Abstract
Phosphatidic acid (PA) level increases during various stress conditions. However, the physiological roles of this lipid in stress response remain largely unknown. In this study, we report that PA induced leaf cell death and elevated the levels of reactive oxygen species (ROS) in the whole leaf and single cells. To further elucidate the mechanism of PA-induced cell death, we then examined whether Rho-related small G protein (ROP) 2, which enhanced ROS production in an in vitro assay, is involved in PA-induced ROS production and cell death. In response to PA, transgenic leaves of Arabidopsis expressing a constitutively active rop2 mutant exhibited earlier cell death and higher levels of ROS than wild type (WT), whereas those expressing a dominant-negative rop2 mutant exhibited later cell death and lower ROS. However, in the absence of exogenous PA, no spontaneous cell death or elevated ROS was observed in constitutively active rop2 plants, suggesting that the activation of ROP GTPase alone is insufficient to activate the ROP-mediated ROS generation pathway. These results suggest that PA modulates an additional factor required for the active ROP-mediated ROS generation pathway. Therefore, PA may be an important regulator of ROP-regulated ROS generation and the cell death process during various stress and defense responses of plants.
Phosphatidic acid (PA) is implicated in numerous stress responses of plants. Intracellular PA levels increase under various biotic and abiotic stress conditions, including pathogen elicitation (Young et al., 1996; van der Luit et al., 2000), wounding (Ryu and Wang, 1996, 1998; Lee et al., 1997), freezing (Welti et al., 2002), hyperosmotic stress (Munnik et al., 2000), and water deficit (Frank et al., 2000; Munnik et al., 2000). It is suggested that PA is a second messenger in a broad range of stress-signaling pathways in plants and mediates important responses to stresses (Munnik, 2001).
PA has many regulatory functions in animal cells, including the regulation of reactive oxygen species (ROS) generation, protein kinase, phosphatase, lipid kinase, phospholipases, intracellular Ca2+ levels, vesicle trafficking, cell proliferation, and cytoskeletal rearrangement (Liscovitch et al., 2000). This lipid also regulates a wide range of important cellular processes in plants, including regulation of ROS generation (Sang et al., 2001a), MAPK activity (Lee et al., 2001), K+ channels (Jacob et al., 1999), and actin organization (Lee et al., 2003). Therefore, PA is likely to be involved in numerous physiological processes in plants, including stomatal movement (Jacob et al., 1999), leaf senescence (Fan et al., 1997), various stress responses, and hormone signaling (for review, see Munnik, 2001). In vivo roles of PA in leaf senescence and stomatal closing movement were demonstrated using PLDα antisense plants; this mutant plant is delayed in ABA- and ethylene-induced senescence (Ryu and Wang, 1995; Fan et al., 1997; Zien et al., 2001) and in ABA-induced stomatal closing movement (Sang et al., 2001b).
A potential mechanism of PA action in plant stress responses is the activation of an NADPH oxidase that produces ROS, similar to the system in neutrophils (Regier et al., 1999; Palicz et al., 2001). A recent report shows that PA promotes ROS formation in Arabidopsis (Sang et al., 2001a). Pharmacological and biochemical studies suggest that the enzyme responsible for ROS generation in plants is similar to the neutrophil NADPH oxidase (Doke, 1983; Levine et al., 1994; Dwyer et al., 1996; Xing et al., 1997). Molecular studies revealed some similarities and differences between the mechanisms regulating NADPH oxidases in plants and animals. Although homologs of the gp91phox catalytic subunit of the neutrophil NADPH oxidase (Keller et al., 1998; Torres et al., 1998) and putative homologs of the Rac small G protein regulatory subunit (Yang, 2002) are present in Arabidopsis, the genome does not encode homologs of the remaining components of the neutrophil NADPH oxidase, p22phox, p67phox, p47phox, or p40phox (Arabidopsis Genome Initiative, 2000). Therefore, triggering of ROS generation by PA in Arabidopsis is not likely to be achieved via the activation of the p22phox and p47phox, as found in neutrophils (Waite et al., 1997; Regier et al., 2000).
Although the detailed mechanism for the regulation of plant NADPH oxidases is possibly different from that in animal ones, one common activator of ROS generation in both systems is small G protein. Plants contain a family of Rac-like GTPases, named ROPs (Rho-related small G proteins in plants) that may be functional homologs of the neutrophil Rac GTPases in the activation of the plasma membrane NADPH oxidases (Kawasaki et al., 1999; Potikha et al., 1999; Park et al., 2000; Baxter-Burrell et al., 2002; for review, see Yang, 2002). A rice (Oryza sativa) ROP, OsRac1, activates ROS generation and cell death in response to fungal and bacterial pathogens, thereby contributing to pathogen resistance (Kawasaki et al., 1999; Ono et al., 2001). GhRac13 of cotton (Gossypium hirsutum) plants triggers ROS production, which may serve as a signal for xylem differentiation (Potikha et al., 1999). One or more ROP GTPases in Arabidopsis plants are involved in the generation of H2O2 required for responses to oxygen deprivation (Baxter-Burrell et al., 2002). It was shown that oxygen deprivation activates ROP GTPases, leading to the generation of H2O2. An interesting question is whether this ROP-mediated ROS generation pathway is a target of PA.
Here, we examine the physiological roles of PA in cell death, and the mechanism of PA activation of ROS generation. We demonstrate that PA induces cell death in leaves via up-regulation of the ROP-mediated ROS generation pathway. These data, together with the fact that PA levels are elevated during a number of stress conditions, suggest that PA plays an important role in inducing cell death during stress responses in plants.
