Abstract
Necrotizing sialometaplasia, is a benign inflammatory lesion primarily involving the minor salivary glands of the hard palate. The lesion often presents itself as a deep-seated palatal ulcer with clinical and histological features similar to those of a malignant neoplasm. Here we report a case of necrotizing sialometaplasia in a 40-year-old female, present on the lateral border of the tongue, mimicking squamous cell carcinoma, clinically. A correct diagnosis to avoid mutilant surgical treatments is essential, considering that it is a self-limiting disease.
Keywords: Necrotizing sialometaplasia, squamous cell carcinoma, tongue
INTRODUCTION
Necrotizing sialometaplasia (NSM) is a benign self-limiting inflammatory lesion of the salivary glands. It was described for the first time by Abrams et al. in 1973.[1] Abrams et al.[1] defined it as a reactive necrotizing inflammatory process involving the minor salivary glands of the hard palate. Since then numerous cases of NSM have been reported, predominantly affecting the palatal minor salivary glands. NSM affecting other oral sites such as major salivary glands, sino nasal mucosa, and larynx, is a very rare occurrence, with only few cases reported in those sites. The significance of this lesion is the fact that it may simulate, both clinically and histologically, malignant lesions like squamous cell carcinoma or mucoepidermoid carcinoma. This becomes especially important in the lesions which occur in unexpected sites where incorrect diagnosis may lead to unnecessary surgical treatment. Here we report a case of NSM present on the lateral border of the tongue, which is an unusual site, highlighting the importance of correct diagnosis to prevent needless mutilant treatment and mental ordeal for the patient.
CASE REPORT
A 40- year-old female reported to Government Dental College, Calicut with a painless growth on the right lateral border of her tongue for the last one month. History revealed trauma due to root stumps of upper right first molar, in the same site, which were extracted few months ago, and paresthesia of the tongue. On examination a growth of size 1.2 × 1 cm was observed on the right lateral border of the tongue [Figure 1]. On palpation fixity of the tongue was noted, however, the lesion was not indurated. The lymph nodes were not palpable. Based on the clinical features, a provisional diagnosis of squamous cell carcinoma was given and incisional biopsy was performed. Microscopic examination demonstrated pseudoepitheliomatous hyperplasia of the surface epithelium. Moving deeper, the connective tissue showed inflamed salivary gland tissue with necrosis of the acini. Squamous metaplasia of the ducts, a bland appearance of the metaplastic squamous cells and maintenance of the lobular morphology was noted [Figure 2, Figure 3]. Periodic acid-Schiff (PAS) positive mucinous material was present in few ductal lumens [Figure 4]. The microscopic evidence supported a diagnosis of necrotizing sialometaplasia. The patient was kept on regular follow-up and nine weeks after the first appearance of the symptoms, the patient showed spontaneous healing of the lesion.
Figure 1.
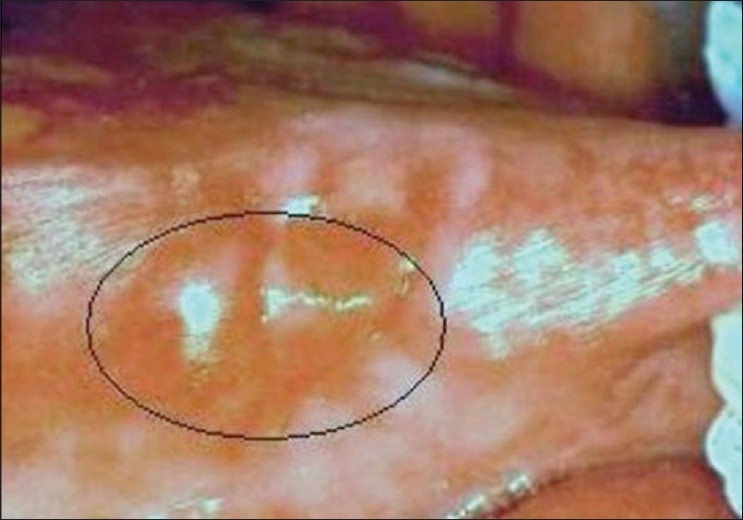
A growth on the right lateral border of the tongue
Figure 2.
