Abstract
Acute pulmonary schistosomiasis affects non-immune individuals returning from endemic areas. Pathogenesis is not well understood. We report a case of acute pulmonary schistosomiasis in which lung biopsy was done 10 weeks after exposure and it identified the presence of schistosomal ovum surrounded by granuloma.
Keywords: Acute schistosomiasis, Pulmonary eosinophilia, Pulmonary schistosomiasis, Schistosomiasis
INTRODUCTION
Schistosomiasis is an endemic parasitic disease in 70 countries and is estimated to infect 200 million worldwide. It is one of the 10 leading causes of morbidity among travelers.[1]
Acute schistosomiasis (Katayama fever) is a self-limited immunologically mediated syndrome first reported in Japan and affects non-immune individuals traveling to endemic areas.[2] It occurs 3–8 weeks after infection, and commonly presents with symptoms such as fever, headache malaise, myalgia, cough, hepatomegaly, splenomegaly and peripheral eosinophilia.[3–5]
Schistosomiasis is one of the major health problems in Saudi Arabia, especially in the province of Asir, south of Saudi Arabia. Awareness of disease presentation, especially in non-endemic provinces, is lacking. This may lead to delay in establishing correct diagnosis and even in using invasive measures to establish diagnosis.
CASE REPORT
A 14-year-old male student presented to our hospital with 5-week history of fever, rigors, cough and malaise for 1 month after returning from a visit to Albaha province (south of Saudi Arabia). He had been evaluated initially in a local hospital and diagnosed with community-acquired pneumonia. He received IV antibiotics and was discharged home on oral antibiotics.
During hospitalization, he had an extensive work-up for fever of unknown etiology that included multiple blood cultures, urine analysis and culture, sputum for acid-fast bacilli (AFB), routine sputum cultures, serology for brucellosis, Monospot test and blood film for malaria. His clinical examination was unremarkable; complete blood count (CBC) and differential count, renal functions and serum electrolytes, chest X-ray and transthoracic echocardiogram were all unremarkable. His liver profile was mildly deranged with aspartate aminotransferase (AST) activity of 48 U/L and alanine aminotransferase (ALT) activity of 82 U/L. He was discharged home and given outpatient appointment for follow-up.
He was re-admitted again 16 days later because of persistence of his symptoms. His clinical examination was also unremarkable. His laboratory investigations, however, were as follows: WBC count 8.1×109/L (eosinophils 30%; lymphocytes 35%; monocytes 7%; neutrophils 25%; and bands 3%), Hb=121 g/L, and platelets 438×109/L)
His chest X-ray showed ill-defined infiltrate. Thus, a chest computed tomography (CT) was requested and revealed peripheral nodular and patchy infiltrates in both lung fields [Figure 1]. There was evidence of splenomegaly in the lower cuts of the scan.
Figure 1.
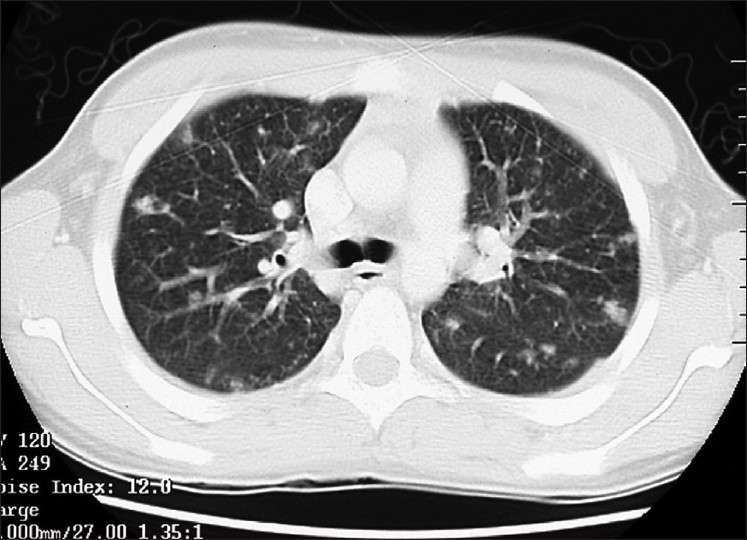
CT of chest showing peripheral nodular and patchy infiltrates in both lung fields
Repeated CBC and differential count was significant for increasing peripheral blood eosinophil count to 63%, with an absolute count of 9.4×109/L and WBC of 14.9×109/L. Blood, urine, sputum cultures and sputum for AFB were negative. Two stool samples for microscopy were negative for parasite. Serum IgE level was 334 U/L, (normal <200 U/L), ANCA-P test was negative, and renal functions and liver function tests were unremarkable.
Bronchoscopy was performed. Bronchoscopic alveolar lavage (BAL) fluid analysis showed marginally elevated eosinophils (7%); however, microbial studies (including AFB, fungal and routine bacterial cultures) were negative. A transbronchial biopsy showed tissue eosinophilia.