RESULTS
PA Induces Death in Arabidopsis Leaf Tissue
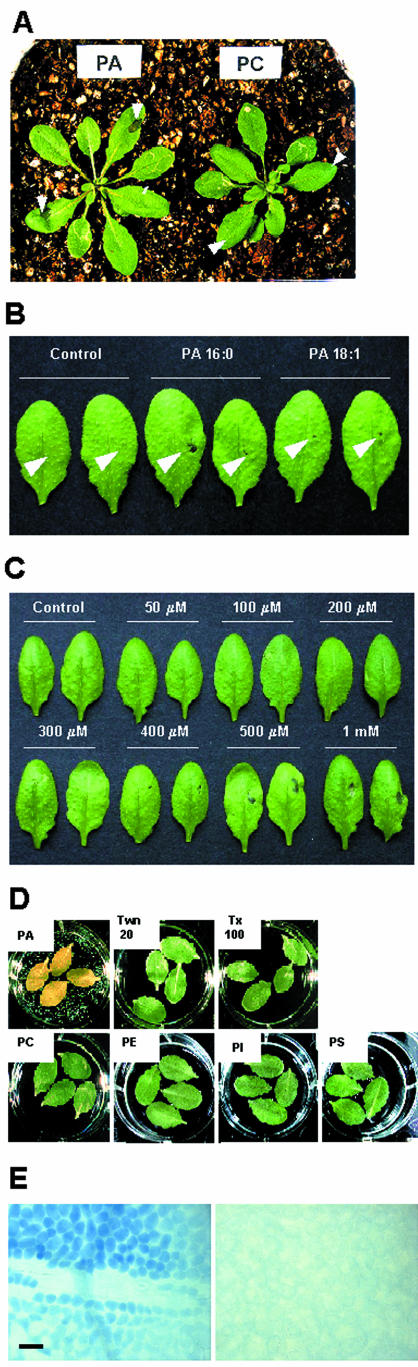
In response to important stress factors, such as pathogen infection or wounding, plant tissues often display regulated cell death. To investigate whether the rise in PA levels during these stress conditions is related to cell death, liposome preparations of PA were infiltrated into Arabidopsis rosette leaves using the pressure of a syringe without a needle. The area of leaves infiltrated with 20 μL of 500 μm dipalmitoyl (di16:0)-PA lost turgor pressure at 1 h after treatment, and displayed loss of chlorophyll and a blighted appearance after 10 to 12 h. In contrast, no evident changes were detected in di16:0-phosphatidylcholine (PC)-treated leaves (Fig. 1A). We then evaluated the influence of fatty acyl tails of PA on leaf cell death. When infiltrated into the leaves, dioleoyl (di18:1)-PA also induced cell death in Arabidopsis leaves, although the lesions were smaller than those generated by di16:0-PA (Fig. 1B). Therefore, the cell death induced by di16:0-PA is not likely to be a specific effect of di16:0-PA but rather a general effect of PA.
Figure 1.
PA specifically induces leaf cell death in Arabidopsis. A, Leaves of WT plants were infiltrated with approximately 20 μLof500 μm PA or PC suspensions. Results shown are representatives of 15 and 12 of PA- and PC-treated individual leaves, respectively. Photograph was taken 24 h after treatment with the lipids. Arrowheads indicate the area of liposome infiltration. B, Leaves of WT plants were infiltrated with di16:0-PA or di18:1-PA. Arrowheads indicate the area of liposome infiltration. C, Leaves of WT plants were infiltrated with various concentrations of PA. In B and C, the control was infiltrated with water. Only one site at the right side of each leaf was infiltrated, and 24 h after, leaves were detached from plants and photographed. Results shown are representative of four (B) and three (C) independent experiments. D, Leaves of WT plants were detached, floated on 500 μm phospholipid liposomes or detergent solutions, and photographed at 24 h after the onset of treatment. A representative result of two independent experiments is shown. E, Trypan blue staining was used to visualize dying cells in areas of turgor loss in PA-treated leaves. Leaves of WT plants were detached, floated on PA (left), or PC (right) suspensions for 2 h, and stained with Trypan blue. Note that PA-treated leaf cells are permeable to Trypan blue, which indicates damage to the cell membrane. Bar = 40 μm. Twn 20, Tween 20; Tx 100, Triton X-100; PE, phosphatidylethanolamine; PI, phosphatidylinositol; and PS, phosphatidyl-Ser.
To test the PA concentration dependence of the response, 20 μL of PA at concentrations ranging from 50 to 1,000 μm was infiltrated into the leaves. The cell death response was apparent at PA concentrations as low as 400 μm, and at higher concentrations of PA tested, up to 1 mm, the area of cell death increased (Fig. 1C). PA-induced cell death also appeared when the leaves were floated on 500 μm lipid suspensions. Leaves floating on a PA solution showed a loss of turgor and chlorosis throughout the entire area after 24 h (Fig. 1D, PA). Cells in the area of turgor loss stained positive with Trypan blue, indicating cell death (Fig. 1E, left). PC-treated leaf cells did not stain with Trypan blue (Fig. 1E, right).
To determine whether PA-induced cell death is a PA-specific effect, we treated detached leaves with many other phospholipids (PC, phosphatidylethanolamine, phosphatidylinositol, and phosphatidyl-Ser) and two types of detergents (Triton X-100 and Tween 20) at the same molar concentration as PA. In contrast to PA-treated leaves that completely lost their turgor pressure and displayed chlorosis, no visible changes were observed in leaves treated with other phospholipids or detergents until 24 h after treatment (Fig. 1D). In addition, metabolites of PA, including lysophosphatidic acid, diacylglycerol, linoleic acid, palmitic acid, and myristic acid, did not induce any noticeable changes in the leaves up to 24 h when the experiment was terminated (data not shown). Our results indicate that the observed cell death is not a detergent effect, nor can it be induced by metabolic products of PA or other phospholipids, but it is specifically triggered by PA.
