 0.039), serum and CSF sL-selectin (P < 0.01, P < 0.000); serum, CSF, ratio and index sVCAM-1 (P < 0.023, P < 0.05, P < 0.01, P < 0.05) were elevated while serum sP-selectin was lower (P < 0.05) than in the non-meningitis group [Table 4].
Table 4.
Adhesion molecules (mean ± SD) in different studied groups
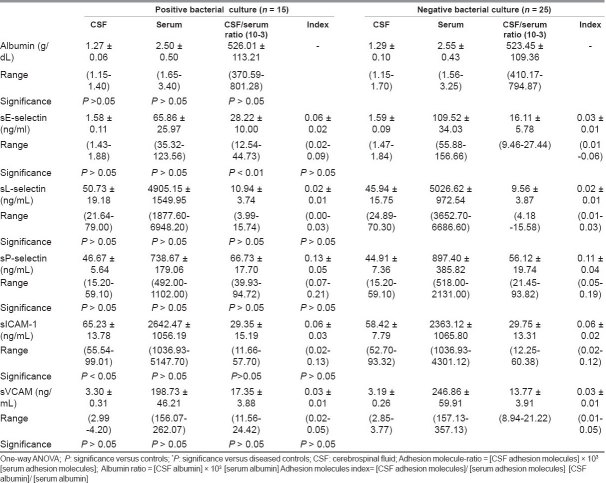
Table 5 compared adhesion molecule profiles of meningitis patients with positive and negative bacterial cultures of CSF. It was found that in the positive-culture meningitis patients, sE-selectin ratio and CSF–sICAM were higher (P < 0.01, P < 0.05) compared to negative-culture meningitis patients. Other measured parameters showed no significant difference between the studied groups.
Table 5.
Adhesion molecules (mean ±SD) in bacteria positive and negative culture meningitis
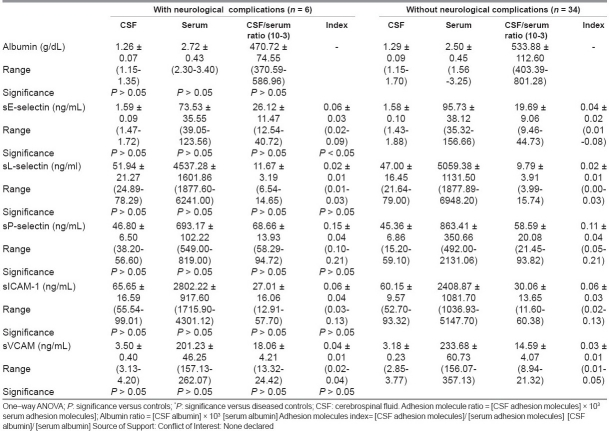
Table 6 compared adhesion molecule profiles of meningitis patients with and without neurological complication. It was found that only the sE-selectin index was higher (P < 0.05) in patients with complications than those without them. Other measured parameters showed no significant difference between the studied groups.
Table 6.
Adhesion molecules (mean ±SD) in meningitis patients with and without neurological complications
In meningitis patients, positive correlations were found between CSF–protein with CSF–WBCs, CSF–sICAM, CSF–sVCAM (r = 0.471, P < 0.002; r = 0.335, P < 0.035; r = 0.353, P < 0.025) and between CSF–sE-selectin with CSF–sICAM (r = 0.559, P < 0.000). In diseased controls, a positive correlation was found between albumin ratio and Glasgow score, CSF protein, sE-selectin ratio and sL-selectin ratio (r = 0.455, P < 0.044; r = 0.474, P < 0.035; r = 0.517, P < 0.021; r = 0.532, P < 0.016).
Discussion
Endothelial cell activation by inflammatory cytokines alters their functions. This, and the subsequent leukocyte–endothelial interaction, is of fundamental importance in the pathology of inflammation and healing.[16] The process of homing and transmigration of leukocytes to their target tissue following bacterial or viral infection involves complex interactions mediated by a variety of chemokines, cytokines, adhesion molecules, and proteases.[17] In healthy people, soluble forms of adhesion molecules are about 100-200 times higher in blood than in CSF.[12] They are increased in the CSF of meningitis patients, up to 1000-fold, in comparison with control values.[18,19] The present study examined the levels of sE-selectin, sL-selectin, sP-selectin, sICAM-1, and sVCAM in sera and CSF samples of meningitis and sepsis children in order to further understand the immunological cascade associated with bacterial meningitis and sepsis.
