Abstract
Object:
The incidence of hydrocephalus requiring shunts in children with myelomeningocele (MMC) is reported to be very high. Shunt-related complications are a significant cause of morbidity and mortality in this population. In order to minimize shunt placements, we used very rigid clinical selection criteria and followed them in all patients who had myelomeningocele and enlarged ventricles. The follow-up outcome of this retrospective study is reported.
Methods:
From 2000 to 2007, 23 patients with myelomeningocele and variable degree of hydrocephalus were treated at our institute with primary surgical closure of their myelomeningoceles without a CSF diversion procedure. Patients with severe hydrocephalus who required immediate shunt insertion, and those with no significant associated hydrocephalus were not included in this study. Data regarding the surgical results and complications, postoperative management, and the outcome at follow-up were obtained from their hospital records.
Results:
Initially increased size of the ventricular system was found to have decreased or stabilized in 17 (81%) patients postoperatively. However, ventriculomegaly continued to progress further in 4 (19%) out of 21 patients. Of 11 patients who presented with enlarged head, eight (73%) patients showed reduction or stabilization in their head circumference. Three (27%) children continued to have progressive head enlargement in the postoperative period and required shunt placement. Signs of raised intracranial pressure observed in six patients on admission, improved in two (33%) and persisted or worsened in four (67%) patients who eventually improved after the insertion of a shunt. Eight (35%) patients experienced wound-related complications following closure of the MMC, including CSF leak in four, wound infection in three, wound breakdown in three, and pseudomeningocele in two patients. Shunt placement was required in the postoperative period in 13 (56.5%) patients to treat raised intracranial pressure in 11 and CSF leak from the wound in two patients.
Conclusions:
Our experience suggests that the placement of shunts can be reduced by adopting a policy with strict clinical and radiographic criteria. Shunt insertion should be reserved for only those patients who have severe hydrocephalus with clinical features of elevated intracranial pressure. Mild to moderate ventricular dilatation, persistent ventriculomagaly, and some increase in ventricular size after myelomeningocele repair can be treated successfully without a shunt.
Keywords: Hydrocephalus, myelomeningocele, shunt placement
Introduction
Hydrocephalus is frequently associated with spinal myelomeningocele (MMC). The association is, in fact, so common that shunt surgery is thought to be almost unavoidable in these patients. However, the rate of shunt placement is reported in literature to be as high as over 85%.[1–9] This means that a large number of children with MMC who have already been suffering from severe disabilities due to spinal MMC, are exposed to the additional burden of serious shunt-related complications, including death. Thus, there exists a rationale to avoid shunt insertion in this population whenever possible. In 2008, Chakraborty et al.[10] suggested that the rate of shunt placement in these patients can be reduced considerably by applying more stringent guidelines.
We have been following the policy of reducing shunt surgery in patients with MMC since 2000. The criteria for shunt placement are strictly followed in our institution and are based on the clinical and radiographic evidence of raised intracranial pressure (ICP) secondary to hydrocephalus. In this retrospective study, we present our experience in the management of children with MMC who have hydrocephalus.
Materials and Methods
All patients with myelomeningocele and hydrocephalus who had been treated surgically at our institution between January 2000 and December 2007 were identified, and their clinical, radiological, and follow-up data were obtained from the hospital records. Only those patients were included in this study who had had primary postnatal repair of their MMC with or without a ventriculo-peritoneal (VP) shunt, and were followed up for at least 12 months postoperatively.
Neurological evaluation, head circumference monitoring, and cranial ultrasound examination / computerized tomography (CT) / magnetic resonance imaging (MRI) scans were performed prior to MMC repair in all patients. Patients with clinical and radiographic evidence of raised ICP due to hydrocephalus underwent ventriculo-peritoneal shunting along with the closure of MMC, and were excluded from this study. The patients who did not have symptoms or signs of intracranial hypertension on presentation were treated for MMC repair alone, regardless of the size of ventricles. These patients were followed up clinically with neurological examination and head size measurement everyday, and ultrasound or CT scans were performed 24–48 h after surgery, at the time of discharge from the hospital, and in the outpatient clinic after three months. Shunt insertion was considered only when raised ICP was suspected on a clinical basis with the appearance of vomiting, irritability, drowsiness, bulging fontanelle, sunset sign, progressively enlarging head, bradycardia, and respiratory irregularities, in association with persistent / progressive ventriculomegaly on serial ultrasound or CT/MRI.