PA Induces H2O2 Production
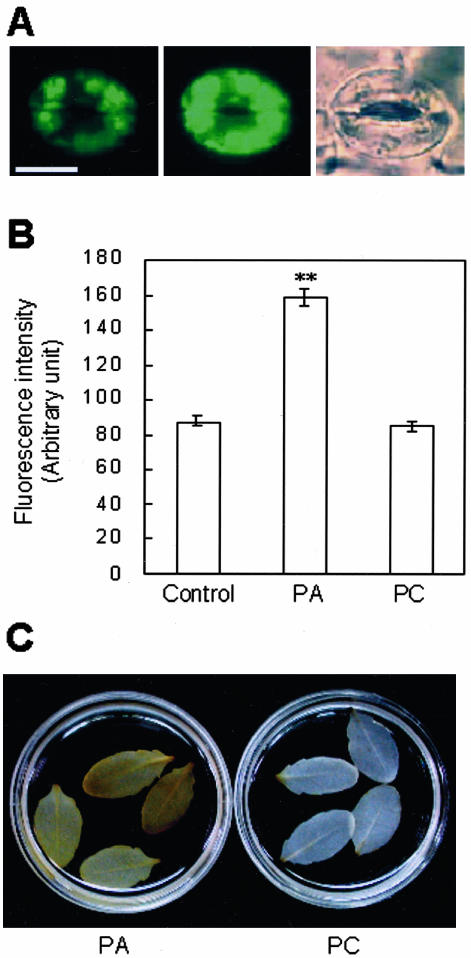
Cell death during defense responses of plants often involves ROS production (Lamb and Dixon, 1997; van Camp et al., 1998). PA enhances ROS generation in vitro and upon infiltration into intact leaves (Sang et al., 2001a). To determine whether PA treatment induces ROS at the single-cell level, we isolated epidermal layers from Arabidopsis rosette leaves and observed ROS production in guard cells. The epidermal layer of Arabidopsis leaf was obtained by sticking the abaxial side of the leaf to a slide glass smeared with medical adhesive and then scraping away all other cell layers, as described in “Materials and Methods.” After this step, only guard cells were still viable. After stabilization for 1 h, guard cells were treated with either 500 μm PA or 500 μm PC, and their ROS levels were compared 10 min later, by measuring the green fluorescence intensity of the ROS-sensitive dye dichlorofluorescin (DCF) from microscopic images of the guard cells. Before PA treatment, DCF fluorescence of guard cells was low and was mostly located in chloroplasts (Fig. 2A, left), whereas after 10 min of PA treatment, the fluorescence level increased throughout the cell (Fig. 2A, center). Fluorescence emission increased to an average of 182% at 10 min after PA treatment but did not change significantly after PC treatment (Fig. 2B). Guard cells of epidermal peels responded similarly to PA as those in scraped epidermis (data not shown).
Figure 2.
PA induces H2O2 production. A, DCF fluorescence in guard cells before (left) and after (center) treatment with 500 μm PA and the corresponding bright-field image of the same cell (right). Bar = 10 μm. B, DCF fluorescence intensity measured from images of guard cells obtained before (control) and 10 min after treatment with 500 μm PA or PC suspensions. PA treatment significantly increased ROS levels over those of the control samples (**, P < 0.01), but PC treatment did not (P > 0.05). The fluorescence intensity from the entire area of guard cells was quantified using Adobe Photoshop 6.0. Results from three independent experiments are combined (averages ± se, n = 200-208). C, DAB staining reveals PA-induced H2O2 production in whole leaves. Detached leaves were preloaded with 1 mg mL-1 DAB solution for 5 to 8 h and then floated on a 500 μm lipid suspension containing 1 mg mL-1 DAB for 10 h. Results are representative of four independent experiments.
We additionally investigated ROS generation in intact Arabidopsis leaves. In this assay, the detached leaves were preincubated with diaminobenzidine (DAB) for 5 h, and treated with suspensions of PA or PC. Deep-brown DAB polymer products were detectable only in PA-treated leaves (Fig. 2C). The results collectively confirm that PA promotes ROS production in Arabidopsis plants.
Promotion of Superoxide Generation by Active ROP2 in Leaf Extracts
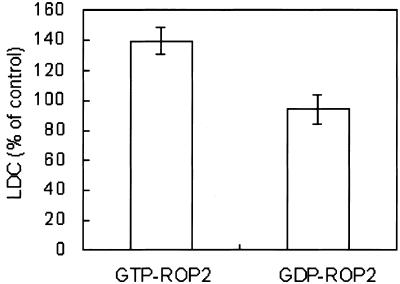
Although specific ROP family members activate ROS generation in rice and cotton fibers (Kawasaki et al., 1999; Potikha et al., 1999; Ono et al., 2001), the specific ROP among the 11 ROP members in Arabidopsis involved in the process has not yet been identified. CA-rop2 Arabidopsis plants have enlarged vascular bundles, which require ROS for development (Y. Fu and Z. Yang, unpublished data). Thus, ROP2 may enhance ROS generation in Arabidopsis. If ROP2 is involved in ROS production, purified active ROP2 should enhance superoxide production. We examined the superoxide production using lucigenin-dependent chemiluminescence (LDC) as an indicator of superoxide (Sang et al., 2001a). Purified ROP2 fused with glutathione S-transferase (GST) proteins were introduced into the reaction mixture of in vitro superoxide assays, either in the active GTP-loaded or inactive GDP-loaded form. GTP-loaded ROP2 increased superoxide production to 140% of that in control without any added ROP (P < 0.01), whereas GDP-loaded ROP2 did not have a significant effect (P > 0.05; Fig. 3). These data show that ROP2 increases ROS generation in Arabidopsis.
Figure 3.
Activated ROP2 enhances superoxide production in an in vitro assay. The superoxide generation activity was assayed by LDC using 5 μg of protein (10,000g supernatant) from WT leaves. One microgram of purified GST-ROP2 protein preloaded with 100 μm GTP or GDP was added to the reaction mixture. Chemiluminescence from the sample was compared with that from the control without added GST-ROP2 protein and expressed as the percentage of the control. Results from two independent experiments with two replicates each are combined (averages ± se, n = 4).