The present investigation demonstrated elevated levels of serum sE-selectin in bacterial meningitis patients compared to sepsis and healthy controls, indicating their enhanced endothelial up-regulation and shedding in this disease and may account for the acuity and magnitude of the inflammatory process observed in meningitis. Surprisingly, in meningitis patients, there was no significant difference in serum and CSF levels of sE selectin between those with positive and negative CSF cultures nor any between those with and without neurological complications. Meanwhile, the CSF/serum ratio was higher in positive than in negative culture patients, and the sE-selectin index was higher in those with neurological complications than those without them. In this respect, Baines and his colleagues[20] reported that among children with severe meningococcal disease, E-selectin concentrations in those children who subsequently died were less than in those who survived. Meanwhile, Cowley and his colleagues[21] reported highest concentrations of E-selectin in some patients who subsequently died. Damage of the blood–CSF barrier through meningeal inflammation has been clearly demonstrated in meningitis. Accordingly, using the albumin CSF/serum ratio, a more sensitive marker of blood–CSF barrier permeability can be determined.[22] The high level of the albumin CSF/serum ratio observed in this study in meningitis and sepsis cases indicated disrupted barrier. This study did not show any correlation between CSF and serum levels of sE-selectin in meningitis. Meanwhile, Mégarbane and his coworkers[23] found close correlations between CSF and serum values for sE-selectin in meningitis patients suggesting an increased diffusion from peripheral blood through a disrupted blood–CSF barrier. In sepsis patients, we reported a positive correlation between the CSF/serum albumin ratio and E-selectin ratio. It has also been proposed that the release of E-selectin is related to the degree of vascular or endothelial injury caused by the sepsis.[19] Endothelial E-selectin-deficient mice demonstrated a dramatic attenuation in the leukocyte influx to the subarachnoid space in response to acute cytokine-induced meningitis.[22]
An important factor essential in rolling, an early stage of leukocyte trafficking into sites of inflammation, is L-selectin. It is expressed on the surface of circulating lymphocytes. In this study, serum sL-selectin level was higher in meningitis and sepsis patients than in healthy controls. Levels of serum and CSF sL-selectin were higher in meningitis compared to the sepsis group. Meanwhile, levels of sL-selectin were not differing in meningitis patients with positive and negative CSF cultures or in those with and without neurological complications. Sulik and his colleagues[5] reported increase in levels of sL-selectin in CSF of meningitis patients but Fassbender et al.[15] did not find. Others reported increased CSF sL-selectin in patients with non-infectious inflammation of CNS, i.e. multiple scoliosis (MS) and systemic lupus erythematosus.[24] As reported previously, L-selectin is rapidly shed from the cell surface, a process that appears to be important for limiting leukocyte accumulation at the sites of inflammation.[25] This shedding is accomplished by the cleavage of cell surface L-selectin which results in an accumulation of the soluble form of the adhesion molecule. The absence of L-selectin on cells extracted from bronchoalveolar lavage suggests that L-selectin is intensively released during migration through the endothelium,[26] which suggests a likely mechanism for the increased levels of sL-selectin in the CSF. The correlation found between the albumin ratio and sL-selectin ratio found in sepsis patients indicates that CSF sL-selectin increased with severity of the disease.
