Abstract
Background:
A major factor in the progression of lymphedema is acute dermatolymphangioadenitis (ADLA).
Aims
To study ADLA episodes and antigenaemia in patients with different grades of filarial lymphedema at pre- and two years post-treatment.
Setting and Design:
A prospectively conducted study from May 2008 through May 2010.
Patients and Methods:
Forty five patients complaining of limb swelling with present or past history of limb redness suggestive of ADLA attacks were included. Patients were clinically examined for lymphedema grading, detection of potential entry points and diagnosis of microfilaraemia. Wuchereria bancrofti antigen titer was estimated by “Trop-Ag W. Bancrofti” ELISA kit. Basic lymphedema management and treatment with antifilarial drugs were applied.
Statistical Analysis
Mann–Whitney test and Chi-square test were used.
Results:
The number of ADLA attacks in the pretreatment period, ranged from one to three per year. Mean duration of the attacks was 3.87±0.79 days. Entry points were detected in 82% of cases. The study revealed statistical significance between extension and grade of lymphedema and number of ADLA attacks per year (P=0.018 and 0.022, respectively). Microfilaraemia was detected in four cases and positive filarial antigenaemia were detected in 29 patients (64.4). The number of ADLA attacks per year significantly decreased from the pre-treatment period (mean: 2.05±0.560) to be 1.23±0.706 after one year and 0.89±0.575 after two years post treatment. There was a significant decrease in the mean antigen titer one year and two years after treatment.
Conclusion:
Basic lymphedema management is effective for controlling ADLA attacks in areas where lymphatic filariasis is endemic.
Keywords: Acute dermatolymphangioadenitis, Filarial, Lymphedema
INTRODUCTION
Lymphatic filariasis characterized by dysfunction of the lymphatics can lead to severe and often irreversible lymphedema and elephantiasis. Decades of research in this field showed that establishment of the adult parasites in the lymphatics triggers a cascade of events that ultimately results in tissue scarring and fibrosis.[1] Lymphatic filariasis is endemic in approximately 80 countries and more than 1.1 billion people worldwide are estimated to be at risk of infection. An estimated 15 million people worldwide have lymphedema and elephantiasis of the extremities of filarial origin.[2]
A major risk factor for development of chronic lymphedema or elephantiasis is thought to be recurrent episodes of acute dermatolymphangioadenitis (ADLA). It is characterized by diffuse inflammation and swelling and occasionally associated with an ascending lymphangitis and adenitis. A distal skin lesion, often an interdigital infection, frequently serves as the entry point for bacteria precipitating ADLA.[3] Frequent ADLA episodes contribute to the progression of lymphedema by repeated damage to superficial lymphatic vessels.[4]
Several studies have reported reduction in acute attacks, lymphedema, and/or hydrocele following mass drug administration, but other studies report no such association. For most of these studies, the primary outcome of interest was microfilaraemia rather than clinical morbidity. Therefore, the studies are often limited by inadequate or non-standardized case definitions, inadequate sample sizes, and intermittent or incomplete follow-up.[5]
The aim of this work is to study the ADLA episodes in patients with filarial lymphedema. The frequency of ADLA attacks and antigenaemia were evaluated after basic lymphedema management and antifilarial drug therapy.
PATIENTS AND METHODS
Selection of cases
This study was prospectively conducted from May 2008 through May 2010. Forty five patients attending Mansoura University Hospitals (Tropical, Internal Medicine, Vascular Surgery and Children Departments), complaining of limb swelling with present or past history of limb redness were included as the study group. The circulating filarial antigenaemia in the study group was compared to a control group of 12 healthy persons and 10 patients having limb oedema of other origin such as heart failure, renal oedema, deep venous thrombosis (DVT), congenital lymphedema, post surgical edema, breast and prostate tumors for judgment of accuracy of antigen detection test.
Clinical examination
After signing an informed written consent, all subjects of the study and control groups were asked for the following; age, residency, occupation, traveling and immigration, duration of the condition, symptoms suggesting ADLA, frequency and duration. Then they were subjected to clinical examination for filarial lesions as; limb lymphedema and elephantiasis. The site (unilateral or bilateral limb affection), extension (ankle, knee, thigh), grade of lymphedema and limb skin condition were determined.