The patient underwent video-assisted thoracoscopic lung biopsies from the left lower lobe which showed a schistosomal ovum within a well-formed granuloma [Figure 2]. Subsequently, the test for schistosoma antibodies serology came positive. The diagnosis of acute pulmonary schistosomiasis was then made.
Figure 2.
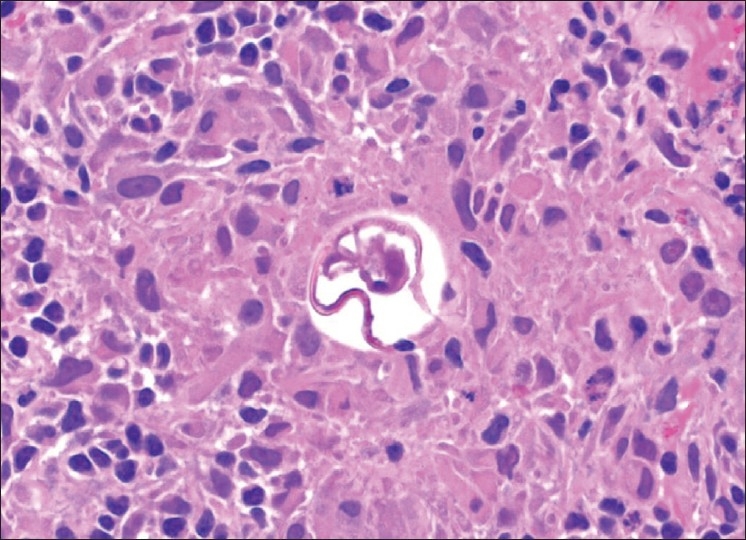
Lung biopsy from the left lower lobe showing a schistosomal ovum within a well-formed granuloma
The patient was given praziquantel (20 mg/kg body weight) twice daily for 2 days. Two days later, he reported to our emergency department with high-grade fever and rigors. His symptoms subsided with the addition of a short course of prednisolone. After this, he visited our outpatient clinic for follow-up and was free of symptoms and had a normal chest X-ray.
DISCUSSION
We present here a case report of a young patient presenting with shortness of breath after visiting a schistosomiasis-endemic area in Saudi Arabia. His chest X-ray showed a bilateral infiltrate, and a lung biopsy done 10 weeks after exposure identified the presence of schistosomal ovum surrounded by granuloma.
Our patient presented with typical features of Katayama fever. He presented with fever, cough and malaise 1 month after returning from Albaha (an endemic area of schistosomiasis in the southern region of Saudi Arabia), had an enlarged spleen as noted in his abdominal CT, and significant peripheral eosinophilia was detected later in the course of his illness. His liver enzymes were mildly impaired.
Churg–Strauss Syndrome, in its early prodromal phase, may present as tissue eosinophilia; however, negative history of bronchial asthma and lack of extrapulmonary manifestations of vasculitis made it unlikely. We were, instead, in favor of the diagnosis of chronic pulmonary eosinophilia.
Lung biopsy was done to get a definitive diagnosis if possible and to avoid using steroid empirically as treatment for idiopathic eosinophilic pulmonary disease and to rule in or out eosinophilic vasculitis.
The lung biopsy showed a well-formed granuloma containing schistosomal ovum. The positive schistosoma antibody serological test done after the biopsy provided further confirmation to the diagnosis of acute pulmonary schistosomiasis. This was not expected and was, in fact, not included in initial differential diagnosis.
Acute schistosomiasis, first described in Japan as Katayama fever, usually presents within an average of 41.5 days after the individuals are exposed to a first infection or a large reinfection or super infection.[2] Cases often occur as epidemics during the rainy season. Individuals present with nocturnal fever peaks, coughing, generalized muscle pain, headache, and a tender, enlarged liver. Splenomegaly occurs in a third of cases. Diffuse pulmonary infiltrates are found clinically and radiologically, and a few cases have signs of meningoencephalitis. All cases have peripheral eosinophilia and a history of water contact 14–84 days before. Other laboratory findings include mild leukocytosis and elevated IgE serum level.[5,6] Nodular lung infiltrates are the most common radiological finding.[7] Patients usually respond promptly to a 6-day course of 20 mg/kg doses of praziquantel.
Diagnosis of Katayama fever is based on clinical grounds[2] and may be missed because of lack of clinician awareness, as in our case. Antibody test for schistosoma may take a few months to be positive. Awareness of the spectrum of presentations is the key element in establishing the diagnosis early and sometimes even without the need for invasive procedures.
As previously mentioned, for patients traveling to an endemic area and presenting with typical clinical manifestations of peripheral eosinophilia, physicians should entertain seriously the diagnosis of acute schistosomiasis and may start an empirical praziquantel therapy while waiting for further serological testing.
