Abstract
Background
Leishmania killicki was originally described in 1980 in southeast Tunisia. It was also recently reported in Lybia and Algeria. Nevertheless, neither vector nor reservoirs of this parasite are known. The identification of the vector and the animal reservoir host of L. killicki is critical for the establishment of an efficient control strategy.
Findings
blood, popliteal lymph node, spleen, bone marrow, liver and skin were collected from 50 rodents in 2009 in south western Tunisia. Samples were smeared onto glass slides, cultured on NNN medium and tested by polymerase chain reaction for Leishmania detection. Parasites were detected by PCR from 10 Psammomys obesus and from two Ctenodactylus gundi. Parasite identification was performed simultaneously by internal transcribed spacer 1 PCR-RFLP and by PCR sequencing. Both Leishmania major and Leishmania killicki were identified from infected Psammomys and Ctenodactylus gundi respectively.
Conclusion
This is the first report of Leishmania killicki identified from Ctenodactylus gundi in Tunisia. This result supports the assumption that C. gundi is a potential reservoir for Leishmania killicki.
Findings
In Tunisia, Leishmania infantum, L. major and L. killicki are responsible for cutaneous leishmaniasis (CL). This last taxon has been described in 1980 in Southeast Tunisia, based on the identification of 29 human strains [1]. It is responsible for CL with a chronic evolution of the lesion and a low endemicity (about 10 cases per year). Since 2004, new foci of CL caused by L. killcki have emerged in different parts of the country. Leishmania killicki was also recently reported in Lybia and in Algeria, two neighbouring countries of Tunisia [2-4]. Nevertheless, until recently, little information was available on the epidemiology of L. killicki CL and neither the vector nor reservoir hosts were known.
The collection of wild animals for detection of a possible parasitic infection seems to be the most effective method for reservoir host identification. Therefore, an epidemiological study was carried out in Metlaoui province in Southwestern Tunisia, a new emerging focus of this taxon, with the objective of detecting and characterizing Leishmania killicki infection in wild rodents.
Wild rodents including Psammomys (Ps.) obesus, Ctenodactylus (C.) gundi, Meriones (Mr.) shawi and Mus (M.) musculus were trapped alive, in 2009 in Metlaoui Province South western Tunisia, by using home-made metal rodent traps covered up with sand and placed at burrow entrances and rock crevices (Figure 1). Trapped rodents were examined for the existence of skin lesions. After euthanizing, samples of blood, liver, spleen, bone marrow, lymph nodes and skin lesion (if present) were obtained for parasite detection. In some animals, samples were not available from all sites. The study that concerned these rodents was conducted adhering to the American Psychological Association's Guidelines for Ethical Conduct in the Care and Use of Animals. The first part was smeared onto a glass slide, fixed with methanol, stained with Giemsa, and examined by microscopy. The second part was inoculated on Novy-MacNeal-Nicolle medium. The cultures were incubated at 26 °C and observed every week for 1 month. The third part was used for DNA extraction. DNA from all tissues was tested for Leishmania spp. infection by internal transcribed spacer 1 (ITS1) PCR according to the protocol of Schönian et al., 2003 [5]. Positive PCR products were analysed by both: i) the Restriction Fragment Length polymorphism (RFLP) method [5], and ii) by sequencing using the same primers than those used for the PCR (Eurofins MWG Operon, Munich, Germany).
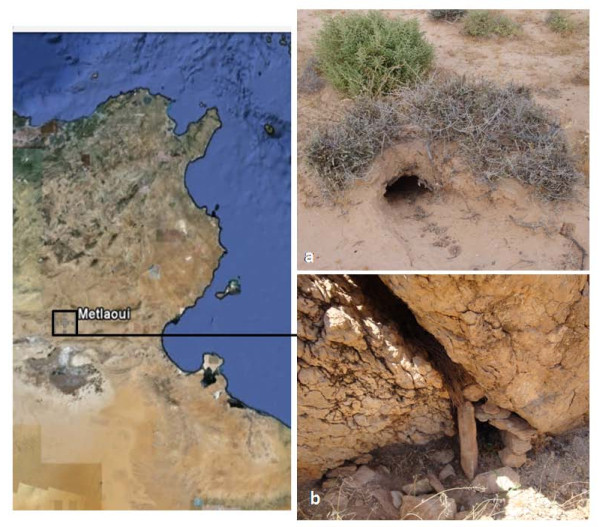
Figure 1.