Clinical grading of lymphedema
Grade of lymphedema was assessed according to WHO four-stage system. Grade I: Pitting edema of the limb that is reversible on elevating the limb. Grade II: Pitting or non-pitting edema that is not reversible on elevating the limb and the skin is normal. Grade III: Non-pitting edema of the limb that is not reversible on elevation and the skin is thickened. Grade IV: Non-pitting edema with fibrotic and verrucous skin changes (elephantiasis).[6]
Diagnosis of ADLA attacks
According to the WHO definition; an ADLA episode would be confirmed if the affected individual presented with localized pain, lymphadenitis and/or lymphangitis and/or cellulites and local warmth, with or without systemic manifestations of fever, nausea and vomiting lasting for at least three days.[7] A suspected ADLA case confirmed if two of the three major criteria are met with or without systemic manifestations for at least three days. The affected limb was inspected for detection of potential entry points (e.g. abrasions, fissures, intertrigo, and ulcers).
Diagnosis of microfilaraemia
Five ml of venous blood were withdrawn (at midnight) for microfilaria detection by thick blood film and membrane filtration technique.[8]
Antigen assay
Three ml of venous blood were withdrawn for detection of circulating filarial antigen in serum by using lymphatic filariasis ELISA kit “Boiling pretreatment Trop-Ag W. bancrofti”. The technique was performed according to the manufacturer's instructions (Trop-Ag W. bancrofti, JCU Tropical Biotechnology Pty Ltd, James Cook University, Townsville, Queensland, Australia).
Treatment
Patients were trained for basic lymphedema management, including daily care of affected foot and leg, treatment of any small wound and abrasions by topical antibiotic, treatment of any fungal infections by topical antifungal, manual draining, massaging of the leg, application of compressive bandage and elevation of the affected leg. During the ADLA attacks, they were instructed to: Rest in bed; apply cold sponges on the affected leg; use antipyretic, analgesic and antibiotic. Patients were instructed to visit the outpatient clinic on experiencing any of the symptoms suggestive of ADLA; otherwise they were regularly checked at 6 months intervals for follow-up.
Patients were treated using ivermectin at a dose of 200 μg/kg, which was repeated after 6 months. After one year patients showing decrease in antigen level were given another dose of ivermectin and those bad responders (stayed high antigenaemia) were given ivermectin (200 μg/kg) and albendazole 400 mg. This was repeated biannually in the second year of treatment.
Statistical analysis
Data were analyzed using the statistical package for social scientist (SPSS 15) for windows (SPSS Inc., Chicago, USA). Significance of difference in quantitative variables between groups was tested with Mann Whitney test and Chi-square test was used for categorical variables. P value less than 0.05 was considered significant.
RESULTS
All of the 45 lymphedema cases had history suggestive of ADLA attacks. Their ages ranged from 12 to 46 years. The mean age of females was 30.97±7.04 years, while in males it was 26.06±9.63 years with no significant difference (P=0.069). Manifestations of ADLA attacks were pain (73.3%), tenderness of the affected limb (86.7%), redness of the skin (97.8%), fever (62.2%), and swelling (66.7%). Mean duration of the attacks was 3.87±0.79 days (range 3–6). The number of ADLA attacks ranged from one to three attacks per year in the pretreatment period. Majority of cases (73.3%) had 2 attacks/year; 15.6% had 3 attacks/year, and 11.1% reported one attack per year. No significant difference was found between site of lymphedema and number of ADLA attacks per year (P=0.243). The same was noticed between gender and number of ADLA attacks per year (P=0.904).
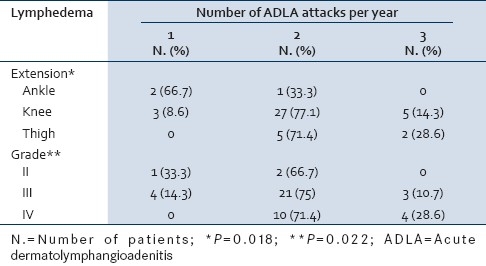
Table 1, summarizes the relation between extension, grade of lymphedema and number of ADLA per year. The extension of lymphedema increased significantly with increasing the numbers of the attacks (P=0.018). The same finding was observed with the grade (P=0.022).
Table 1.
Relation of extension and grade of lymphedema to the number of ADLA per year
Entry points were detected in 82% of cases, some of them had more than one entry point. These lesions were in the form of; web space fungus (46.7%), abrasions (35.6%), fissures (20%), small injuries (11.1%), and ulcers (8.9%).