Mild or moderate hydrocephalus, or persistent ventriculomegaly not associated with intracranial hypertension, and the presence of pseudomeningocele or CSF leak at the closure site were not considered indications for shunt surgery. Similarly, worsening of mental functions was not a criterion for shunting because complete neurocognitive analysis was not performed in all patients.
Case Reports
Case 1
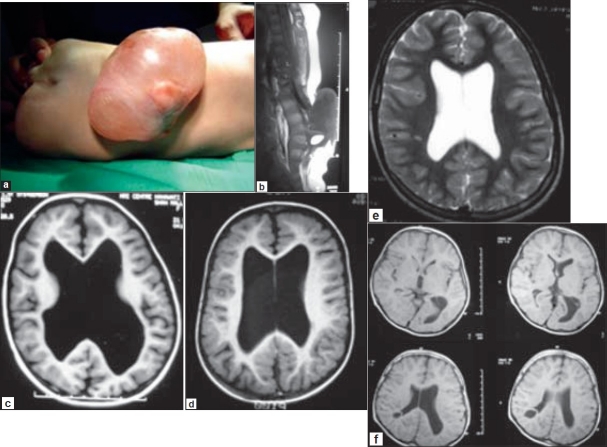
A three week-old neonate was admitted with a lumbar MMC associated with bilateral foot deformities and weakness, impaired sensations and reflexes, and bladder / bowel disturbances [Figure 1a]. The child was fully alert and had a normal head circumference with mildly tense fontanelle. The results of his ocular and neurological examinations were unremarkable. An MRI scan of his spine disclosed a low-lying conus with tethering at L4 and a large MMC in the lumbar region [Figure 1b], and mild to moderate hydrocephalus [Figure 1c and d]. The child underwent surgery for untethering of the cord and repair of MMC without a CSF diversion procedure. The patient did well postoperatively and remained clinically and neurologically stable. MRI of the brain at discharge from the hospital one week later demonstrated no change in the size of the ventricular system [Figure 1e]. He continued to improve subsequently during the follow-up assessment and a repeat MRI scan after four years showed complete resolution of the hydrocephalus [Figure 1f].
Figure 1.
a. Lumbar MMC associated with bilateral foot deformities and weakness, impaired sensations and reflexes, and bladder/bowel disturbances in a three week-old infant. b. MRI scan showing a large MMC with low-lying conus and tethering at L4. c. and d. MRI of the brain showing mild to moderate hydrocephalus. e. MRI of the brain one week after repair of MMC showing no change in the size of the ventricular system. f. MRI scan after four years revealing complete resolution of hydrocephalus
Case 2
A male neonate aged two weeks, was referred to our institution for the management of his lumbar MMC [Figure 2a] associated with hypotonia and lower extremity weakness, diminished reflexes, impaired sensations, foot deformities, and patulous anal sphincter. The child's head size was normal but the fontanelles were tense. The results of his general and neurological examinations were otherwise normal. An MRI of the spine revealed a thickened and low-lying conus with tethering at the L4 level with a large lumbar MMC [Figure 2b]. A CT scan of the brain demonstrated asymmetrical hydrocephalus with severe enlargement of the left lateral ventricle compared to the right lateral ventricle, producing a mass effect [Figure 2c]. During surgery, his MMC was closed along with sectioning of the fatty filum and untethering of the cord, and his hydrocephalus was observed postoperatively. The baby maintained his clinical and neurological status well after surgery, and both his cranial ultrasound after 48 h and at discharge as well as a CT scan of the brain after four weeks [Figure 2d] showed gradual reduction in the size of the ventricular system. His hydrocephalus resolved completely during follow-up [Figure 2e].
Figures 2a.