Geographical situation and landscape of rodent collection sites. The map of Tunisia on the left shows the geographical situation of Metlaoui province, the study area. The photos on the right show rodent burrow, the biotope of Psammomys obesus (a) and crevices within boulder, the biotope of Ctenodactylus gundi (b) where rodent traps were set.
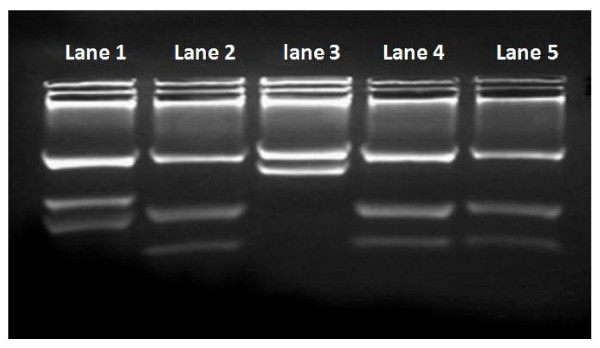
In total, 50 rodents were caught, belonging to the following species: Ps. obesus (n = 40), C. gundi (n = 6), Mr. shawi (n = 3) and M. musculus (n = 1). Among the 205 tissue samples collected from these rodents, only the bone marrow of a single C. gundii was positive by direct examination, but culture was negative for all organs. Sixteen organs were detected positive for Leishmania spp. infection using ITS1-PCR: they were from ten Ps. obesus (cutaneous lesion n = 5, bone marrow n = 3, lymph node n = 3, spleen n = 3) and from two C. gundi (bone marrow). The analysis of the positive PCR products by RFLP showed that all positive organs sampled from Ps. obesus were infected with L. major. The restriction patterns of the two positive PCR products from the C. gundii bone marrow were identical to the L. killicki reference strain profile (Figure 2). Sequencing of the positive PCR products has shown the same result as RFLP analysis.
Figure 2.
Restriction pattern of the amplified ribosomal Internal Transcribed Spacer 1 using HaeIII. Reference strains used were: Leishmania infantum MHOM/FR/78/LEM75 (Lane 1) (three fragments of 187 bp, 72 bp and 55 bp), Leishmania killicki MHOM/TN/LEM163 (Lane 2) (three fragments of 188 bp, 57 bp and 26 bp) and L. major MHOM/MA/81/LEM265 (Lane 3) (two fragments of 206 bp and 132 bp). Restriction pattern of the PCR products amplified from the two Ctenodactylus gundi bone marrow (Lane 4 and 5) are the same as Leishmania killicki profile (Lane 2).
If the detection of L. major in Ps. obesus was an expected result, since this rodent was already described as the reservoir of this Leishmania taxon [6], our study is the first report of L. killicki infecting a rodent. The detection of this Leishmania species in the bone marrow of asymptomatic animals indicates that it can visceralize in C. gundi. Consequently, this rodent species could be a natural host of L. killicki, and an efficient reservoir host as two out of six specimens were found infected.
Ctenodactylus gundi is a gregarious rock-dwelling rodent found in northern Africa. In Tunisia it exists in the mountainous area of Tataouine (the original focus of L. killicki) [7] as well as in all emerging Tunisian foci of CL caused by L. killicki [8]. C. gundi populations inhabit crevices within boulder mounds in the vicinity of villages. The rock crevices and caves present suitable breeding sites for sand flies. This rodent species was suspected to be the L. killicki reservoir since the end of the last century [7,9,10]; however no evidence has been given before. The detection of L. killicki in C. gundi by both direct examination and PCR method is the first proof of the potential role of this wild rodent as a natural host of this parasite.
Conclusion
Cutaneous leishmaniasis due to L. killicki seems to be a zoonotic disease involving C. gundi in its life cycle. However, the isolation of the parasite from this rodent is crucial for the confirmation of this first result. Further investigation, such collecting wild rodents in other L. killicki foci, is required to discern the potential epidemiologic role of Ctenodactylus gundi in spreading infection.
Competing interests
The authors declare that they have no competing interests
Authors' contributions
Conceived and designed the experiments: HB, NH, KJ, DA, FP and JPD. Performed the experiments: KJ, DC, NH, NC, HM. Drafted the manuscript: NH, KJ, HB, FP, JPD. Participated in field missions: KJ, DC, MG, DA, HB, NH. All authors approved the final version of the manuscript.