Differential Responses to PA of Transgenic Plants Expressing rop2 Mutant Genes
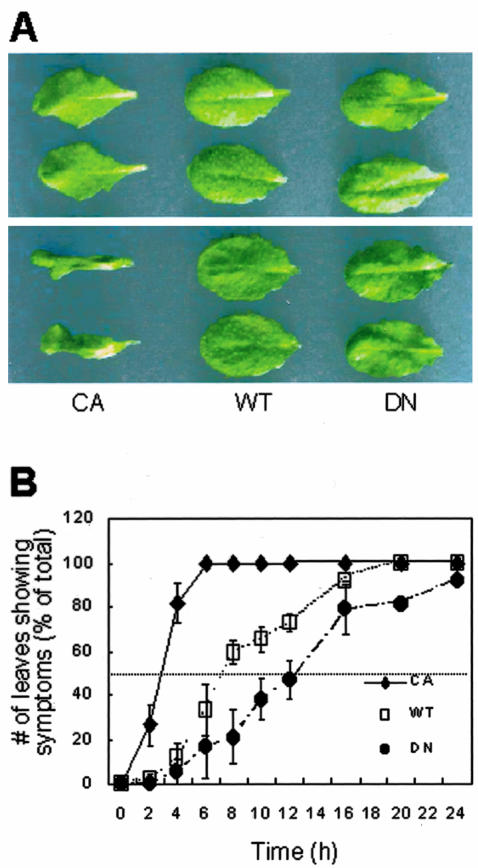
A possible involvement of the ROP2 small G protein in PA-induced cell death was examined by floating rosette leaves of rop2 transgenic plants on a PA suspension. We used heterozygous plants of constitutively active rop2 (CA-rop2) and dominant-negative rop2 (DN-rop2) transformants, because heterozygous plants show only mild alterations in leaf shape (Fig. 4A, top) when compared with WT, unlike homozygous plants (Li et al., 2001). CA-rop2 showed symptoms associated with cell death within 3.5 h, when WT and DN-rop2 showed no indications of cell death or turgor loss (Fig. 4A, bottom). At each time point, we counted numbers of leaves that lost turgor or showed chlorosis in areas broader than 50% of the total leaf area. CA-rop2 displayed early symptoms of cell death, followed by WT and DN-rop2; more than 50% of the tested leaves developed PA-induced death symptoms after 4 h of PA treatment for CA-rop2, 8 h for WT, and 16 h for DN-rop2 plants (Fig. 4B). This result indicates that plants with more active ROP2 responded with greater sensitivity to PA treatment.
Figure 4.
ROP2 transgenic leaves are altered in response to PA treatment. A, Only CA-rop2 transgenic leaves lose turgor pressure when treated with 500 μm PA for 3.5 h. Leaves were detached and then floated on PA suspension for 3.5 h. Photographs were taken before (top) and after (bottom) the PA treatment. A representative result of 10 independent experiments is shown. B, Time-dependent changes in development of symptoms induced by 500 μm PA. Percentages of leaves showing loss of turgor and/or chlorosis in areas broader than 50% of the total leaf area are plotted. Results from four independent experiments are combined (averages ± se, n = 48).
WT and rop2 transgenic plants display different morphology. Leaves of heterozygous CA-rop2 transgenic plants are slender, thicker, and twisted at the edge, whereas those of heterozygous DN-rop2 transgenic plants are ruffled and thinner than WT. The possibility that the differences in leaf morphology lead to varying amounts of PA absorption and thus different response times was tested by comparing phospholipid absorption among transgenic plants using [glycerol-U-14C]PA. No significant differences were observed in the amount of [14C]PA absorbed among transgenic lines (data not shown), ruling out the possibility that the variable rates of PA-induced necrosis among the rop2 transgenic lines are caused by distinct lipid absorbing rates.
PA Induces ROS Production in rop2 Transgenic Plants
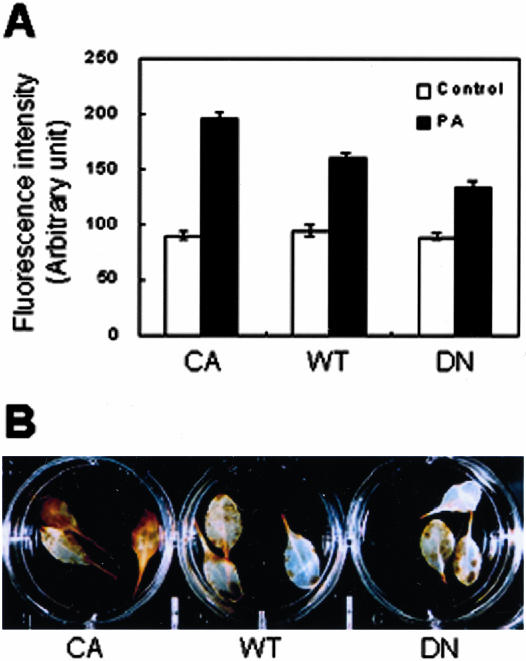
We investigated whether the different responses of rop2 transgenic plants to PA were caused by their difference in ROS generation and/or accumulation. H2O2 production in guard cells of rop2 transgenic plants was assayed, as described for Figure 2. Before PA treatment, fluorescence levels in guard cells of the CA-rop2, WT, and DN-rop2 plants were similar (Fig. 5A; P > 0.05). After treatment with PA, fluorescence emission from guard cells of CA-rop2, WT, and DN-rop2 plants increased to 217%, 168%, and 150% of control levels, respectively (Fig. 5A). Compared with WT plants, the change in ROS levels was significantly higher and lower for CA-rop2 (P < 0.01) and DN-rop2 (P < 0.01) plants, respectively. PC did not stimulate ROS production in any rop2 transgenic plants (data not shown). Similar results were obtained in the assay using whole leaf. CA leaves exhibited more intense deposition of brown DAB polymers than WT or DN-rop2 leaves in response to PA treatment (Fig. 5B). These results support that PA activates the ROP-mediated ROS generation pathway, resulting in leaf cell death.
Figure 5.
Plants transformed with mutant rop2 display altered H2O2 production rates upon 500 μm PA treatment. A, DCF fluorescence intensity measured from images of guard cells before (control) and 10 min after treatment with PA suspension. Results from three independent experiments are combined (averages ± se, n = 200-238). B, DAB staining of H2O2 in leaves of rop2 transgenic plants treated with 500 μm PA for 5.5 h. A representative result of three independent experiments is shown.