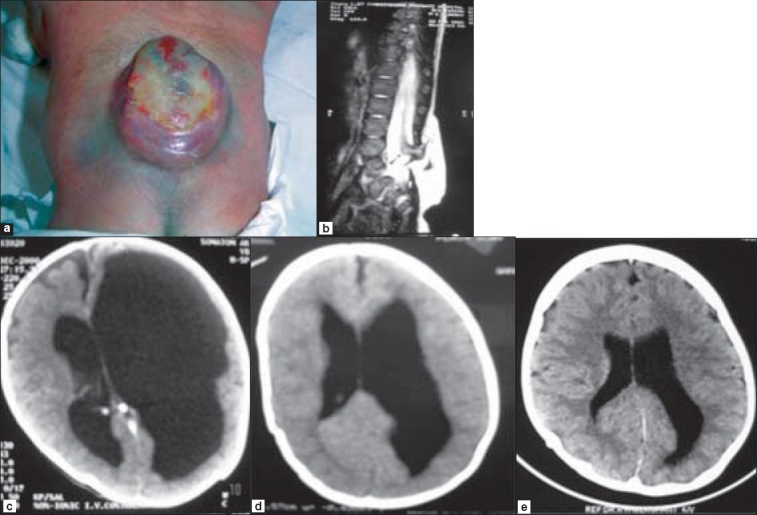
Lumbar MMC in a two week-old child. b. MRI showing a thickened filum and low-lying conus and tethering at L4 level. c. CT scan of the brain demonstrating asymmetrical hydrocephalus with left lateral ventricle severely enlarged as compared to the right lateral ventricle, producing mass effect. d and e. CT scans of the brain after four weeks and at follow-up respectively, showing gradual resolution of hydrocephalus
Case 3
A five month-old male child presented with a large MMC in the lumbosacral region [Figure 3a] with distal weakness in both the lower extremities (L > R), diminished reflexes and anal tone, as well as sensory impairment. His head circumference was normal and stable, with full and tense fontanelle. An MRI scan revealed a lumbar MMC with low-lying conus and tethering of the cord at L3 [Figure 3b], along with moderate hydrocephalus [Figure 3c]. During surgery, his conus was untethered and the MMC was repaired. He remained clinically and neurologically stable for 48 h postoperatively, but then started deteriorating gradually. Initially, he developed vomiting and became irritable with increased head circumference and bulged out fontanelle. Subsequently, he also developed the setting-sun sign. A repeat CT scan of the brain on the 5th postoperative day demonstrated further enlargement of the ventricular system with periventricular CSF ooze and pressure effect [Figure 3d]. However, the patient's condition stabilized immediately after placement of a ventriculo-peritonial shunt and he was discharged after three days in a completely recovered condition [Figure 3e].
Figure 3.
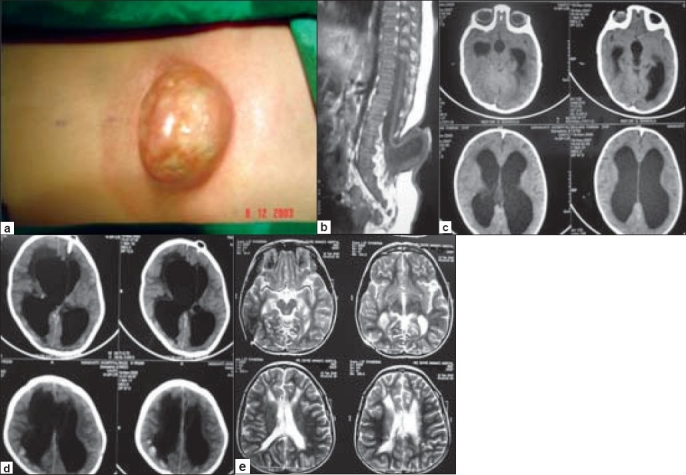
a. A large lumbosacral MMC in a five month-old child. b. Sagittal MRI scan showing a lumbar MMC with low-lying conus and tethering of the cord at L3-4. c. MRI of the brain demonstrating moderate hydrocephalus. d. CT scan of the brain on the 5th postoperative day showing enlargement of the ventricular system with periventricular CSF ooze and pressure effect. e. MRI brain at follow up showing a shunt catheter in the right lateral ventricle and completely normal ventricular system.
Results
Between January 2000 and December 2007, 23 patients were treated surgically for repair of MMC without any CSF diversion procedure. There were 13 male and 10 female children with a mean age of two months (range: three days to 14 months). The mean follow-up period was 36 months with a range of three months to 4.5 years.
All patients had congenital myelomeningocele that was located in the lumbosacral region in 17 patients and in the dorsolumbar region in six patients. None of these patients had overt signs of hydrocephalus, chiari malformation, or any other similar lesion in the craniospinal axis. Other associated anomalies included talipes deformities in eight (35%) and hip dislocation in five (22%) patients. All patients had varying degrees of motor and sensory deficits in their lower extremities on presentation, and 11 (48%) patients showed evidence of bladder and bowel dysfunctions.
The MRI findings were consistent with MMC, low-lying conus, and tethering of the cord. The majority of the patients also demonstrated a combination of intradural lipoma, fatty filum, or syrinx in the conus. Mild to moderate ventriculomegaly was documented in all patients.