Contributor Information
Kaouther Jaouadi, Email: kaoutherj@gmail.com.
Najoua Haouas, Email: najoua.h@laposte.net.
Dhekra Chaara, Email: chaara.dhekra@yahoo.fr.
Mohamed Gorcii, Email: gorcii2002@yahoo.fr.
Najla Chargui, Email: najla_ch@yahoo.fr.
Denis Augot, Email: Denis.AUGOT@anses.fr.
Francine Pratlong, Email: f-pratlong@chu-montpellier.fr.
Jean-Pierre Dedet, Email: parasito@univ-montp1.fr.
Selim Ettlijani, Email: selimettlijani@gmail.com.
Habib Mezhoud, Email: habib.mezhoud@laposte.net.
Hamouda Babba, Email: hamouda.babba@gnet.tn.
Acknowledgements
This study was supported by a grant from WHO/TDR-EMRO (Operational Research in Tropical and Communicable Disease) Ref. SGSS 09/118 and by DGRS/CNRS Project, Ref. 09/R 09-01.
References
- Rioux JA, Lanotte G, Pratlong F. In: Leishmania taxonomie et phylogenèse. Applications éco-épidemiologiques. Rioux JA, editor. Montpellier (France): Institut Méditerranéen d'études Épidémiologiques and Écologiques; 1986. Leishmania killicki n. sp. (Kinetoplastida- Trypanosomatidae) pp. 139–142. [Google Scholar]
- Aoun K, Bouslimi N, Haouas N, Babba H, El Buni A, Bouratbine A. First report of Leishmania killicki Rioux, Lanotte & Pratlong, 1986 in Libya. Parasite. 2006;13:87–88. [PubMed] [Google Scholar]
- Harrat Z, Boubidi SC, Pratlong F, Benikhlef R, Selt B, Dedet JP, Ravel C, Belkaid M. Description of a dermotropic Leishmania close to L. killicki (Rioux, Lanotte and Pratlong 1986) in Algeria. Trans R Soc Trop Med Hyg. 2009;103:716–720. doi: 10.1016/j.trstmh.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Pratlong F, Dereure J, Ravel C, Lami P, Balard Y, Serres G, Lanotte G, Rioux JA, Dedet JP. Geographical distribution and epidemiological features of Old World cutaneous leishmaniasis foci, based on the isoenzyme analysis of 1048 strains. Trop Med Int Health. 2009;14(9):1071–1085. doi: 10.1111/j.1365-3156.2009.02336.x. [DOI] [PubMed] [Google Scholar]
- Schönian G, Nasereddin A, Dinse N, Carola Schweynoch C, Schallig HDFH, Presber W, Jaffe CL. PCR diagnosis and characterization of Leishmania in local and imported clinical samples. Diag Microbio. Infect Dis. 2003;47:349–358. doi: 10.1016/s0732-8893(03)00093-2. [DOI] [PubMed] [Google Scholar]
- Ismail Ben, Ben Rachid MS, Gradoni L, Helal H, Bach Hamba D. La leishmaniose cutanée zoonotique en Tunisie: étude du réservoir dans le foyer de Douara. Ann Soc Belg Med Trop. 1987;67:335–343. [PubMed] [Google Scholar]
- Ben Ismail R, Ben Rachid MS. In: Maladies Tropicales Transmissibles. Aupelf-Uref, editor. John Libbey Enrobex, Paris; 1989. Epidémiologie des leishmanioses en Tunisie; pp. 73–80. [Google Scholar]
- Bourathbine A, Aoun K, Gharbi M, Harrat Z, Ezzedini MS, Etlijeni S. Spread of Leishmania killicki to Central and South West Tunisia. Parasite. 2005;12:59–63. doi: 10.1051/parasite/2005121059. [DOI] [PubMed] [Google Scholar]
- Ashford RW. Cutaneous leishmaniasis: strategies of prevention. Clinics dermatol. 1999;17:327–323. doi: 10.1016/s0738-081x(99)00051-6. [DOI] [PubMed] [Google Scholar]
- Tabbabi A, Ghrab J, Aoun K, Ready PD, Bouratbine A. Habitats of the sandfly vectors of Leishmania tropica and L. major in a mixed focus of cutaneous leishmaniasis in southeast Tunisia. Acta Tropica. 2011. in press . [DOI] [PubMed]