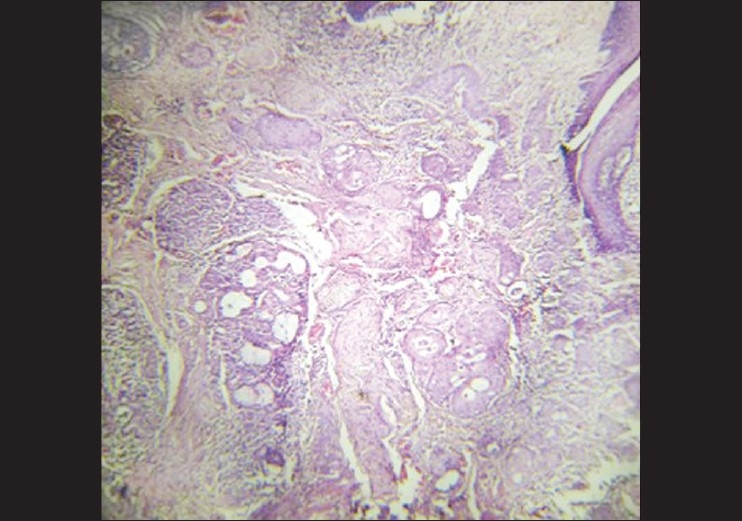
Photomicrograph showing infl amed salivary gland tissue with necrosis of the acini. Squamous metaplasia of the ducts, a bland appearance of metaplastic squamous cells and maintenance of the lobular morphology was noted (H and E, 10×)
Figure 3.
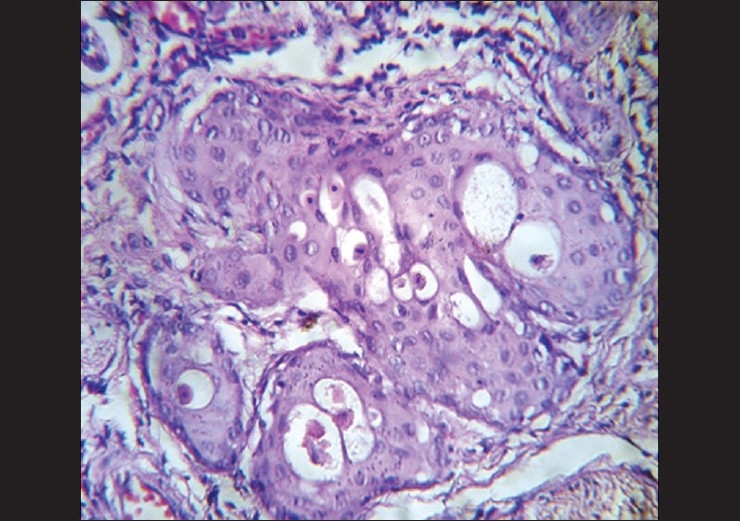
Photomicrograph showing squamous metaplasia of the ducts, a bland appearance of the metaplastic squamous cells (H and E, 40×)
Figure 4.