Cytokines, including tumor necrosis factor-alpha (TNF-α) and interleukin (IL)-1β, activate endothelial cells, altering function in several ways. Presynthesized P-selectin, which is stored in the Weibel-Palade bodies of endothelial cells and the a-granules of platelets, is released on to the endothelial cell surface on activation. It subsequently disappears from the cell surface and at least a proportion is released into the bloodstream, where it may be detected.[27] In this investigation, serum sP-selectin was higher in meningitis and sepsis children than in controls. Surprising, the serum levels of sP-selectin was higher in those with sepsis than with meningitis. Meanwhile, in this study, no difference was found in sP-selectin levels neither between patients with positive and negative CSF cultures nor between patients with and without neurological complications. Our results similar to those of Sakamaki and his colleagues,[27] who found higher concentrations of P-selectin in critically ill adults with sepsis. Baines and his coworkers[20] found that serum P-selectin was not raised in children with meningococcal disease and, more particularly, was low in those with meningococcal disease who died. Also, low P-selectin concentrations in adults with meningococcal disease was reported.[28] The lack of an increase in P-selectin concentrations contrasts with the rise seen in shed E-selectin and ICAM-1, which can be explained by that both E-selectin and ICAM-1 depend on de novo protein synthesis and so have a slower time course than P-selectin, which is released from preformed stores.[20]
Both ICAM-1 and VCAM-1 mediate leukocyte activation events that damage tissues, such as release of lysozomal enzymes and production of oxygen radicals.[29] The role of ICAM-1 is not limited to mediating leukocyte–endothelial cell interactions but also behaves as signal transducers.[30] In this study, serum ICAM-1 concentrations in children with meningitis and sepsis were higher than in healthy controls. Meanwhile, there was no significant difference in ICAM-1 concentrations between meningitis and sepsis patients. The lack of significant difference concerning ICAM-1 could be explained by that not only the activated endothelium but also various other cell types express ICAM-1 as cells of the CNS, e.g., astrocytes[6] as well as fibroblasts, leukocytes, and epithelial cells.[7] In meningitis children, CSF sICAM level was higher in those with positive than those with negative CSF bacterial culture indicating that bacteria present in the CSF activates more endothelial cells to release ICAM-1. Meanwhile, there was no significant difference between sICAM levels between meningitis patients with and without neurological complications. Previous studies[31,23,5] reported a rise in CSF concentrations of sICAM-1 in CNS infections of bacterial, viral, or fungal origin. In consistency with ours, Baines study[20] reported raised serum ICAM-1 concentrations in children with meningococcal disease than controls with no significant difference in ICAM-1 concentrations between those with mild and severe meningococcal disease, nor between those who survived and those who died. Previous work[32] has shown a rise of up to 4-fold in ICAM-1 levels in critically ill adults with sepsis, which was highest in those who subsequently died.
In this study, meningitis patients showed positive correlations between CSF sICAM-1 with CSF protein and CSF sE-selectin. ICAM-1 and E-selectin are both dependent on protein synthesis for increased synthesis and share similar time courses, which may explain their relation. The correlation between CSF sICAM-1 concentrations and CSF protein content found suggests injury to the blood–brain barrier, and CNS increases CSF ICAM-1 concentrations alongside CSF protein concentrations.[33] Although ICAM-1 has been the most extensively studied of all adhesion molecules, confusing data about source of CSF sICAM-1 in meningitis exist in the literature. Previous studies,[34,15,35] demonstrated that CSF concentration of sICAM-1 was related to an increased diffusion through a disrupted blood–CSF barrier in meningitis. Fassbender and his colleagues[15] reported that the CSF/serum index evaluation suggested a significant intrathecal release that did not occur; hence, infiltration through the blood–CSF barrier was determined to be the cause. Later studies[12] provided indirect evidence of an intrathecally produced fraction (20-40%) of sICAM-1 in the CSF of normal controls and demonstrated in 59 meningitis cases that the brain-derived fraction of sICAM-1 was up to 12-fold higher than in controls. Mégarbane and his coworkers[23] observed that sICAM-1, despite a comparable molecular weight (80-92 kD), had a lower CSF/serum correlation and a higher CSF/serum ratio, suggesting an associated mechanism, which could be an intrathecal release. This mechanism had been suspected by Jander et al.