For diagnosis of microfilaraemia; using the traditional direct smear method revealed one positive case. Four cases (8.9%) were microfilaraemic by using the membrane filtration (nucleopore) technique; they were all males with unilateral lymphedema.
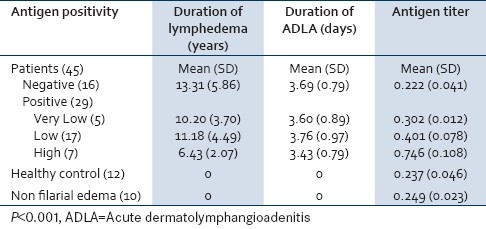
Positive filarial antigenaemia was detected in 29 of 45 patients (64.4%). The remaining 16 patients (35.6%) were negative (non reactive titer). Table 2 shows antigen positivity in relation to mean antigen titer, duration of lymphedema, and ADLA attacks. The mean antigen titer was significantly higher for patients (0.380±0.189) than controls (0.244±0.041) whether normal controls or controls with non filarial lymphedema (P<0.001). Duration of lymphedema was significantly higher in patients with negative antigenaemia (13.3 years) than patients with positive high antigenaemia (6.4 years). Duration of ADLA attacks was not significantly different between all groups.
Table 2.
Antigen positivity among subjects of the study in relation to mean antigen titer, duration of lymphedema and ADLA attacks
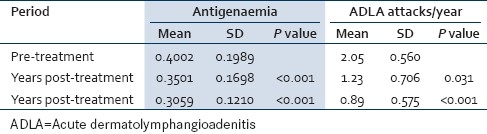
Number of patients with positive antigen titer decreased from 29 in pre-treatment to 20 (51.3%) after the first year of treatment, and further decreased to 15 (41.7%) two years post-treatment. There was a significant decrease in the mean antigen titer one year and two years after treatment. Number of ADLA attacks significantly decreased one year and two years post-treatment [Table 3].
Table 3.
Changes in antigen level and number of ADLA attacks pre- and post-treatment
DISCUSSION
Number of ADLA attacks in this study ranged from 1 to 3 attacks per year in the pretreatment period. Mean duration of ADLA attacks was 3.87±0.79 days without gender difference regarding attack number or duration. Our results supported that of Addis et al., who reported that during the 12 months before entering the program, 175 patients reported a mean of 2.1 episodes of ADLA (range 0–13), Mean reported duration of each ADLA episode was 2.6 days (range 1–44 days), and ADLA incidence by 12-month recall was not associated with patient age or sex.[9]
In the present study, statistical significance was observed between extension and grade of lymphedema in relation to the number of ADLA attacks/year [Table 1]. These results are in agreement with most studies that showed positive association between lymphedema stage and observed or patient-reported incidence of ADLA.[10] On the other hand, other studies found no such association.[11] However, all of which relied on patient recall of ADLA incidence rather than objective clinical examination during the attacks.
In the present study, 82.2% of the cases were positive for lesions in the foot and legs, which could be potential entry points. This is in agreement with Dreyer et al. and McPherson et al.[12] who found that the entry lesions are those skin lesions in the affected limb, which serve as portals of entry for pathogens that are responsible for causing the distressing symptom of ADLA. High prevalence of intertrigo in our study (46.7%) could be due to impairment of lymphatic function, narrow web space in the toes due to the swelling causing constant friction and chafing of the skin forming niches beneath skin folds that act as portals for pathogens. The prevalence of entry lesions (especially intertrigo) was significantly higher in limbs with ADLA attacks than those without the attacks.[13]
Concerning microfilaraemia, negative results were expected because the chronic nature of the cases. Four cases (8.9%) were microfilaraemic, they were all males with unilateral lymphedema. Reports on the association of lymphedema and microfilaraemia have often been conflicting. Some studies have reported that persons with lymphedema frequently had no detectable microfilaraemia,[14] whereas others reported microfilaraemia among individuals with lymphedema.[15] Some authors explained the presence of chronic lymphatic pathology and microfilaraemia by re-infection of diseased persons especially in highly endemic areas.[16]
Positive filarial antigenaemia was detected in 64.4% of patients; among them 15.6%, 48.8% and 13.3% had very low, low positive and high antigenaemia respectively [Table 2]. In the present study, low antigen levels in lymphedematous patients are in agreement with that of Dissanayake et al,[17] and Zheng et al.[18] as patients with chronic manifestation had a relatively higher levels of circulating immune complexes and/or may be no longer harbor adult parasites to produce antigenaemia.