DISCUSSION
In this study, we examined the effect of PA at the whole-leaf and single-cell levels to elucidate its physiological role and mechanism of action. The lipid induces ROS and cell death in Arabidopsis leaves and generates ROS at the single-cell level (Figs. 1 and 2). We suggest that these responses involve ROP2-mediated ROS-generating machinery, based on the finding that rop2 transgenic plants display distinct responses to PA (Figs. 4 and 5). However, an activation of ROP small G protein alone is not sufficient to induce ROS production and cell death, because activated ROP2 by itself did not induce ROS generation or cell death (Fig. 4A and 5A); PA was required to initiate ROS generation and cell death even in CA-rop2 plants. In addition, ROS levels differed between the mutant and the WT plants only in the presence of PA (Fig. 5A). Therefore, the effect of PA on ROS production and cell death does not seem to be mediated via a direct activation of ROP small G protein by PA. The direct target of PA for activation of ROS generation remains to be determined.
PA specifically induced leaf cell death in Arabidopsis (Fig. 1, A and D). PAs with either di16:0 or di18:1 fatty acyl chains induced cell death, suggesting that the cell death induced by PA is independent of the fatty acyl chains (Fig. 1B). This result is also consistent with a previous report, which showed that PAs with various fatty acyl tails stimulated ROS production (Sang et al., 2001a). However, because the lesions generated by di18:1-PA were consistently smaller than those generated by di16:0-PA, we cannot exclude the possibility that the fatty acyl chains may change the effective concentration of PA on cell death. Alternatively, the difference in the magnitude of the effect of the two PAs may have been due to different rates of metabolism or incorporation of different PA types.
Because PA has a tendency to form hexagonal II phase in the presence of Ca2+ (Verkleij et al., 1982) that may lead to membrane destabilization, we additionally tested unsaturated phosphatidylethanolamine with a similar propensity to destabilize membranes (Cullis and de Kruijff, 1979), along with detergents, other phospholipids, and PA metabolic products. The cell death response was detected only in PA-treated leaves, implying that the observed cell death is not due to membrane destabilization or a general effect of phospholipids or their metabolites, but is a specific response to PA itself (Fig. 1D).
Next, the mechanism of PA-induced leaf cell death was investigated. An important factor that induces cell death in plant cells is ROS including superoxide anion and hydrogen peroxide (Lamb and Dixon, 1997; van Camp et al., 1998). Earlier data show that PA promotes superoxide anion production in Arabidopsis leaves (Sang et al., 2001a). In this study, we confirm that PA promotes ROS production in Arabidopsis, both at the leaf and single guard cell level (Fig. 2). PA-induced ROS production was observed within 10 min of treatment in guard cells before cell death became apparent (data not shown), suggesting that ROS is a mediator of PA-induced cell death.
Guard cells actively participate in defense and stress responses because stomata are an exit pathway for water and a major entry site for pathogens. Therefore, guard cells respond to very diverse stimuli including hormones, abiotic stresses such as cadmium (Perfus-Barbeoch et al., 2002) and drought (Schroeder et al., 2001), and elicitors such as chitosan and oligosaccharides (Lee et al., 1999). Among the many signal mediators that have been identified (for review, see Hetherington, 2001), PA and ROS mediate stomatal closing movement in response to ABA (Jacob et al., 1999; Pei et al., 2000). However, there has been no report of PA effect on ROS generation in guard cells. We demonstrated the link between PA and ROS generation, which may be important for many signaling processes in guard cells (Figs. 2 and 5).
ROP activation is implicated in defense responses and developmental processes that involve ROS generation (Kawasaki et al., 1999; Potikha et al., 1999; Baxter-Burrell et al., 2002). In our in vitro assay, exogenously added active ROP2 protein enhanced ROS production (Fig. 3), thus confirming its role in this process. At the intact leaf level, rop2 transgenic plants displayed different cell death response times upon application of PA. CA-rop2 leaves were the earliest to lose turgor pressure, whereas DN-rop2 leaves were the latest (Fig. 4). This pattern correlated clearly with ROS production responses of each transgenic line (Fig. 5); more ROS was observed in CA-rop2 leaves than in WT or DN-rop2 leaves when treated with PA. The data support a possible involvement of ROP2 in PA-induced ROS production and cell death.
Leaf cell death progression differed between the mutant and WT plants when floated in 500 μm PA solution (Fig. 4). This concentration is much higher than the PA concentration required to induce other physiological responses in other plant materials (Ritchie and Gilroy, 1998; Jacob et al., 1999; Lee et al., 2001, 2003; Sang et al., 2001a). However, we suggest that the cell death response to PA we observed is a natural cell death cascade instead of necrosis for the following reasons. First, infiltrated PA is metabolized rapidly (Sang et al., 2001a), reducing the PA concentration to much lower concentrations than what we applied. Second, stress may cause the intracellular PA level to increase to very high levels in a micro-environment. In wounded leaves, for example, the PA level goes up 4- to 6-fold (Lee et al., 1997, 2001). Third, treatment with unsaturated PE, which has a similar toxicity profile as PA, did not induce a PA-generated response. Fourth, whereas the PA uptake level among the three plant genotypes did not differ, the symptoms associated with death induced by 500 μm PA correlated well with the ROS production levels of different genotypes (Figs. 4 and 5). In contrast, PA at 1 mm rapidly induced symptoms associated with death in all genotypes (data not shown), indicating that PA at this concentration induced toxicity directly. These results collectively indicate that the response shown in this study most likely simulates a natural biological response of plants to various stresses.
In contrast to the pro-death effect of PA that we found, many animal studies found that PA at low concentrations has an anti-death function (Kishikawa et al., 1999; Lee et al., 2000; Siddiqui et al., 2001; Popescu et al., 2002). Because our experimental scheme did not allow anti-death effect to become apparent, it remains possible that PA at lower concentrations may be inhibitory to cell death.