[31] who found an elevation in the sICAM-1 index in nine meningitis cases, in comparison with non-inflammatory neurological disease. Sulik and his colleagues[5] reported a considerable correlation between sICAM-1 and albumin CSF/serum ratios suggesting an important role for diffusion through the blood–CSF barrier; however, the presence of a brain-derived fraction was also suggested as CSF/albumin concentration ratios of sICAM-1 in meningitis patients exceed corresponding albumin quotients. Considering that these molecules have similar molecular weights, this observation suggests that there is a significant intrathecal release of sICAM-1. The study research group of the Fassbender et al.[15] consisted of patients with meningitis caused by different bacterial agents, and in some patients, no etiological agent was found. It is possible that materials released from various pathogens or direct interactions between micro-organisms and host cells result in an over expression of adhesion molecules. Methods not sensitive enough to detect sICAM-1 concentrations in normal CSF and insufficient methods for blood–CSF barrier evaluation were suggested as reasons for the controversial results in CSF sICAM-1 measurements.[12] Variations in soluble adhesion molecule level during the progression of the disease may represent another reason for the confusing results .[31] An anti-ICAM-1 monoclonal antibody has been shown to reduce early inflammation and edema, intracranial pressure, regional cerebral blood flow, and white blood cell invasion into the CSF.[36] Moreover, ICAM-1 deficiency significantly modifies bacteremia and survival in hematogenous bacterial meningitis in infant mouse models,[37] suggesting a prominent role for this molecule in meningitis. Soluble forms of adhesion molecules may act as competitors and inhibit ongoing immune responses or conversely act as stimulators by binding to specific ligands and triggering cellular activation.[38]
The principal source of VCAM-1 is the endothelial cell.[7] In the present study, there were elevated serum levels of sVCAM-1 in meningitis patients compared to sepsis and healthy controls. Also, levels of sVCAM-1 in CSF, ratio, and index were higher in meningitis compared to the sepsis group. The increased in VCAM-1 ratio and index in meningitis patients compared to sepsis obtained in this study suggests its intrathecally released. A rise in the concentration of VCAM-1 had been described in adults with meningococcal sepsis or meningitis.[28] In meningitis patients, a close correlation between CSF sVCAM-1 concentrations and indicators of blood–brain barrier damage (CSF total protein) was found. VCAM-1 also plays a critical role in the pathophysiology of viral meningitis, as assessed by VCAM-1 up-regulation of endothelial cells in sites of lymphocytic choriomeningitis virus infection and inhibitory effect of anti-VLA-4 monoclonal antibodies on leukocyte migration.[29]
While the above-mentioned mechanisms provide explanations for increased levels of adhesion molecules in the CSF of meningitis patients, a reason of increased levels of adhesion molecules (sL-selectin, sP-selectin, and sICAM-1) in the CSF of children with sepsis without meningitis presents a distinct problem. One explanation is that adhesion molecules production is upregulated by various proinflammatory cytokines that enter the CNS upon infection. There is also a suggestion that L-selectin may be shed in moderate amounts as part of the rolling process at an early stage of leukocyte recruitment, which would allow the leukocytes to return to the circulation.[40] This process could likely be responsible for the rise in sL-selectin concentration in children with sepsis and current lack of pleocytosis.
In summary, the present study suggests that elevated levels of adhesion molecules may in concert contribute to leukocyte extravasation during bacterial meningitis. The more prominent increase in serum levels of sE-selectin, sL-selectin, and sVCAM-1 in bacterial compared to non-meningeal sepsis infection may reflect both the differences in intensity and diversity of the immune mechanism involved. The intrathecal indices of sL-selectin and sVCAM-1 are higher in meningitis than in sepsis are evidence of the inflammatory response within the CSF including recruitment of leukocytes and their transmigration through the vascular barrier into the meninges in response to bacterial pathogens. An increase in the sVCAM-1 ratio and the index in meningitis compared to septic children indicates their intrathecal formation. The presence of a discrepancy of the CSF/serum ratios for molecules of the same molecular weight may suggest intrathecal shedding in addition to diffusion through the blood–CSF barrier. The present study contributes to the understanding of factors involved in the inflammatory process associated with bacterial meningitis, and if supported by further research, may lead to the use of distinct profiles of mediators as possible biomarkers for the various forms of meningeal infections. These markers may also lead to the development of specific targeted therapy to improve the clinical outcome, critical especially in bacterial meningitis; this area of research suggests it to be a promising approach.