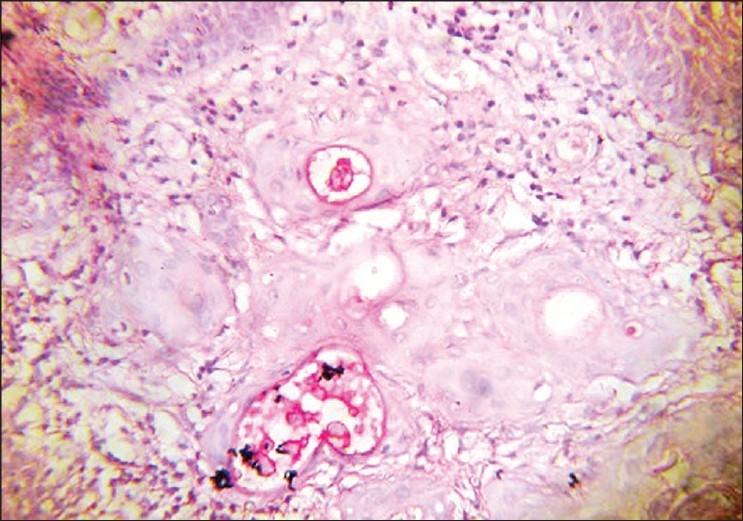
Periodic acid-Schiff positive mucinous material present in ductal lumens (PAS, 40×)
DISCUSSION
In 1973 Abrams et al., reported a clinicopathologic study of seven cases with a previously unreported disease of a minor salivary gland and introduced the term NSM to describe this lesion.[1] In the largest study to date, Brannon et al.[2] analyzed the clinicopathologic findings of 69 cases and reviewed the literature on another 115 cases of NSM. The mean age of these patients at diagnosis was 46 years; men outnumbered women by a ratio of nearly 2:1. Seventy-seven percent of these cases presented with a deep-seated ulcer on the palate;[2] however, an antecedent swelling or mass was not uncommon. Other involved sites included the maxillary sinus, retromolar pad, lower lip, tongue, and buccal mucosa.[3–9]
The most common clinical presentation of NSM is an ulcer presenting as a crateriform lesion with indurated and well-delineated borders, usually present on the palate.[10,11] These features are strongly suggestive of a carcinoma, leading to frequent erroneous diagnosis of carcinoma. The ulcer is tender, but not as overtly painful as its appearance might suggest. However, a precursor swelling is also not uncommon. Parasthesia or anesthesia of the involved area has also been reported.[12] NSM is solitary in the majority of cases but metachronous lesions have also been reported. The present case presented with a complaint of swelling, this can be present in the initial stages of the lesions, with paraesthesia and fixity of tongue, all these features have been previously reported in the literature for NSM.
The most widely accepted theory explaining the etiology of NSM is ischemia of the blood vessels, leading to infarction of the gland tissues.[13] The factors believed to lead to ischemia are trauma; administration of local anesthetics; smoking, alcohol and cocaine use; infection; intubation; and surgical procedures for various lesions.[13–20] NSM has also been reported in a patient with Buerger's disease and Raynaud's phenomenon supporting the role of ischemia as an important etiological factor.[21] In the nonpalatal lesions, the disease is often traced to a cause such as surgery, trauma or irradiation. The trauma caused by the upper first molar leading to ischemia may account for the lesion seen in this case.
Anneroth and Hansen classified the histopathogenesis of NSM by proposing five histological stages: infarction, sequestration, ulceration, reparative, and healed.[22] It is not possible to observe these well-demarcated stages in any given biopsy specimen.
More commonly observed is a spectrum of histological features that include ulceration, pseudoepitheliomatous hyperplasia, necrotic acini, squamous metaplasia of the ducts, a bland appearing morphology of the metaplastic squamous cells, and maintenance of the lobular morphology in spite of the inflammatory and metaplastic changes.
As a patient's history and clinical presentation may be confusing, all such lesions should be biopsied. An incisional biopsy of the base of the ulcer and the edge that is most indurated and raised will yield the most representative specimen. It is important to take a large specimen and to include the base of the lesion because NSM may occur over a true neoplasm. While many lesions of NSM in the past were overdiagnosed as invasive malignancies, the opposite can also occur, that is, a NSM can obscure a malignancy.