The mean antigen titer was 0.237±0.046 in healthy control group and 0.249±0.023 in control group with limb edema (causes other than filariasis); this may be attributed to habitation in an endemic area and the presence of asymptomatic carriers. The detection of low levels of antigen in subjects supposed to be not infected with filariasis may either be due to the presence of cross-reacting antigen in serum, or to be a false positive reaction caused by serum components such as rheumatoid factors or unrelated immune complexes or exposure to animal filariae and infections with non-filarial nematodes.[17]
Comparison between different categories of antigen positivity [Table 2] revealed that the duration of lymphedema was significantly higher in negative versus high antigenaemia patients (P=0.004). This is in agreement with the data reporting that moderate levels of antigen were detected in the early clinical cases of lymphedema while lower levels were present in chronic form of elephantiasis. This was attributed to the presence of living adult worms producing antigens during active or early infection.[17] The highest percentage of antigen positivity was reported to be in the early stage of filariasis (microfilaraemia) while symptomatic amicrofilaraemia had the lowest antigenaemia.[19,20]
In the present study, number of ADLA attacks per year [Table 3] significantly decreased from the pre-treatment period (mean 2.05±0.560) to be 1.23±0.706 after one year (P=0.031) and 0.89±0.575 two years post-treatment (P<0.001). These results are in agreement with previous studies stated that simple foot hygiene and prevention of secondary bacterial infections, via early treatment of any lesions by topical antibiotics or antifungal, lower the incidence of ADLA attacks.[21,22] Similar results were obtained when proper hygiene and skin care were emphasized, the incidence of ADLA decreased rapidly to 31% of earlier levels and these reductions were sustained over time.[9]
Careful cleaning of the affected limbs can be extremely helpful in healing the infected surface area and slowing and even reversing much of the damage that has occurred already. Therefore, training the patients as well as health professionals to deal properly with those potential portals of entry for pathogens became an important part of lymphedema management to reduce the frequency of ADLA attacks, and hence, significantly reducing the morbidity of lymphatic filariasis.[23]
In the present study, number of cases with positive antigen titer, decreased from 29 (64.4%) to 20 (51.3%) after the first year of treatment and further decreased to 15 (41.7%) two years post treatment [Table 3]. There was a significant decrease in the mean antigen titer after treatment, (P<0.001). When the mean antigen titre of the first and second years was compared, there was also a significant reduction (P<0.001). According to Ismail et al,[24] ivermectin had dramatic effects on parasite antigen levels, antigen levels decreased after treatment with ivermectin to 59%, 77%, and 84% of the pre-treatment levels after 6 months, 1 year, and 2 years, respectively. According to Simonsen et al.[25] during the first 6 months after treatment with ivermectin, the circulating filarial antigens (CFA) intensities decreased significantly to 84%, while treatment with the combination (ivermectin and albendazole) decreased the CFA intensities significantly compared to pre-treatment values. On contrary, others reported that the antifilarial drugs (ivermectin and albendazole) even though important in the sustained reduction of blood microfilaria levels, have no role in the management of lymphedema or acute attacks.[26] Epidemiologic associations between transmission intensity and the prevalence of lymphedema have suggested to some investigators that third-stage larvae trigger lymphedema.[27,28] This hypothesis is supported by observations of decreases in lymphedema prevalence and severity following mass treatment with antifilarial drugs. Although such reductions are not always observed, these findings suggest that mass drug administration could have therapeutic benefits on filarial morbidity.[5]
Further investigations are needed to assess the role of ivermectin in preventing new infections and prophylaxis against re-infection in old cases that may worsen the condition. More efforts should be done to use basic lymphedema management earlier and on a larger scale in our endemic area.
CONCLUSION
Recurrent episodes of ADLA are considered to be a major risk factor for the development and progression of lymphedema of the lower extremities, evidenced by the significant associations between extension and grade of lymphedema with increasing number of ADLA attacks per year.
Basic lymphedema management is feasible and effective for reducing the number of ADLA attacks in patients from lymphatic filariasis endemic areas. Number of ADLA attacks per year and number of cases with positive antigen titer significantly decreased following basic lymphedema management and treatment with antifilarial drug therapy.
Footnotes
Source of Support: Authors own finance.
Conflict of Interest: No conflict of interest.