Acknowledgments
This work was supported by financial grant No. 426/008 from University Research Board in King Abdulaziz University, Jeddah, Saudi Arabia.
Footnotes
Source of Support: Supported by fi nancial grant No. 426/008 from University Research Board in King Abdulaziz University, Jeddah, Saudi Arabia.
Conflict of Interest: None declared.
References
- 1.van Furth AM, Roord JJ, van Furth R. Roles of proinflammatory and anti-inflammatory cytokines in pathophysiology of bacterial meningitis and effect of adjunctive therapy. Infect Immun. 1996;64:4883–90. doi: 10.1128/iai.64.12.4883-4890.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geneva: WHO; 2003. World Health Organization. State of the art of new vaccines; research and development initiative for vaccine research; pp. 34–5. [Google Scholar]
- 3.Roos KL, Tunkel AR, Scheld WM. Acute bacterial meningitis in children and adults. In: Scheld WM, Whitley RJ, Durack DT, editors. Textbook of Infections of the Central Nervous System. 2nd ed. Philadelphia: Lippincott-Raven; 1997. pp. 335–403. [Google Scholar]
- 4.Oberholzer A, Oberholzer C, Moldawer LL. Cytokine signaling: Regulation of the immune respone in normal and critically ill states. Crit Care Med. 2000;28:N3–12. doi: 10.1097/00003246-200004001-00002. [DOI] [PubMed] [Google Scholar]
- 5.Sulik A, Wojtkowska M, Rozkiewicz D, Oldak E. Increase in adhesion molecules in cerebrospinal fluid of children with mumps and mumps meningitis. Scand J Immunol. 2006;64:420–4. doi: 10.1111/j.1365-3083.2006.01797.x. [DOI] [PubMed] [Google Scholar]
- 6.Bevilacqua MP, Nelson RM, Mannori G, Cecconi O. Endothelial-leukocyte adhesion molecules in human disease. Ann Rev Med. 1994;45:361–78. doi: 10.1146/annurev.med.45.1.361. [DOI] [PubMed] [Google Scholar]
- 7.Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. [PubMed] [Google Scholar]
- 8.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–14. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 9.Etzioni A. Adhesion molecules in leukocyte endothelial interaction. Adv Exp Med Biol. 1996;408:151–7. doi: 10.1007/978-1-4613-0415-9_17. [DOI] [PubMed] [Google Scholar]
- 10.Ley K. Pathways and bottlenecks in the web of inflammatory adhesion molecules and chemoattractants. Immunol Res. 2001;24:87–95. doi: 10.1385/IR:24:1:87. [DOI] [PubMed] [Google Scholar]
- 11.Engelhardt B, Vestweber D, Hallmann R, Schulz M. E- and P-selectin are not involved in the recruitment of inflammatory cells across the blood brain barrier in experimental autoimmune encephalomyelitis. Blood. 1997;90:4459–4472. [PubMed] [Google Scholar]
- 12.Lewczuk P, Reiber H, Tumani H. Intercellular adhesion molecule-1 in cerebrospinal fluid-the evaluation of blood derived and brain derived fractions in neurological diseases. J Neuroimmunol. 1998;87:156–61. doi: 10.1016/s0165-5728(98)00084-8. [DOI] [PubMed] [Google Scholar]
- 13.Sinclair JF, Skeoch CH, Hallworth D. Prognosis of meningococcal septicaemia. Lancet. 1987;2:38. doi: 10.1016/s0140-6736(87)93067-4. [DOI] [PubMed] [Google Scholar]
- 14.Members of the American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 15.Fassbender K, Schminke U, Ries S, Ragoschke A, Kischka U, Fatar M, et al. Endothelial-derived adhesion molecules in bacterial meningitis: Association to cytokine release and intrathecal leukocyte-recruitment. J Neuroimmunol. 1997;74:130–4. doi: 10.1016/s0165-5728(96)00214-7. [DOI] [PubMed] [Google Scholar]