The classical histopathological differential diagnosis of NSM includes squamous cell carcinoma and mucoepidermoid carcinoma. The histopathological features most helpful in the differential diagnosis are the bland appearing morphology of metaplastic squamous cells, absence of features of cellular atypia and preservation of lobular morphology. In addition low-grade mucoepidermoid carcinomas usually have cystic elements partially lined by mucocytes rather than being incorporated into the squamous islands, as in NSM. There is a benign lesion termed subacute necrotizing sialadenitis (SANS) requiring differentiation from NSM. SANS is a nonspecific inflammatory condition of unknown etiology. Microscopically it is characterized by focal acinar cell necrosis secondary to the inflammatory process and slight atrophy of ductal cells; neither ductal squamous metaplasia nor pseudoepitheliomatous hyperplasia is observed in SANS. Clinically they are nonulcerated nodular lesions located in the palate accompanied by an abrupt onset of pain.[23] In the present case histopathological findings like inflammation of salivary gland tissue with necrosis of the acini, squamous metaplasia of the ducts, with a bland appearance of metaplastic squamous cells, and maintenance of the lobular morphology suggested the diagnosis of NSM. PAS positive mucinous material present in few ductal lumens confirmed the diagnosis. Sometimes these clinical and histopathological findings and the unusual site may mislead the pathologist.
NSM is a self-limiting lesion and does not require any specific treatment other than follow-up. NSM fills in with granulation tissue and completely epithelializes its surface within three months. However, regular follow-up over the subsequent three months is extremely important because an NSM would resolve in that time. In this case the swelling was of a four-week duration and in the next five weeks there was spontaneous healing. This is consistent with the literature, which reports a course of four-to-ten weeks for the lesion, after which it heals spontaneously. Lesions that persist for a longer duration require rebiopsy and a second review of the original histopathological features.
CONCLUSION
Awareness of the presence of such an entity is essential for avoiding the misdiagnosis of malignancy. A simple incisional biopsy is required to confirm the histological diagnosis and to rule out more serious disease processes.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Abrams AM, Melrose RJ, Howell FV. Necrotizing sialometaplasia: A disease simulating malignancy. Cancer. 1973;32:130–5. doi: 10.1002/1097-0142(197307)32:1<130::aid-cncr2820320118>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Brannon RB, Fowler CB, Hartman KS. Necrotizing sialometaplasia: A clinicopathologic study of sixty-nine cases and review of the literature. Oral Surg Oral Med OralPathol. 1991;72:317–25. doi: 10.1016/0030-4220(91)90225-2. [DOI] [PubMed] [Google Scholar]
- 3.Johnson WH. Necrotizing sialometaplasia involving the mucous glands of the nasal cavity. Hum Pathol. 1977;8:589–92. doi: 10.1016/s0046-8177(77)80119-6. [DOI] [PubMed] [Google Scholar]
- 4.Forney SK, Foley JM, Sugg WE, Oatis GW., Jr Necrotizing sialometaplasia of the mandible. Oral Surg Oral Med Oral Pathol. 1977;43:720–6. doi: 10.1016/0030-4220(77)90056-1. [DOI] [PubMed] [Google Scholar]
- 5.Anneroth G, Bystedt H, Hammarstrom L. Necrotizing sialometaplasia: a malignancy simulating oral lesion. Swed Dent J. 1986;10:53–8. [PubMed] [Google Scholar]