Interestingly, under normal conditions, CA-rop2 guard cells contained similar levels of ROS as WT or DN-rop2 (Fig. 5A). Moreover, CA-rop2 leaves did not display spontaneous cell death (Fig. 4A), indicating that activation of ROP alone was not sufficient to induce ROS production and cell death in Arabidopsis. At first glance, this result appears to contradict the data presented in Figure 3, where ROP2 enhanced ROS generation in an in vitro experiment. However, this discrepancy is very probably due to the presence in the in vitro assay of a high level of PA artificially generated during the process of plant homogenization. This interpretation is consistent with the previous report that the addition of PA induced significant effects on ROS production only when PLDα-deficient plants were employed, presumably because they release reduced amounts of PA during homogenization (Sang et al., 2001a). Therefore, activated ROP appears to enhance ROS generation only in the presence of sufficient amounts of PA. Therefore, PA is not an upstream effector of ROP but is more likely to act downstream of activated ROP in the ROS-generating pathway. PA may regulate ROS-generating machinery at the assembly of active ROP into the active NADPH oxidase complex under various stress conditions. Further research on the identity of the target site of PA action is likely to be highly rewarding.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Seeds of WT, CA-rop2-, and DN-rop2-transformed Arabidopsis ecotype Columbia were sown in soil, grown in a growth chamber or greenhouse at 21°C ± 1°C under light/dark cycles of 16/8 h, and used at the 4- or 6-week-old stage.
Preparation of Liposomes and Treatments
Chemicals were purchased from Sigma-Aldrich (St. Louis) unless specifically indicated. Lipids and fatty acids tested in this study included di16:0-PA, di18:1-PA, di16:0-PC, di18:1 phosphatidylethanolamine, phosphatidylinositol from soybean (Glycine max), di16:0 phosphatidyl-Ser, 1-oleoyl lysophosphatidic acid (Avanti Polar Lipids, Birmingham, AL), 1-palmitoyl-2-oleoyl diacylglycerol, linoleic acid, palmitic acid, and myristic acid. The compounds were dissolved in organic solvents, dried under nitrogen gas, and then sonicated with water. Prepared lipid suspensions were infiltrated into rosette leaves of intact plants with the pressure of needleless syringe and, in the case of detached leaves, introduced by floating the leaves on the suspension. Unless indicated, di16:0-PA was used in all experiments.
Assay of H2O2 Levels in Guard Cells
The production of H2O2 in guard cells was examined using 2′,7′-dichlorofluorescein diacetate (Molecular Probes, Eugene, OR; Lee et al., 1999). The epidermal layer of Arabidopsis leaf was obtained by sticking the abaxial side of the leaf to a slide glass smeared with medical adhesive (Hollister 7730, Hollister, Libertyville, IL) and scraping away all other cell layers with a single-edge razor blade. The epidermal layer was incubated with 50 μm 2′,7′-DCF diacetate including 0.1% (w/v) p-phenylenediamine (anti-fading agent) for 10 min and treated with a drop of lipid suspension for another 10 min. After a brief wash, the tissue was observed under a fluorescent microscope (Axioskop 2, Zeiss, Welwyn Garden City, UK), and pictures were taken using an Axio Cam CCD camera (Zeiss). Green fluorescence intensity was measured from the images of the whole guard cells with Adobe Photoshop 6.0, according to the method described previously (Park et al., 2003). In brief, we delineated regions of individual pairs of guard cells from pictures of epidermal strips and calculated the mean green fluorescence intensity for these regions.
Detection of H2O2 in Arabidopsis Leaves
H2O2 was visualized in Arabidopsis leaf, using DAB as a substrate (Thordal-Christensen et al., 1997). Leaves were excised and then abraded at the adaxial side with a water emulsion of ground sea sand. After 2 h of stabilization in water, the leaves were supplied with a 1 mg mL-1 DAB solution for 5 to 8 h at 25°C through the abraded side. DAB-loaded leaves were floated on lipid suspensions containing 1 mg mL-1 DAB. The treatment was terminated by immersion of the leaves in boiling ethanol (95%) for 10 min. After cooling, the leaves were retained in ethanol and photographed.
Lucigenin Assay for Superoxide Synthesis
The superoxide generation activity was assayed by the LDC method (Sang et al., 2001a) with some modifications. GST-ROP2 fusion proteins were expressed in Escherichia coli and purified as described previously (Wu et al., 2000), and then 1 μg of GST-ROP2 protein was preloaded in nucleotide binding buffer (50 mm Tris-HCl, pH 7.5, 10 mm MgCl2, 1 mm dithiothreitol, 5 mm EDTA, 1 mg mL-1 bovine serum albumin, 1 mm Na3VO4, 5 mm NaF, 1 mm phenylmethylsulfonyl fluoride, and 100 μm GTP or GDP). Proteins were extracted from five rosette leaves of 4-week-old plants by grinding in mortar and pestle under liquid nitrogen. After hydration with 1 mL of extraction buffer (50 mm Tris-HCl, pH 7.5, 10 mm KCl, 1 mm EDTA, 0.5 mg mL-1 bovine serum albumin, 0.5 mm phenylmethylsulfonyl fluoride, 10 mm β-mercaptoethanol, and 0.6% [w/v] PVP-40), the sample was centrifuged at 10,000g at 4°C for 45 min, and the supernatant was collected. Protein content was determined by the Bradford method (Bio-Rad Laboratories, Hercules, CA). A standard assay mixture contained 5 μg of Arabidopsis leaf protein, 1 μg of GST-ROP2 protein preloaded with nucleotide, 0.1 m Gly-NaOH, pH 9.0, 1 mm EDTA, 0.02% (v/v) Triton X-100, 0.2 mm lucigenin, and 80 μm NADPH to make a total volume of 500 μL. The reaction was initiated by the addition of NADPH. In the control reaction mixture, GST-ROP2 protein was omitted. LDC was measured in a luminometer (Lumat LB9501, Berthold, Bad Wildbad, Germany) with counts reported every 10 s for 1 min, and the last two values were averaged.
Acknowledgments
We thank Eunsook Jeong for managing the plants.
Article, publication date, and citation information can be found at www.plantphysiol.org/cgi/doi/10.1104/pp.103.031393.