REFERENCES
- 1.Bennuru S, Nutman TB. Lymphatics in human lymphatic filariasis: In vitro models of parasite-induced lymphatic remodeling. Lymphat Res Biol. 2009;7:215–9. doi: 10.1089/lrb.2009.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chandrasena TG, Premaratna R, Muthugala MA, Pathmeswaran A, de Silva NR. Modified dermatology life quality index as a measure of quality of life in patients with filarial lymphoedema. Trans Roy Soc Trop Med Hyg. 2007;101:245–9. doi: 10.1016/j.trstmh.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 3.Ananthakrishnan S, Das LK. Entry lesions in bancroftian filarial lymphoedema patients-a clinical observation. Acta Trop. 2004;90:215–8. doi: 10.1016/j.actatropica.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 4.Suma TK, Shenoy RK, Varghese J, Kuttikkal VV, Kumaraswami V. Estimation of ASO titer as an indicator of streptococcal infection precipitating acute adenolymphangitis in Brugian lymphatic filariasis. Southeast Asian J Trop Med Public Health. 1997;28:826–30. [PubMed] [Google Scholar]
- 5.Addiss DG, Brady MA. Morbidity management in the Global Programme to Eliminate Lymphatic Filariasis: A review of the scientific literature. Filaria J. 2007;6:2. doi: 10.1186/1475-2883-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lymphatic filariasis: The disease and its control. Fifth report of the WHO Expert Committee on Filariasis. World Health Organ Tech Rep Ser. 1992;821:1–71. [PubMed] [Google Scholar]
- 7.WHO. Lymphatic filariasis: Diagnosis and pathogenesis. WHO Expert Committee on Filariasis. Bull World Health Organ. 1993;71:135–41. [PMC free article] [PubMed] [Google Scholar]
- 8.Desowitz RS, Jenkins C, Anian G. Bancroftian filariasis in an isolated hunter-gatherer shifting horticulturist group in Papua New Guinea. Bull World Health Organ. 1993;71:55–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Addiss DG, Louis-Charles J, Roberts J, LeConte F, Wendt JM, Milord MD, et al. Feasibility and effectiveness of basic lymphedema management in leogane, Haiti, an Area Endemic for bancroftian filariasis. PLoS Negl Trop Dis. 2010;4:668. doi: 10.1371/journal.pntd.0000668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreyer G, Noroes J, Figueredo-Silva J, Piessens WF. Pathogenesis of lymphatic disease in bancroftian filariasis: A clinical perspective. Parasitol Today. 2000;16:544–8. doi: 10.1016/s0169-4758(00)01778-6. [DOI] [PubMed] [Google Scholar]
- 11.Gyapong JO, Gyapong M, Adjei S. The epidemiology of acute adenolymphangitis due to lymphatic filariasis in northern Ghana. Am J Trop Med Hyg. 1996;5:591–5. doi: 10.4269/ajtmh.1996.54.591. [DOI] [PubMed] [Google Scholar]
- 12.McPherson T, Fay MP, Singh S, Penzer R, Hay R. Health workers’ agreement in clinical description of filarial lymphedema. Am J Trop Med Hyg. 2006;74:500–4. [PubMed] [Google Scholar]
- 13.Harichandrakumar KT, Krishnamoorthy K, Kumari AK, Das LK. Health status of lymphatic filariasis assessed from patients using seven domains five levels (7D5L) instrument. Acta Tropica. 2006;99:137–43. doi: 10.1016/j.actatropica.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Addiss DG, Dimock KA, Eberhard ML, Lammie PJ. Clinical, parasitologic, and immunologic observations of patients with hydrocele and elephantiasis in an area with endemic lymphatic filariasis. J Infect Dis. 1995;171:755–8. doi: 10.1093/infdis/171.3.755. [DOI] [PubMed] [Google Scholar]
- 15.Simonsen PE, Meyrowitsch DW, Jaoko WG, Malecela MN, Mukoko D, Pedersen EM, et al. Bancroftian filariasis infection, disease, and specific antibody response patterns in a high and a low endemicity community in East Africa. Am J Trop Med Hyg. 2002;66:550–9. doi: 10.4269/ajtmh.2002.66.550. [DOI] [PubMed] [Google Scholar]