- 6.Matilla A, Flores T, Nogales FF, Jr, Galera H. Necrotizing sialometaplasia affecting the minor labial glands. Oral Surg Oral Med Oral Pathol. 1979;47:161–3. doi: 10.1016/0030-4220(79)90172-5. [DOI] [PubMed] [Google Scholar]
- 7.Gad A, Willen H, Willen R, Thorstensson S, Ekman L. Necrotizing sialometaplasia of the lip simulating squamous cell carcinoma. Histopathology. 1980;4:111–21. doi: 10.1111/j.1365-2559.1980.tb02903.x. [DOI] [PubMed] [Google Scholar]
- 8.Willen H, Willen R, Ekman L. Necrotizing sialometaplasia of the bucca. Acta Pathol Microbiol Scand Am. 1981;89:199–201. doi: 10.1111/j.1699-0463.1981.tb00209.x. [DOI] [PubMed] [Google Scholar]
- 9.Papanayotou PH, Kayavis JG, Edivatianos AA, Trigonidis G. Necrotizing sialometaplasia of the cheek: Report of case and review of literature. J Oral Surg. 1980;38:538–40. [PubMed] [Google Scholar]
- 10.Rougi GJ, Kessler S. Necroting sialometaplasia: A condition simulating malignancy. Arch Dermatol. 1979;115:329–31. [PubMed] [Google Scholar]
- 11.Samit AM, Mashberg A, Orange E, Greene GW. Necrotizing sialometaplasia. J Oral Surg. 1979;37:353–6. [PubMed] [Google Scholar]
- 12.Lamey PJ, Lewis MA, Crawford DJ, MacDonald DG. Necrotizing sialometaplasia presenting as greater palatine nerve anesthesia. Int J Oral Maxillofac Surg. 1989;18:70–2. doi: 10.1016/s0901-5027(89)80131-6. [DOI] [PubMed] [Google Scholar]
- 13.Suckiel JM, Davis WH, Patakas BM, Kaminishi RM. Early and late manifestations of necrotizing sialometaplasia. J Oral Surg. 1978;36:902–5. [PubMed] [Google Scholar]
- 14.Shigematsu H, Shigematsu Y, Noguchi Y, Fujita K. Experimental study on necrotizing sialometaplasia of the palate in rats: Role of local anesthesic injections. Int Oral Maxillofac Surg. 1996;25:239–41. doi: 10.1016/s0901-5027(96)80038-5. [DOI] [PubMed] [Google Scholar]
- 15.De Saint Aubain Somerhausen N, Larsimont D, Tant L, Verhest A. Necrotizing sialometaplasia: Apropos of a case and review of literature. Acta Stomatol Belg. 1996;93:61–3. [PubMed] [Google Scholar]
- 16.Schoning H, Emshoff R, Kreczy A. Necrotizing sialometaplasia in two patients with bulimia and chronic vomiting. Int J Oral Maxillofac Surg. 1998;27:463–5. doi: 10.1016/s0901-5027(98)80039-8. [DOI] [PubMed] [Google Scholar]
- 17.Arguelles MT, Viloria JB, Talens MJ, Mc Crory TP. Necrotizing sialometaplasia. Oral Surg. 1976;42:86–90. doi: 10.1016/0030-4220(76)90034-7. [DOI] [PubMed] [Google Scholar]
- 18.Fechner RE. Necrotizing sialometaplasia. Am J Clin Pathol. 1977;67:315–7. doi: 10.1093/ajcp/67.4.315. [DOI] [PubMed] [Google Scholar]
- 19.Yoshimura Y, Matsuura R, Sugihara T, Matsumoto K. Necrotizing sialometaplasia: report of a case and review of the Japanese literature. J Osaka Univ Dent Sch. 1985;25:171–6. [PubMed] [Google Scholar]
- 20.Imbery TA, Edwards PA. Necrotizing sialometaplasia: Literature review and case reports. J Am Dent Assoc. 1996;127:1087–92. doi: 10.14219/jada.archive.1996.0334. [DOI] [PubMed] [Google Scholar]
- 21.Rye LA, Calhoun NR, Sedman RS. Necrotizing sialometaplasia in a patient with BuergerÕs disease and RaynaudÕs phenomenon. Oral Surg. 1980;49:233–6. doi: 10.1016/0030-4220(80)90054-7. [DOI] [PubMed] [Google Scholar]
- 22.Anneroth G, Hansen LS. Necrotizing sialometaplasia: The relationship of its pathogenesis to its clinical characteristics. Int J Oral Surg. 1982;11:283–91. doi: 10.1016/s0300-9785(82)80027-6. [DOI] [PubMed] [Google Scholar]
- 23.Werning JT, Waterhouse JP, Mooney JW. Subacute necrotizing sialoadenitis. Oral Surg Oral Med Oral Pathol. 1990;70:756–9. doi: 10.1016/0030-4220(90)90015-k. [DOI] [PubMed] [Google Scholar]