- 16.Frenette PS, Wagner DD. Molecular medicine: Adhesion molecules. N Engl J Med. 1996;334:836–43. doi: 10.1056/NEJM199606063342308. [DOI] [PubMed] [Google Scholar]
- 17.Shapiro S, Miller A, Lahat N, Sobel E, Lerner A. Expression of matrix metalloproteinases, sICAM-1 and IL-8 in CSF from children with meningitis. J Neurol Sci. 2003;206:43–8. doi: 10.1016/s0022-510x(02)00317-9. [DOI] [PubMed] [Google Scholar]
- 18.Lopez-Cortes LF, Cruz-Ruiz M, Gomez-Mateos J, Jimenez-Hernandez D, Palomino J, Jimenez E. Measurement of levels of tumor necrosis factor-alpha and interleukin-1beta in CSF of patients with meningitis of different etiologies: Utility in the differential diagnosis. Clin Infect Dis. 1993;16:534–9. doi: 10.1093/clind/16.4.534. [DOI] [PubMed] [Google Scholar]
- 19.Newman W, Beall LD, Carson CW, Hunder GG, Graben N, Randhawa ZI, et al. Soluble E-selectin is found in supernatants of activated endothelial cells and is elevated in serum of patients with septic schock. J Immunol. 1993;150:644–54. [PubMed] [Google Scholar]
- 20.Baines PB, Marzouk O, Thomson AP, Sills JA, Riordan FA, Hart CA. Endothelial cell adhesion molecules in meningococcal disease. Arch Dis Child. 1999;80:74–6. doi: 10.1136/adc.80.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cowley HC, Heney D, Gearing AJ, Hemingway I, Webster NR. Increased circulating adhesion molecule concentrations in patients with the systemic inflammatory response syndrome: A prospective cohort study. Crit Care Med. 1994;22:651–7. doi: 10.1097/00003246-199404000-00022. [DOI] [PubMed] [Google Scholar]
- 22.Tang T, Frenette PS, Hynes RO, Wagner DD, Mayadas TN. Cytokine-induced meningitis is dramatically attenuated in mice deficient in endothelial selectins. J Clin Invest. 1996;97:2485–90. doi: 10.1172/JCI118695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mégarbane B, Marchal P, Marfaing-Koka A, Belliard O, Jacobs F, Chary I, et al. Increased diffusion of soluble adhesion molecules in meningitis, severe sepsis and systemic inflammatory response without neurological infection is associated with intrathecal shedding in cases of meningitis. Intensive Care Med. 2004;30:867–74. doi: 10.1007/s00134-004-2253-1. [DOI] [PubMed] [Google Scholar]
- 24.Baraczka K, Pozsonyi T, Nékám K, Virányi M, Seszták M, Szongoth M, et al. Soluble L-selectin levels in serum and cerebrospinal fluid in patients with multiple sclerosis and systemic lupus erythematosus. Acta Neurol Scand. 2000;102:114–7. doi: 10.1034/j.1600-0404.2000.102002114.x. [DOI] [PubMed] [Google Scholar]
- 25.Walcheck B, Kahn J, Fisher JM, Wang BB, Fisk RS, Payan DG, et al. Neutrophil rolling altered by inhibition of L-selectin shedding in vitro. Nature. 1996;380:720–3. doi: 10.1038/380720a0. [DOI] [PubMed] [Google Scholar]
- 26.Prieto J, Eklund A, Patarroyo M. Regulated expression of integrins and other adhesion molecules during differentiation of monocytes into macrophages. Cell Immunol. 1994;156:191–211. doi: 10.1006/cimm.1994.1164. [DOI] [PubMed] [Google Scholar]
- 27.Sakamaki F, Ishizaka A, Handa M, Fujishima S, Urano T, Sayama K, et al. Soluble form of P-selectin in plasma is elevated in acute lung injury. Am J Respir Crit Care Med. 1995;151:1821–6. doi: 10.1164/ajrccm.151.6.7539327. [DOI] [PubMed] [Google Scholar]