This work was supported by the Korea Research Foundation (grant no. KRF-2001-015-DS0052) and by the Korea Science and Engineering Foundation (grant no. 2000-6-203-01-2) awarded to Y.L. and Z.Y.
References
- Arabidopsis Genome Initiative (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796-815 [DOI] [PubMed] [Google Scholar]
- Baxter-Burrell A, Yang Z, Springer PS, Bailey-Serres J (2002) RopGAP4-dependent Rop GTPase rheostat control of Arabidopsis oxygen deprivation tolerance. Science 296: 2026-2028 [DOI] [PubMed] [Google Scholar]
- Cullis PR, de Kruijff B (1979) Lipid polymorphism and the functional roles of lipids in biological membranes. Biochim Biophys Acta 559: 399-420 [DOI] [PubMed] [Google Scholar]
- Doke N (1983) Involvement superoxide anion generation in hypersensitive response of potato tuber tissues to infection with an incompatible race of Phytopthora infestans. Physiol Plant Pathol 23: 345-347 [Google Scholar]
- Dwyer SC, Legendre L, Low PS, Leto TL (1996) Plant and human neutrophil oxidative burst complexes contain immunologically related proteins. Biochim Biophys Acta 1289: 231-237 [DOI] [PubMed] [Google Scholar]
- Fan L, Zheng S, Wang X (1997) Antisense suppression of phospholipase D alpha retards abscisic acid- and ethylene-promoted senescence of post-harvest Arabidopsis leaves. Plant Cell 9: 2183-2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank W, Munnik T, Kerkmann K, Salamini F, Bartels D (2000) Water deficit triggers phospholipase D activity in the resurrection plant Craterostigma plantgineum. Plant Cell 12: 111-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AM (2001) Guard cell signaling. Cell 107: 711-714 [DOI] [PubMed] [Google Scholar]
- Jacob T, Ritchie S, Assmann SM, Gilroy S (1999) Abscisic acid signal transduction in guard cells is mediated by phospholipase D activity. Proc Natl Acad Sci USA 96: 12192-12197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T, Henmi K, Ono E, Hatakeyama S, Iwano M, Satoh H, Shimamoto K (1999) The small GTP-binding protein Rac is a regulator of cell death in plants. Proc Natl Acad Sci USA 96: 10922-10926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T, Damude HG, Wener D, Doerner P, Dixon RA, Lamb C (1998) A plant homolog of the neutrophil NADPH oxidase gp91phox subunit gene encodes a plasma membrane protein with Ca2+ binding motifs. Plant Cell 10: 255-266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishikawa K, Chalfant CE, Perry DK, Bielawska A, Hannun YA (1999) Phosphatidic acid is a potent and selective inhibitor of protein phosphatase 1 and an inhibitor of ceramide-mediated responses. J Biol Chem 274: 21335-21341 [DOI] [PubMed] [Google Scholar]
- Lamb C, Dixon RA (1997) The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48: 251-275 [DOI] [PubMed] [Google Scholar]
- Lee S, Choi H, Suh S, Doo I-S, Oh K-Y, Choi EJ, Taylor ATS, Low PS, Lee Y (1999) Oligogalacturonic acid and chitosan reduce stomatal aperture by inducing the evolution of reactive oxygen species from guard cells of tomato and Commelina communis. Plant Physiol 121: 147-152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Hirt H, Lee Y (2001) Phosphatidic acid activates a wound-activated MAPK in Glycine max. Plant J 26: 479-486 [DOI] [PubMed] [Google Scholar]
- Lee S, Park J, Lee Y (2003) Phosphatidic acid induces actin polymerization by activating protein kinases in soybean cells. Mol Cell 15: 313-319 [PubMed] [Google Scholar]
- Lee S, Suh S, Kim S, Crain RC, Kwak JM, Nam H-G, Lee Y (1997) Systemic elevation of phosphatidic acid and lysophospholipid levels in wounded plants. Plant J 12: 547-556 [Google Scholar]
- Lee SD, Lee BD, Han JM, Kim JH, Kim Y, Suh PG, Ryu SH (2000) Phospholipase D2 activity suppresses hydrogen peroxide-induced apoptosis in PC12 cells. J Neurochem 75: 1053-1059 [DOI] [PubMed] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C (1994) H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 79: 583-593 [DOI] [PubMed] [Google Scholar]
- Li H, Shen J-J, Zheng Z-L, Lin Y, Yang Z (2001) The Rop GTPase switch controls multiple developmental processes in Arabidopsis. Plant Physiol 126: 670-684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscovitch M, Czarny M, Fiucci G, Tang X (2000) Phospholipase D: molecular and cellular biology of a novel gene family. Biochem J 345: 401-415 [PMC free article] [PubMed] [Google Scholar]
- Munnik T (2001) Phosphatidic acid: an emerging plant lipid second messenger. Trends Plant Sci 6: 227-233 [DOI] [PubMed] [Google Scholar]
- Munnik T, Meijer HJ, Ter Riet B, Hirt H, Frank W, Bartels D, Musgrave A (2000) Hyperosmotic stress stimulates phospholipase D activity and elevates the levels of phosphatidic acid and diacylglycerol pyrophosphate. Plant J 22: 147-154 [DOI] [PubMed] [Google Scholar]
- Ono E, Wong HL, Kawasaki T, Hasegawa M, Kodama O, Shimamoto K (2001) Essential role of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA 98: 759-764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palicz A, Foubert TR, Jesaitis AJ, Marodi L, McPhail LC (2001) Phosphatidic acid and diacylglycerol directly activate NADPH oxidase by interacting with enzyme components. J Biol Chem 276: 3090-3097 [DOI] [PubMed] [Google Scholar]
- Park J, Choi H-J, Lee S, Lee T, Yang Z, Lee Y (2000) Rac-related GTP-binding protein in elicitor-induced reactive oxygen generation by suspension-cultured soybean cells. Plant Physiol 124: 725-732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-Y, Jung J-Y, Park J, Hwang J-U, Kim Y-W, Hwang I, Lee Y (2003) A role of phosphatidylinositol 3-phosphate in ABA-induced reactive oxygen species generation in guard cells. Plant Physiol 132: 92-98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature 406: 731-734 [DOI] [PubMed] [Google Scholar]