- 16.Bundy DA, Grenfell BT, Rajagopalan PK. Immunoepidemiology of lymphatic filariasis: The relationship between infection and disease. Immunol Today. 1991;12:71–5. doi: 10.1016/S0167-5699(05)80021-0. [DOI] [PubMed] [Google Scholar]
- 17.Dissanayake S, Forsyth KP, Ismail MM, Mitchell GF. Detection of circulating antigen in bancroftian filariasis by using a monoclonal antibody. Am J Trop Med Hyg. 1984;33:1130–40. doi: 10.4269/ajtmh.1984.33.1130. [DOI] [PubMed] [Google Scholar]
- 18.Zheng HJ, Tao ZG, Reddy MV, Harinath BC, Piessens WF. Parasite antigens in sera and urine of patients with bancroftian and brugian filariasis detected by sandwich ELISA with monoclonal antibodies. Am J Trop Med Hyg. 1987;36:554–60. doi: 10.4269/ajtmh.1987.36.554. [DOI] [PubMed] [Google Scholar]
- 19.Weil GJ, Jain DC, Santhanam S, Malhotra A, Kumar H, Sethumadhavan KV, et al. A monoclonal antibody-based enzyme immunoassay for detecting parasite antigenaemia in bancroftian filariasis. J Infect Dis. 1987;156:350–5. doi: 10.1093/infdis/156.2.350. [DOI] [PubMed] [Google Scholar]
- 20.Hassan MM, Ata M, Ramzy RM, El-Gendi AM, Hegab MH, Gabr NS, et al. Evaluating the detection of circulating filarial antigen in diagnosis of bancroftian filariasis and filarial hydrocoele. J Egypt Soc Parasitol. 1996;26:687–96. [PubMed] [Google Scholar]
- 21.Dreyer G, Dreyer P, Noroes J. Recommendations for the treatment of bancroftian filariasis in symptomless and diseased patients. Rev Soc Bras Med Trop. 2002;35:43–50. doi: 10.1590/s0037-86822002000100009. [DOI] [PubMed] [Google Scholar]
- 22.Rath K, Swain BK, Mishra S, Patasahani T, Kerketta AS, Babu BV. Peripheral health workers’ knowledge and practices related to filarial lymphedema care: A study in an endemic district of Orissa, India. Am J Trop Med Hyg. 2005;72:430–3. [PubMed] [Google Scholar]
- 23.Dreyer G, Addiss D, Gadelha P, Lapa E, Williamson J, Dreyer A. Interdigital skin lesions of the lower limbs among patients with lymphoedema in an area endemic for bancroftian filariasis. Trop Med Int Health. 2006;11:1475–81. doi: 10.1111/j.1365-3156.2006.01687.x. [DOI] [PubMed] [Google Scholar]
- 24.Ismail MM, Weil GJ, Jayasinghe KS, Premaratne UN, Abeyewickreme W, Rajaratnam HN, et al. Prolonged clearance of microfilaraemia in patients with bancroftian filariasis after multiple high doses of ivermectin or diethylcarbamazine. Trans Roy Soc Trop Med Hyg. 1996;90:684–8. doi: 10.1016/s0035-9203(96)90437-x. [DOI] [PubMed] [Google Scholar]
- 25.Simonsen PE, Magesa SM, Dunyo SK, Malecela-Lazarod MN, Michaele E. The effect of single dose ivermectin alone or in combination with albendazole on Wuchereria bancrofti infection in primary school children in Tanzania. Trans Roy Soc Trop Med Hyg. 2004;98:462–72. doi: 10.1016/j.trstmh.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 26.Shenoy RK, Suma TK, Rajan K, Kumaraswami V. Prevention of acute adenolymphangitis in brugian filariasis: Comparison of the efficacy of ivermectin and diethylcarbamazine, each combined with local treatment of the affected limb. Ann Trop Med Parasitol. 1998;92:587–94. doi: 10.1080/00034989859285. [DOI] [PubMed] [Google Scholar]
- 27.Bockarie MJ, Tisch DJ, Kastens W, Alexander ND, Dimber Z, Bockarie F, et al. Mass treatment to eliminate filariasis in Papua New Guinea. N Engl J Med. 2002;347:1841–8. doi: 10.1056/NEJMoa021309. [DOI] [PubMed] [Google Scholar]
- 28.Kazura JW, Bockarie M, Alexander N, Perry R, Bockarie F, Dagoro H, et al. Transmission intensity and its relationship to infection and disease due to Wuchereria bancrofti in Papua New Guinea. J Infect Dis. 1997;176:242–7. doi: 10.1086/514030. [DOI] [PubMed] [Google Scholar]