- 28.Bruserud O, Aksela PE, Bergheim J, Nesthus I. Serum concentrations of E-selectin, P-selectin, ICAM-1 and interleukin 6 in acute leukaemia patients with chemotherapy-induced leucopenia and bacterial infections. Br J Hematol. 1995;91:394–402. doi: 10.1111/j.1365-2141.1995.tb05309.x. [DOI] [PubMed] [Google Scholar]
- 29.Whalen MJ, Carlos TM, Dixon CE, Robichaud P, Clark RS, Marion DW, et al. Reduced brain edema after traumatic brain injury in mice deficient in P-selectin and intercellular adhesion molecule-1. J Leukoc Biol. 2000;67:160–8. doi: 10.1002/jlb.67.2.160. [DOI] [PubMed] [Google Scholar]
- 30.Greenwood J, Etienne-Manneville S, Adamson P, Couraud PO. Lymphocyte migration into the central nervous system: Implication of ICAM-1 signalling at the blood-brain barrier. Vasc Pharmacol. 2002;38:315–22. doi: 10.1016/s1537-1891(02)00199-4. [DOI] [PubMed] [Google Scholar]
- 31.Jander S, Heidenreich F, Stoll G. Serum and CSF levels of soluble intercellular adhesion molecule-1 (ICAM-1) in inflammatory neurological diseases. Neurology. 1993;43:1809–13. doi: 10.1212/wnl.43.9.1809. [DOI] [PubMed] [Google Scholar]
- 32.Sessler CN, Windsor AC, Schwartz M, Watson L, Fisher BJ, Sugerman HJ, et al. Circulating ICAM-1 is increased in septic shock. Am J Respir Crit Care Med. 1995;151:1420–7. doi: 10.1164/ajrccm.151.5.7735595. [DOI] [PubMed] [Google Scholar]
- 33.Biihrer C, Herold R, Stibenz D, Henze G, Obladen M. Cerebrospinal fluid soluble L-selectin (sCD62L) in meningoencephalitis. Arch Dis Child. 1996;74:288–92. doi: 10.1136/adc.74.4.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trojano M, Avolio C, Simone IL. Soluble intercellular adhesion molecule-1 in serum and cerebrospinal fluid of critically active relapsing-remitting multiple sclerosis: Correlation with Gd-DTPA magnetic resonance imaging-enhancement and cerebrospinal fluid findings. Neurology. 1996;47:1535–41. doi: 10.1212/wnl.47.6.1535. [DOI] [PubMed] [Google Scholar]
- 35.Pleines UE, Stover JF, Kossman T, Trentz O, Morganti-Kossmann MC. Soluble ICAM-1 in CSF coincides with the extent of cerebral damage in patients with severe traumatic brain injury. J. Neurotrauma. 1998;15:399–409. doi: 10.1089/neu.1998.15.399. [DOI] [PubMed] [Google Scholar]
- 36.Weber JR, Angstwurm K, Burger W, Einhaupl KM, Dirnagl U. Anti ICAM-1 (CD54) monoclonal antibody reduces inflammatory changes in experimental bacterial meningitis. J Neuroimmunol. 1995;63:63–8. doi: 10.1016/0165-5728(95)00131-x. [DOI] [PubMed] [Google Scholar]
- 37.Tan TQ, Smith CW, Hawkins EP, Mason EO, Kaplan SL. Hematogenous bacterial meningitis in an intercellular adhesion molecule-1-deficient infant mouse model. J Infect Dis. 1995;171:342–9. doi: 10.1093/infdis/171.2.342. [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, Benveniste EN. Adhesion molecule expression and regulation on cells of the central nervous system. J Neuroimmunol. 1999;98:77–88. doi: 10.1016/s0165-5728(99)00084-3. [DOI] [PubMed] [Google Scholar]
- 39.Christensen JP, Andersson EC, Scheynius A, Marker O, Thomsen AR. Alpha 4 integrin directs virus-activated CD8+ T cells to sites of infections. J Immunol. 1995;154:5293–301. [PubMed] [Google Scholar]
- 40.Rainer TH. L-selectin in health and disease. Resuscitation. 2002;52:127–41. doi: 10.1016/s0300-9572(01)00444-0. [DOI] [PubMed] [Google Scholar]