Table 1 shows the postoperative state of the hydrocephalus and its management in all 23 patients who underwent surgical closure of MMC as a primary procedure. Initially, the increased size of the ventricular system was found to decrease or be stabilized postoperatively in 17 (81%) patients, but the increase continued to progress further in four (19%) out of 21 patients. Of 11 patients who presented with an enlarged head, eight (73%) patients showed reduction or stabilization in their head circumference. Three (27%) children, however, continued to have progressive head enlargement in the postoperative period and required shunt placement. Signs of raised intracranial pressure observed in six patients on presentation, improved in two (33%) patients but persisted or worsened in four (67%) patients who finally improved after the insertion of a shunt.
Table 1.
Postoperative outcome of hydrocephalus in 23 patients

Eight (35%) patients experienced wound-related complications following the closure of the MMC, including CSF leak in four, wound infection in three, wound breakdown in three, and pseudomeningocele in two patients [Table 2].
Table 2.
Complications of myelomeningocele repair in eight patients

The decision to use a CSF diversion procedure was based entirely on the patient's clinical condition and supported by radiographic findings. Shunt placement was required in 13 (56.5%) out of the 23 patients [Table 3]. Of these 13 patients who underwent shunt placement, 11 had symptoms of elevated intracranial pressure. Vomiting and ocular signs with persistent hydrocephalus were observed postoperatively in five patients, irritability and bulged out fontanelle in two patients, and a progressive hydrocephalus with rapidly enlarging head circumference in three patients. One patient worsened neurologically and developed altered sensorium, bradycardia, respiratory irregularities, and enlarged ventricles more than 24 h after surgery. Out of four patients who developed postoperative CSF leak from the wound, two required shunt insertion after all other measures to control CSF leakage failed. All these patients improved immediately after shunting and were discharged in a stable condition after a few days.
Table 3.
Indications of shunt placement in 13 patients

The shunt insertion was performed within first 48 h of the MMC repair in one patient, after the first week in four patients, after two weeks in five patients, after three weeks in two patients, and after four weeks in one patient.
Discussion
Although shunts are the mainstay of treatment for hydrocephalus, their management is fraught with a high rate of complications, which include serious disabilities and death.[11] The failure rate of shunts has been estimated to be over 50%. In a prospective, randomized shunt design trial, the overall one-year failure rate was approximately 40% for a group of patients whose median corrected age was 55 days;[12–14] the two-year failure rate was 50%. These studies also demonstrate that younger children, particularly those less than six months of age, are at significantly increased risk of shunt complications.
The incidence of shunt-related complications has been reported to be greater in patients with myelomeningocele than in those who required shunt placement for the treatment of other conditions.[15,16] Iskandar et al.[15] as well as other authors observed that complications due to shunts in these children were more common, more serious, and often life-threatening. Recent studies also suggest that the overall survival in patients with MMC without hydrocephalus is significantly better and that the patients with MMC who have had shunt surgery, have reduced longevity compared to patients who have not required shunt placement for CSF diversion.[17,18] Moreover, there seems to be an adverse correlation between shunt placement and neurocognitive outcome. Mapstoneet al .[19] have shown that children with MMC who did not require shunt placement, have been reported to have higher mean IQs than those who did. There is also good evidence that shunt complications such as infection and multiple revisions have greater adverse effects on intelligence in this population.[20,21]
Although some of these complications observed in children with MMC who require shunt placement only reflect the severity of the disease,[8,22,23] it is difficult to ignore the long-term adverse effects of shunt-related complications in this group of patients.[4,10,24] The cost effectiveness of the treatment is also an important consideration. The economic burden of shunt surgery is apparently very high because of the high incidence of complications and the associated failures that result in multiple hospitalizations and shunt revisions. It is therefore necessary to justify shunt insertion on the basis of cost-effectiveness and its clinical benefit.
It thus appears reasonable and appropriate to consider the possibilities of avoiding or reducing shunt surgery in children with myelomeningocele. Shunt surgery performed only in carefully selected patients may reduce an additional burden of shunt-related complications in a significant proportion of these children. A more critical evaluation of the indications of shunt placement is necessary to ensure that shunts are only placed in patients who truly need them and who can benefit from them. By using a stringent shunt placement policy, recent studies by Chakraborty and co-workers[10] as well as Dirks et al.,[3] have demonstrated that the shunt insertion rate in patients with myelomeningocele is lower than that reported in the literature. [1–9,25]
According to the theory for the etiology of hydrocephalus proposed by McLone and Knepper,[23] the chronic egress of CSF through an open myelomeningocele defect impairs the development of the normal CSF pathways and acts as the driving force for the chiari-II malformation, both of which contribute to the development of hydrocephalus. It was therefore hypothesized that early, perhaps in utero repair of myelomeningocele could potentially reduce the incidence of chiari-II malformation and hydrocephalus. The rate of shunt placement in some of the largest series of in utero repair of myelomeningocele published in 2003–2004 was 54%,[8,22] which is significantly lower than the shunt placement rate in patients treated postnatally, i.e., 63–91% (1-9,25 ). The shunt placement rate in the study published by Chakraborty et al.[10] was comparable to the incidence of shunt insertion reported after in utero repair.