- Perfus-Barbeoch L, Leonhardt N, Vavasseur A, Forestier C (2002) Heavy metal toxicity: cadmium permeates through calcium channels and disturbs the plant water status. Plant J 32: 539-548 [DOI] [PubMed] [Google Scholar]
- Popescu AT, Vidulescu C, Stanciu CL, Popescu BO, Popescu LM (2002) Selective protection by phosphatidic acid against staurosporine-induced neuronal apoptosis. J Cell Mol Med 6: 433-438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potikha TS, Collins CC, Johnson DI, Delmer DP, Levine A (1999) The involvement of hydrogen peroxide in the differentiation of secondary walls in cotton fibers. Plant Physiol 119: 849-858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regier DS, Greene DG, Sergeant S, Jesaitis AJ, McPhail LC (2000) Phosphorylation of p22phox is mediated by phospholipase d-dependent and -independent mechanisms: correlation of NADPH oxidase activity and p22phox phosphorylation. J Biol Chem 275: 28406-28412 [DOI] [PubMed] [Google Scholar]
- Regier DS, Waite KA, Wallin R, McPhail LC (1999) A phosphatidic acid-activated protein kinase and conventional protein kinase C isoforms phosphorylate p22phox, an NADPH oxidase component. J Biol Chem 274: 36601-36608 [DOI] [PubMed] [Google Scholar]
- Ritchie S, Gilroy S (1998) Abscisic acid signal transduction in the barley aleurone is mediated by phospholipase D activity. Proc Natl Acad Sci USA 95: 2697-2702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu SB, Wang X (1995) Expression of phospholipase D during castor bean leaf senescence. Plant Physiol 108: 713-719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu SB, Wang X (1996) Activation of phospholipase D and the possible mechanism of activation in wound-induced lipid hydrolysis in castor bean leaves. Biochim Biophys Acta 1303: 243-250 [DOI] [PubMed] [Google Scholar]
- Ryu SB, Wang X (1998) Increase in free linolenic and linoleic acids associated with phospholipase d-mediated hydrolysis of phospholipids in wounded castor bean leaves. Biochim Biophys Acta 1393: 193-202 [DOI] [PubMed] [Google Scholar]
- Sang Y, Cui D, Wang X (2001a) Phospholipase D and phosphatidic acid-mediated generation of superoxide in Arabidopsis. Plant Physiol 126: 1449-1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang Y, Zheng S, Li W, Huang B, Wang X (2001b) Regulation of plant water loss by manipulating the expression of phospholipase D alpha. Plant J 28: 135-144 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Kwak JM, Allen GJ (2001) Guard cell abscisic acid signaling and engineering drought hardiness in plants. Nature 410: 327-330 [DOI] [PubMed] [Google Scholar]
- Siddiqui RA, Jenski LJ, Wiesehan JD, Hunter MV, Kovacs RJ, Stillwell W (2001) Prevention of docosahexaenoic acid-induced cytotoxicity by phosphatidic acid in Jurkat leukemic cells: the role of protein phosphatase-1. Biochim Biophys Acta 1541: 188-200 [DOI] [PubMed] [Google Scholar]
- Thordal-Christensen H, Zhang Z, Wei Y, Collinge DB (1997) Subcellular localization of H2O2 in plants: H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew interaction. Plant J 11: 1187-1194 [Google Scholar]
- Torres MA, Onouchi H, Hamada S, Machida C, Hammond-Kosack KE, Jones JD (1998) Six Arabidopsis thaliana homologues of human respiratory burst oxidase (gp91phox). Plant J 14: 365-370 [DOI] [PubMed] [Google Scholar]
- van Camp W, van Montagu M, Inzé D (1998) H2O2 and NO: redox signals in disease resistance. Trends Plant Sci 3: 330-334 [Google Scholar]
- van der Luit AH, Piatti T, van Doorn A, Musgrave A, Felix G, Boller T, Munnik T (2000) Elicitation of suspension-cultured tomato cells triggers the formation of phosphatidic acid and diacylglycerol pyrophosphate. Plant Physiol 123: 1507-1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkleij AJ, de Maagd R, Leunissen-Bijvelt J, de Kruijff B (1982) Divalent cations and chlorpromazine can induce non-bilayer structures in phosphatidic acid-containing model membranes. Biochim Biophys Acta 684: 255-262 [DOI] [PubMed] [Google Scholar]
- Waite KA, Walline R, Qualliotine-Mann D, McPhail LC (1997) Phosphatidic acid-mediated phosphorylation of the NADPH oxidase component p47-phox: evidence that phosphatidic acid may activate a novel kinase. J Biol Chem 272: 15569-15578 [DOI] [PubMed] [Google Scholar]
- Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou H-E, Rajashekar CB, Williams TD, Wang X (2002) Profiling membrane lipids in plant stress responses. J Biol Chem 277: 31994-32002 [DOI] [PubMed] [Google Scholar]
- Wu G, Li H, Yang Z (2000) Arabidopsis RopGAPs are a novel family of rho GTPase-activating proteins that require the Cdc42/Rac-interactive binding motif for rop-specific GTPase stimulation. Plant Physiol 124: 1625-1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing T, Higgins VJ, Blumwald E (1997) Race-specific elicitors of Cladosporium fulvum promote translocation of cytosolic components of NADPH oxidase to the plasma membrane of tomato cells. Plant Cell 9: 249-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z (2002) Small GTPase: versatile signaling switches in plants. Plant Cell 14: S375-S388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young SA, Wang X, Leach JE (1996) Changes in the plasma membrane distribution of rice phopholipase D during resistant interactions with Xanthomonas oryzae pv oryzae. Plant Cell 8: 1079-1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zien CA, Wang C, Wang X, Welti R (2001) In vivo substrates and the contribution of the common phospholipase D, PLDalpha, to wound-induced metabolism of lipids in Arabidopsis. Biochim Biophys Acta 1530: 236-248 [DOI] [PubMed] [Google Scholar]