The pathological data of acute pulmonary schistosomiasis available so far are scanty. The first report of lung granuloma secondary to bilharziasis was given by S. H. ElMallah and M. Hashem from Egypt, which was published in 1953.[8] They demonstrated the presence of bilharzial pseudotubercles. Added to that report, we could only identify four other cases. The first one was reported by Davidson et al.[7] and the second one by Schwartz.[4] In both the cases, histopathological specimens were obtained by transbronchial biopsy. Both revealed histopathological features compatible with eosinophilic pneumonia but failed to demonstrate the presence of ova or parasite and no granuloma was detected. In the third case reported by Soares Souza et al., open lung biopsy was performed and showed parenchymal granulomatous inflammation and numerous schistosome ova.[9] The fourth case was again from Egypt which was a young patient presenting with hemoptysis. Histopathology revealed pulmonary venous congestion with bilharzial ova.[10].
Acute pulmonary schistosomiasis is thought to be an immune-mediated disease similar to other forms of eosinophilic pneumonia. This postulation is supported by the presence of eosinophilia, immune complexes and elevated IgE levels.[11]
CONCLUSION
Our aim of presenting this case is to add to the limited literature of acute pulmonary schistosomiasis with histopathological confirmation of the fact that schistosomal ova could be found in the lung tissue in acute phase of pulmonary schistosomiasis and could participate in the pathogenesis of these conditions. We, in fact, speculated that the nodular lung lesion in our case was due to the presence of schistosomal granuloma.
By presenting this case, we would like to increase the awareness of the physicians to the diagnosis of acute schistosomiasis among individuals returning from endemic areas. We could have saved much time and effort if we were optimally aware about it.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Freedman DO, Kozarsky PE, Weld LH, Cetron MS. GeoSentinel: The global emerging infections sentinel network of the International Society of Travel Medicine. J Travel Med. 1999;6:94–8. doi: 10.1111/j.1708-8305.1999.tb00839.x. [DOI] [PubMed] [Google Scholar]
- 2.Ross AG, Vickers D, Olds GR, Shah SM, McManus DP. Katayama syndrome. Lancet Infect Dis. 2007;7:218–24. doi: 10.1016/S1473-3099(07)70053-1. [DOI] [PubMed] [Google Scholar]
- 3.Doherty JF, Moody AH, Wright SG. Katayama fever: An acute manifestation of schistosomiasis. BMJ. 1996;313:1071–2. doi: 10.1136/bmj.313.7064.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooke GS, Lalvani A, Gleeson FV, Conlon CP. Acute pulmonary schistosomiasis in travelers returning from Lake Malawi, sub-Saharan Africa. Clin Infect Dis. 1999;29:836–9. doi: 10.1086/520445. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz E, Rozenman J, Perelman M. Pulmonary manifestations of early schistosome infection among nonimmune travelers. Am J Med. 2000;109:718–22. doi: 10.1016/s0002-9343(00)00619-7. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen LQ, Estrella J, Jett EA, Grunvald EL, Nicholson L, Levin DL. Acute schistosomiasis in nonimmune travelers: Chest CT findings in 10 patients. Am J Roentgenol. 2006;186:1300–3. doi: 10.2214/AJR.05.0213. [DOI] [PubMed] [Google Scholar]
- 7.Davidson BL, El-Kassimi F, Uz-Zaman A, Pillai DK. The “lung shift” in treated schistosomiasis.Bronchoalveolar lavage evidence of eosinophilic pneumonia. Chest. 1986;89:455–7. doi: 10.1378/chest.89.3.455. [DOI] [PubMed] [Google Scholar]
- 8.El-Mallah SH, Hashem M. Localized bilharzial granuloma of the lung simulating a tumour. Thorax. 1953;8:148–51. doi: 10.1136/thx.8.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Souza SA, Jr, Marchiori E, Maluf CP, Gasparetto EL, Escuissato DL. Acute pulmonary schistosomiasis: Correlation between the high-resolution CT and pathological findings. Rev Port Pneumol. 2007;13:741–4. doi: 10.1016/s2173-5115(07)70369-x. [DOI] [PubMed] [Google Scholar]
- 10.Sersar SI, Abulmaaty RA, Elnahas HA, Moussa SA, Shiha UA, Ghafar WA, et al. A diagnostic dilemma of right lower lobe collapse caused by pulmonary bilharsiasis. Heart Lung Circ. 2006;15:50–2. doi: 10.1016/j.hlc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 11.de Jesus AR, Silva A, Santana LB, Magalhães A, de Jesus AA, de Almeida RP, et al. Clinical and immunologic evaluation of 31 patients with acute schistosomiasis mansoni. J Infect Dis. 2002;185:98–105. doi: 10.1086/324668. [DOI] [PubMed] [Google Scholar]