Our experience is quite similar to that of the authors of the above studies.[3,10] We also believe that by using strict indication criteria, the rate of shunt placement can be reduced significantly in children with myelomeningocele. Only 13 out of 23 (56.5%) patients in our study required shunting as opposed to > 80% described in the literature. We eliminated shunting in patients who had mild or moderate ventriculomegaly and in those who did not have elevated intracranial pressure. Persistent ventriculomegaly and some increase in ventricular size is common following repair of myelomeningocele. Our experience suggests that postoperative ventriculo-megaly is generally nonprogressive and can be stabilized and managed conservatively without any surgical intervention. Similarly, we also avoided shunt insertion in our patients who had wound-related complications, including pseudomeningocele and CSF leakage.
Conclusions
This study has demonstrated that not all children with myelomeningocele and ventriculomegaly require CSF shunting. Shunt-related complications can be reduced significantly by avoiding unnecessary shunt placements. Strict clinical and radiological criteria should be applied to ensure that shunts are only placed in patients who really need them and who can benefit from them.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Bowman RM, McLone DG, Grant JA, Tomita T, Ito JA. Spina bifida outcome: A 25-year prospective. Pediatr Neurosurg. 2001;34:114–20. doi: 10.1159/000056005. [DOI] [PubMed] [Google Scholar]
- 2.Bruner JP, Tulipan N, Paschall RL, Boehm FH, Walsh WF, Silva SR, et al. Fetal surgery for myelomeningocele and the incidence of shunt-dependent hydrocephalus. JAMA. 1999;282:1873–4. doi: 10.1001/jama.282.19.1819. [DOI] [PubMed] [Google Scholar]
- 3.Dirks PB, Drake JM, Lamberti-Pasculli M, Rutka JT, Humphreys RP, McDonald P. Falling ventriculoperitoneal shunt rates in myelomeningocele, in The International Society for Pediatric Neurosurgery 31st Annual Meeting Abstracts. International Society for Pediatric Neurosurgery. 2007. Available from: http//www.ispneurosurgery.org/ database/View/AbstractProgram.asp?AbstractID=479)
- 4.Hunt GM, Oakeshott P, Kerry S. Link between the CSF shunt and achievement in adults with spina bifida. J Neurol Neurosurg Psychiatry. 1999;67:591–5. doi: 10.1136/jnnp.67.5.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Machado HR, Santos de Oliveira R. Simultaneous repair of myelomeningocele and shunt insertion. Childs Nerv Syst. 2004;20:107–9. doi: 10.1007/s00381-003-0853-7. [DOI] [PubMed] [Google Scholar]
- 6.Rintoul NE, Sutton LN, Hubbard AM, Cohen B, Melchionni J, Pasquariello PS, et al. A new look at myelomeningoceles: Functional level, vertebral level, shunting and the implications for fetal intervention. Paediatrics. 2002;109:409–13. doi: 10.1542/peds.109.3.409. [DOI] [PubMed] [Google Scholar]
- 7.Steinbok P, Irvine B, Cochrane DD, Irvine BJ. Long-term outcome and complications of children born with meningomyelocele. Childs Nerv Syst. 1992;8:92–6. doi: 10.1007/BF00298448. [DOI] [PubMed] [Google Scholar]
- 8.Tulipan N, Sutton LN, Bruner JP, Cohen BM, Johnson M, Adzick NS. The effect of intrauterine myelomeningocele repair on the incidence of shunt-dependent hydrocephalus. Pediatr Neurosurg. 2003;38:27–33. doi: 10.1159/000067560. [DOI] [PubMed] [Google Scholar]
- 9.Wakhlu A, Ansari NA. The prediction of postoperative hydrocephalus in patients with spina bifida. Childs Nerv Syst. 2004;20:104–6. doi: 10.1007/s00381-003-0849-3. [DOI] [PubMed] [Google Scholar]
- 10.Chakraborty A, Crimmins D, Hayward R, Thompson D. Toward reducing shunt placement rates in patients with myelomeningocele. J Neurosurg Pediatr. 2008;1:361–5. doi: 10.3171/PED/2008/1/5/361. [DOI] [PubMed] [Google Scholar]
- 11.Duncan CC. Management of proximal shunt obstruction: Technical note. J Neurosurg. 1988;68:817–9. doi: 10.3171/jns.1988.68.5.0817. [DOI] [PubMed] [Google Scholar]
- 12.Drake JM, Kestle J. Determining the best cerebrospinal fluid shunt valve design: The pediatric valve design trial. Neurosurgery. 1996;38:604–7. doi: 10.1097/00006123-199603000-00042. [DOI] [PubMed] [Google Scholar]
- 13.Drake JM, Kestle J. Rationale and methodology of the multicenter pediatric cerebrospinal fluid shunt design trial. Childs Nerv Syst. 1996;12:434–47. doi: 10.1007/BF00261620. [DOI] [PubMed] [Google Scholar]
- 14.Kestle J, Drake JM, Group PH. Long term follow-up for the shunt design trial. Childs Nerv Syst. 1999;15:426. [Google Scholar]
- 15.Iskandar BJ, Tubbs S, Mapstone TB, Grabb PA, Bartolucci AA, Oakes WJ. Death in shunted hydrocephalic children in the 1990s. Pediatr Neurosurg. 2005;28:173–6. doi: 10.1159/000028644. [DOI] [PubMed] [Google Scholar]
- 16.Tuli S, Drake J, Lambert-Pasculli M. Long-term outcome of hydrocephalus management in myelomeningoceles. Childs Nerv Syst. 2003;19:286–91. doi: 10.1007/s00381-003-0759-4. [DOI] [PubMed] [Google Scholar]
- 17.Davis BE, Daly CM, Shurtleff DB, Duguay S, Seidel K, Loeser JD, et al. Long-term survival of individuals with myelomeningocele. Pediatr Neurosurg. 2005;41:186–91. doi: 10.1159/000086559. [DOI] [PubMed] [Google Scholar]
- 18.Tuli S, Tuli J, drake J, Spears J. Predictors of death in pediatric patients requiring cerebrospinal fluid shunts. J Neurosurg. 2004;100:442–6. doi: 10.3171/ped.2004.100.5.0442. [DOI] [PubMed] [Google Scholar]
- 19.Mapstone TB, Rekates HL, Nulsen FE, Dixon MS, Jr, Glaser N, Jaffe M. The relationship of CSF shunting and IQ in children with myelomeningocele: A retrospective analysis. Childs Nerv Syst. 1984;11:112–8. doi: 10.1159/000120166. [DOI] [PubMed] [Google Scholar]
- 20.Barf HA, Verhoef M, Jennekens-Schinkel A, Post MW, Gooskens RH, Prevo AJ. Cognitive status of young adults with spina bifida. Dev Med Child Neurol. 2003;45:813–20. doi: 10.1017/s0012162203001518. [DOI] [PubMed] [Google Scholar]
- 21.McLone DG, Czyzewski D, Raimondi AJ, Sommers RC. Central nervous system infections as a limiting factor in the intelligence of children with myelomeningocele. Paediatrics. 1982;70:338–42. [Google Scholar]
- 22.Bruner JP, Tulipan N, Reed G, Davis GH, Bennett K, Luker KS, et al. Intrauterine repair of spina bifida: Preoperative predictors of shunt-dependent hydrocephalus. Am J Obstet Gynecol. 2004;190:1305–12. doi: 10.1016/j.ajog.2003.10.702. [DOI] [PubMed] [Google Scholar]
- 23.McLone DG, Knepper PA. The cause of Chiari II malformation: A unified theory. Pediatr Neurosci. 1989;15:1–12. doi: 10.1159/000120432. [DOI] [PubMed] [Google Scholar]
- 24.Hetherington R, Dennis M, Barnes M, Drake J, Gentili F. Functional outcome in young adults with spina bifida. J Neurol Neurosurg Psychiatry. 1999;67:591–5. doi: 10.1007/s00381-005-1231-4. [DOI] [PubMed] [Google Scholar]
- 25.Mirzai H, Ersahin Y, Mutluer S, Kayahan A. Outcome of patients with myelomeningocele: The Ege University experience. Childs Nerv Syst. 1998;14:120–3. doi: 10.1007/s003810050192. [DOI] [PubMed] [Google Scholar]